Abstract
Insights into immune-mediated thrombotic thrombocytopenic purpura (iTTP) pathophysiology have led to novel targeted therapies. Immunomodulatory strategies target anti-ADAMTS13 antibodies: rituximab is effective in inducing responses in refractory/relapsed TTP and increasing relapse-free survival; caplacizumab targets the von Willebrand factor–platelet interaction to hasten platelet count recovery and reduce mortality and TTP-related ischemic events. Bortezomib and recombinant ADAMTS13 are under investigation. This review examines how targeted therapies are disrupting current treatment paradigms to improve outcomes of iTTP.
Introduction
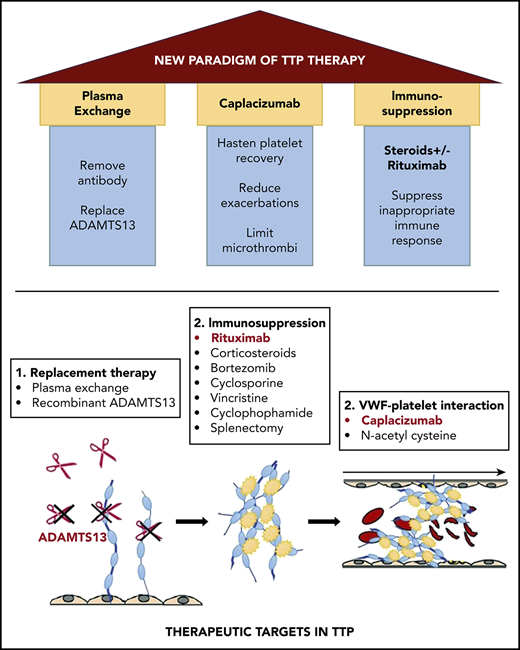
Immune-mediated thrombotic thrombocytopenic purpura (iTTP), also called acquired TTP, is an acute, life-threatening disorder characterized by microangiopathic hemolytic anemia (MAHA), thrombocytopenia, and ischemic organ impairment resulting from platelet-rich microthrombi. iTTP is caused by a deficiency of ADAMTS13, a von Willebrand factor (VWF) cleaving protease, from inhibitory and/or clearing autoantibodies.1 The advent of plasma exchange (PEX) reduced mortality of acute iTTP from >90% to <20%2 and established a new standard of care for this historically fatal disorder. Insights into iTTP pathophysiology have led to novel targeted treatments. Recognition of the central role of anti-ADAMTS13 antibodies ushered in immunomodulatory therapies, of which rituximab is the most successful.3 VWF multimers have also emerged as a therapeutic target with caplacizumab, a nanobody targeting the VWF–platelet interaction.4,5 Here, we examine how targeted therapies are changing the current paradigm of iTTP management (Figure 1).
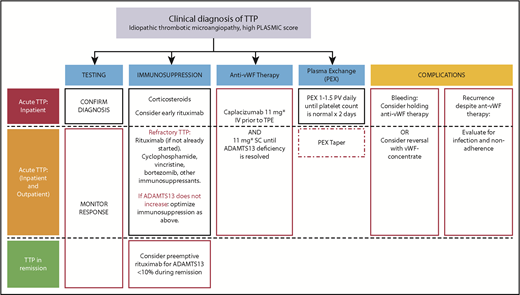
The impact of targeted therapy on treatment of TTP. Changes to current management are indicated in red. iTTP remains a clinical diagnosis, and the PLASMIC score can be useful in estimating the probability of ADAMTS13 deficiency. Laboratory testing: ADAMTS13 activity <10% confirms the diagnosis. Monitoring ADAMTS13 can determine safety for stopping caplacizumab and guide preemptive rituximab during remission. Immunosuppression: Early addition of rituximab has previously been studied to prevent exacerbation (though a large RCT has not been completed) and relapse. Early addition of rituximab may be justified to shorten duration of caplacizumab to reduce overall cost and relapse rate. For patients with persistent ADAMTS13 deficiency while on caplacizumab, or those with persistent/recurring thrombocytopenia while on PEX and immunosuppression (refractory ITP), consider agents with reported efficacy in refractory iTTP. These include bortezomib, cyclophosphamide, and vincristine as well as other immunosuppressant agents such as cyclosporine, azathioprine, and mycophenolate. Preemptive rituximab for patients in remission with ADAMTS13 deficiency is an emerging strategy in iTTP. Anti-vWF therapy: Caplacizumab was started on day 1 in the RCTs that showed reduced mortality and refractoriness, and should be used if feasible. Risk of recurrence of TTP is high in patients with persistent ADAMTS13 deficiency; therefore, if caplacizumab is initiated, it should be continued until ADAMTS13 deficiency is resolved. PEX: Many centers taper PEX; this area remains controversial. Although PEX taper is not required (red dashed box) to maintain a normal platelet count, the optimal duration of PEX is not currently known. The high rate of persistent ADAMTS13 deficiency at 30 days after stopping PEX may suggest underuse of PEX (and/or immunosuppression). A tapering schedule of PEX after achieving a normal platelet count has been used to prevent exacerbations, however, this is less likely to be needed if caplacizumab is used although the high cost of caplacizumab may be a barrier to its widespread use. Complications: Caplacizumab is associated with mucocutaneous bleeding. Although rarely serious, the drug has a relatively short half-life and can be withheld until symptoms resolve. vWF concentrates can be used for serious bleeding. iTTP recurrence on anti-vWF therapy is very uncommon and should prompt evaluation for nonadherence and infections, which are known triggers for exacerbation. *The daily dose of caplacizumab was 10 mg in the phase 2 and 3 clinical trials, which was the dose approved by the European Medicines Agency. The US Food and Drug Administration, however, approved a dose of 11 mg daily. IV, intravenously; SC, subcutaneously.
The impact of targeted therapy on treatment of TTP. Changes to current management are indicated in red. iTTP remains a clinical diagnosis, and the PLASMIC score can be useful in estimating the probability of ADAMTS13 deficiency. Laboratory testing: ADAMTS13 activity <10% confirms the diagnosis. Monitoring ADAMTS13 can determine safety for stopping caplacizumab and guide preemptive rituximab during remission. Immunosuppression: Early addition of rituximab has previously been studied to prevent exacerbation (though a large RCT has not been completed) and relapse. Early addition of rituximab may be justified to shorten duration of caplacizumab to reduce overall cost and relapse rate. For patients with persistent ADAMTS13 deficiency while on caplacizumab, or those with persistent/recurring thrombocytopenia while on PEX and immunosuppression (refractory ITP), consider agents with reported efficacy in refractory iTTP. These include bortezomib, cyclophosphamide, and vincristine as well as other immunosuppressant agents such as cyclosporine, azathioprine, and mycophenolate. Preemptive rituximab for patients in remission with ADAMTS13 deficiency is an emerging strategy in iTTP. Anti-vWF therapy: Caplacizumab was started on day 1 in the RCTs that showed reduced mortality and refractoriness, and should be used if feasible. Risk of recurrence of TTP is high in patients with persistent ADAMTS13 deficiency; therefore, if caplacizumab is initiated, it should be continued until ADAMTS13 deficiency is resolved. PEX: Many centers taper PEX; this area remains controversial. Although PEX taper is not required (red dashed box) to maintain a normal platelet count, the optimal duration of PEX is not currently known. The high rate of persistent ADAMTS13 deficiency at 30 days after stopping PEX may suggest underuse of PEX (and/or immunosuppression). A tapering schedule of PEX after achieving a normal platelet count has been used to prevent exacerbations, however, this is less likely to be needed if caplacizumab is used although the high cost of caplacizumab may be a barrier to its widespread use. Complications: Caplacizumab is associated with mucocutaneous bleeding. Although rarely serious, the drug has a relatively short half-life and can be withheld until symptoms resolve. vWF concentrates can be used for serious bleeding. iTTP recurrence on anti-vWF therapy is very uncommon and should prompt evaluation for nonadherence and infections, which are known triggers for exacerbation. *The daily dose of caplacizumab was 10 mg in the phase 2 and 3 clinical trials, which was the dose approved by the European Medicines Agency. The US Food and Drug Administration, however, approved a dose of 11 mg daily. IV, intravenously; SC, subcutaneously.
Current management of iTTP
iTTP is a medical emergency, and its prompt diagnosis and treatment are critical. ADAMTS13 activity <10% confirms the diagnosis; however, results take several days because few centers have this assay available in-house. Laboratory confirmation of iTTP should not delay institution of PEX and immunosuppression (with high-dose glucocorticoids) in a patient who presents with unexplained MAHA and severe thrombocytopenia.6,7 Clinical prediction rules, such as the PLASMIC score which includes a platelet count <30 × 109/L, hemolysis, no active cancer, no history of solid-organ or stem-cell transplant, mean corpuscle volume <90 fL, international normalized ratio <1.5, and creatinine <2.0 mg/dL,8 can aid in discriminating iTTP from other thrombotic microangiopathies. An external validation of the score in patients with MAHA and thrombocytopenia confirmed that a score of 6 or 7 (high) has sensitivity and specificity >90% and a negative predictive value of 98% for iTTP9 and can help identify patients likely to respond to PEX. However, these metrics are true only when applied to a population with suspected thrombotic microangiopathy and cannot replace clinical judgment or examination of the blood smear for schistocytes.8 The benefit of PEX was conclusively established in a randomized clinical trial (RCT),2 but the use of corticosteroids is based primarily on expert opinion supported by the autoimmune nature of the disease and a series in which 30 of 54 patients with less severe TTP responded to steroid therapy alone in 48 to 72 hours.10 Moreover, a study comparing cyclosporine to prednisone as an adjunct to PEX showed better suppression of ADAMTS13 autoantibodies and an improvement in ADAMTS13 activity in the first month after stopping PEX in patients in the steroid arm.11 The current standard of care is to continue daily PEX until the platelet count normalizes for 2 consecutive days.6,12 Refractory iTTP generally refers to patients who continue to be thrombocytopenic with an accompanying high lactate dehydrogenase after 5 plasma exchanges.13 Some centers offer a tapering schedule of PEX14,15 based on the observation that early recurrences of iTTP within 30 days of stopping plasma exchange (termed exacerbations) are common, and are presumed to be due to persistent anti-ADAMTS13 antibodies.12 Evidence supporting this practice is limited, and exacerbations are expected to be less common in patients treated with caplacizumab or rituximab early in the course of acute TTP.16 Remission in iTTP is defined by a normal platelet count and lactate dehydrogenase after cessation of PEX.6,13 Although central to TTP pathogenesis, ADAMTS13 activity is not routinely used to guide duration of PEX or immunosuppression. Relapses (defined as recurrence >30 days after stopping PEX) occur in 30% to 50% of patients17 and are most common in the first 2 years after the initial diagnosis, although late relapses have been described, and can occur at any point after the initial diagnosis. ADAMTS13 activity during iTTP remission is emerging as a predictor of relapse,18,19 and preemptive immunosuppression for ADAMTS13 deficiency in remission is an area of active investigation.
Rituximab
Rituximab, a chimeric monoclonal antibody directed against CD20, depletes B cells and suppresses the production of anti-ADAMTS13 antibodies, thereby restoring function of the ADAMTS13 protease. It has been used off-label for acquired iTTP since 2002.20 Although no RCTs have evaluated rituximab vs placebo as an adjunct to plasma exchange, rituximab is the current standard of care for patients with relapsed and/or refractory iTTP based on excellent outcomes in patients with a poor response to PEX and high-dose steroids in observational studies.17,21-23 In a British series of 25 patients with refractory or relapsed TTP, all patients achieved remission and none relapsed over a median follow-up of 19 months.21 The French Thrombotic Microangiopathies reference center also reported a higher rate of recovery in 21 patients treated with rituximab for refractory TTP vs 53 historical controls (100% vs 78%) and a lower relapse rate (0% vs 9.4%) at 1 year.24
More recently, rituximab has gained traction as an adjunct to plasma exchange in the upfront treatment of newly diagnosed iTTP based on a single-arm phase 2 trial of 40 patients who received rituximab along with daily plasma exchange for an initial episode of iTTP and had lower relapse rate compared with historical controls (10% vs 57%).17,25,26 Median days of PEX to achieve remission was 16.5 (range, 4-34) compared with 18 (range, 6-92) in historical controls, although this did not meet statistical significance. Rituximab is also used as preemptive therapy to prevent relapses in patients with ADAMTS13 deficiency during remission.26-29 The most commonly used dose and schedule for rituximab is 375 mg/m2 weekly for 4 to 8 doses, based on the dosing for lymphoma. Recent studies suggest that lower doses (100-,16,30 200-,28 and 500-mg fixed doses)28 may have comparable efficacy.
Caplacizumab
Caplacizumab is an anti-VWF nanobody, a humanized, bivalent variable-domain-only immunoglobulin fragment, which inhibits interaction between VWF multimers and platelets, reducing platelet aggregation and microvascular thrombosis.4,5 The safety and efficacy of caplacizumab has been evaluated in 2 double-blind, placebo-controlled RCTs: the Study to Assess Efficacy and Safety of Anti-von Willebrand Factor Nanobody in Patients with Acquired Thrombotic Thrombocytopenic Purpura (phase 2 TITAN study) and Phase 3 Trial with Caplacizumab in Patients with Acquired Thrombotic Thrombocytopenic Purpura (HERCULES trial). Subjects received PEX plus caplacizumab or placebo (10 mg intravenous loading dose followed by 10 mg subcutaneously daily) along with PEX and immunosuppression until normalization of the platelet count and for 30 days thereafter. The primary end point, time to platelet count normalization, was shorter in the caplacizumab vs placebo group in both trials, with a platelet normalization rate ratio of 1.55 (85% confidence interval, 1.10-2.20) in HERCULES.5 Median days of PEX of were 5 (range, 1-35) and 7 (range, 3-46) in the caplacizumab and placebo groups, respectively.5 The incidence of the major secondary end point, a composite of iTTP-related death, iTTP recurrence, and major thromboembolic event, was also lower in caplacizumab treated patients vs placebo (12% vs 49%, P < .001).5 Notably, there were no deaths or refractory TTP in the caplacizumab arm (vs 4 deaths and 7 refractory disease with placebo). Although recurrence rates for patients that received rituximab were not reported, rituximab therapy is not expected to have skewed recurrence rates in favor of caplacizumab because fewer patients in the caplacizumab vs placebo groups received rituximab (39% vs 48%).
Bleeding was the primary adverse effect of caplacizumab therapy and occurred in 65% (vs 48% in the placebo arm) in HERCULES.4 Mucocutaneous bleeding including epistaxis and gingival bleeding were the most common events, and most bleeding was of mild to moderate severity that resolved without intervention. Three subjects that developed severe bleeding on caplacizumab received VWF concentrate (severe epistaxis), tranexamic acid (for gingival bleeding), and a red cell transfusion (for upper gastrointestinal bleeding). Overall, most caplacizumab-related bleeding resolve without intervention (though it may be necessary to hold the drug), whereas topical vasoconstrictors and antifibrinolytics are effective in others, with VWF concentrates reserved for patients with severe, refractory bleeding.
Interestingly, the rate of recurrence after stopping caplacizumab was 22% in TITAN and only 8% in HERCULES. A “catch-up” effect was noted in TITAN, with more recurrences in the caplacizumab vs placebo arm after stopping therapy, highlighting that caplacizumab does not affect autoantibody production and concurrent immunosuppression is crucial. HERCULES investigators were encouraged to continue caplacizumab/placebo for an extension period of up to 4 weeks for patients with ADAMTS13 activity <10% at the end of the treatment period, preventing early recurrences in those with unresolved immunological disease activity. Six subjects had exacerbations on caplacizumab, all of which occurred in the setting of infection or nonadherence. Therefore, hematologists new to the use of the drug should be wary of these factors for patients with recurrences on caplacizumab.
How we anticipate new targeted strategies will change the treatment paradigm
Integrating caplacizumab into the treatment of acute iTTP
Caplacizumab is now approved in Europe and the United States.31 Ideally, caplacizumab would be initiated at diagnosis based on the TITAN and HERCULES trials, which showed that initiating caplacizumab early led to faster platelet count recovery, reduced iTTP-related death, relapses, and major thromboembolic events.4,5 This recommendation is based solely on the assessment that the risk of mucocutaneous bleeding is outweighed by its benefit in reducing (and possibly eliminating) refractoriness to PEX and mortality in iTTP, both of which are can be unpredictable.23,32 However, the absolute reduction in median hospital days (9 vs 12 days), median duration of PEX (5 vs 7 days), mortality (0% vs 4%), and refractoriness (0% vs 4%) is relatively small. The proposed cost of caplacizumab is high at US$270 000 per course (assuming a median of 35 doses and approximately US$7700/dose), which is likely to limit use. Although there is a considerable reduction in the rate of recurrence, particularly early recurrence, which is the most compelling argument for use, this might be offset by earlier use of rituximab. If limited by cost, we would offer caplacizumab to patients with cardiac or neurological involvement, which are predictors of severe disease.32,33 It is tempting to reserve caplacizumab for patients with confirmed ADAMTS13 deficiency and refractoriness; however, there are currently no data to support salvage therapy. For patients who receive caplacizumab, ADAMTS13 activity may be used to tailor duration of therapy similar to the HERCULES trial in which patients with ADAMTS13 <10% at the end of the treatment period were offered extended therapy to provide protection until ADAMTS13 recovery. Although a threshold for stopping therapy was not evaluated, we suggest that ADAMTS13 >20% to 30% for at least 2 consecutive weeks may be a reasonable starting point.
When should rituximab be used in iTTP treatment?
Acute iTTP
Rituximab improves the response rate in iTTP refractory to PEX, but caplacizumab is likely to make this less common. Rituximab appears to increase relapse-free survival, regardless of when it is administered in the course of iTTP (upfront or relapse), with the best evidence being in relapsed iTTP. Although 2 small studies suggest that adding rituximab early in the course of acute iTTP may reduce the duration of PEX and hospitalization, this has not been demonstrated in RCTs. We use rituximab in relapsed and refractory iTTP. Perhaps with the addition of caplacizumab to the treatment of iTTP, it might be justified to use rituximab early in the course and for upfront therapy in patients with their initial episode of iTTP with the goal of increasing ADAMTS13 activity more rapidly, thus allowing faster (saving health care costs) and safer cessation of caplacizumab. This is an important question that warrants investigation.
iTTP during remission
Although some groups advocate that ADAMTS13 deficiency during clinical remission is a predictor of relapse,18,19 there is considerable variability in the natural history of TTP and reduced ADAMTS13 activity does not invariably indicate an imminent relapse.34 No RCTs have evaluated preemptive rituximab to prevent relapses of iTTP, but it is reasonable to consider this strategy in select patients. The French TMA Reference Centre recently reported the long-term outcome of 92 patients with iTTP who received preemptive rituximab during clinical remission, after identification of a severe ADAMTS13 deficiency (activity <10%) during remission.29 During a follow-up period of 35.8 months, rituximab significantly decreased the median number of iTTP relapses (0 episodes per year compared with 0.33 episodes per year on 37 patients before the use of rituximab). This was not an RCT; however, the relapse rate compared with 23 historical controls with ADAMTS13 <10% in remission was 15% vs 74%.29 Our practice is to discuss ADAMTS13 monitoring and preemptive rituximab with our patients. For patients interested in this strategy, we suggest monitoring ADAMTS13 activity every 3 months and offer preemptive rituximab when ADAMTS13 activity is <10% to 20%. The appropriate frequency for monitoring ADAMTS13, the optimal ADAMTS13 activity threshold, and the effects of rituximab on the long-term sequelae of TTP remain to be determined.
ADAMTS13 is an emerging end point for clinical trials in iTTP
To this point, the platelet count has served as the marker of remission and as evidence of efficacy in iTTP trials. For example, in the phase 2 clinical trial of early rituximab for iTTP, the primary end point was time to normal platelet count.25 With caplacizumab, platelet count recovery is less dependent on ADAMTS13 activity, if at all, and thus is not a reliable measure of effective immunosuppression. Moreover, persistent or recurrent ADAMTS13 deficiency is a strong predictor of TTP recurrence, and earlier recovery of ADAMTS13 activity could help tailor duration of immunosuppression (and caplacizumab). We propose that ADAMTS13 activity, as the most reliable marker of TTP disease activity, is an important end point that should be integrated in future therapeutic trials in TTP. However, it is not known whether faster recovery of ADAMTS13 activity correlates with durability of normal ADAMTS13 activity.
Other novel therapies
Bortezomib has reported efficacy in TTP refractory to PEX, corticosteroids, rituximab, and often other immunosuppressive therapies.35,36 It is an attractive agent for multirefractory TTP because it depletes plasma cells that can continue to produce anti-ADAMTS13 antibody even after CD20+ B cells have been depleted by rituximab. However, it needs to be evaluated in prospective studies. Recombinant ADAMTS13 has demonstrated efficacy in increasing ADAMTS13 activity in congenital TTP37 and may have efficacy as an adjunct to PEX in iTTP to minimize the volume and duration of plasma therapy.38
Conclusions and future perspectives
It is an exciting time for iTTP research. Rituximab has an established role in refractory and relapsed iTTP. It also reduces the relapse rate when used in the frontline treatment of iTTP and preemptively during remission in patients who have persistent or recurrent ADAMTS13 deficiency. Results of a recently completed low-dose (100 mg weekly × 4) rituximab pilot study (NCT01554514) are encouraging and, if as effective as the standard dose, would reduce costs and possibly toxicity from infusion reactions.16 Caplacizumab hastens recovery from iTTP and may mitigate ischemic organ damage. Other agents such as bortezomib35,39,40 and recombinant ADAMTS13 are under development.37 We are cautiously optimistic that these developments (Table 1) will further improve survival and prevent the morbidity and mortality associated with TTP recurrences.
Targeted therapies for iTTP: risks, benefits, and unknowns
| Drug . | Risks . | Benefits . | Unknowns . |
|---|---|---|---|
| Rituximab (cost, ∼$20 000 per 4-dose course) | Infusion reactions (first dose: 12%-77%, mostly mild-moderate; decreases with subsequent infusions) | Likely increases relapse-free survival | Consequences of immunosuppression especially with retreatment |
| HBV reactivation (2%) | Upfront for acute iTTP (10% vs 57% at 5 y) | Risk of progressive multifocal leukoencephalopathy (never reported in iTTP) | |
| Preemptively for ADAMTS13 deficiency in remission (15% vs 74%) | |||
| Caplacizumab (cost, ∼$270 000 for a 4-wk course) | Mucosal bleeding (65%, mostly mild to moderate) | Reduction in mortality (0% vs 4%) | Real-world safety/efficacy outside of expert centers |
| Reduction in refractoriness (0% vs 4%) | Effect of cost on utilization | ||
| Reduction in median hospital days (9 vs 12 d) | Adherence in nonclinical trial | ||
| Reduction in PEX days (5 vs 7 d) | Optimal duration of therapy | ||
| (all vs placebo) | |||
| Bortezomib (cost, ∼$5200 for 4 doses) | Thrombocytopenia (16%-52%) | Targets plasma cells | Identifying patients likely to benefit |
| Drug-induced TMA (rare) | Extensive experience with drug (from use in plasma cell dyscrasias) | Durability of response | |
| Peripheral neuropathy (37% with subcutaneous dosing) | Optimal delivery and schedule | ||
| Varicella zoster reactivation (6%-11%) | Utility of combining with other immunosuppressive agents | ||
| Recombinant ADAMTS13 (cost unknown) | None reported | Raises ADAMTS13 activity in congenital TTP | Currently no evidence on use in iTTP |
| Drug . | Risks . | Benefits . | Unknowns . |
|---|---|---|---|
| Rituximab (cost, ∼$20 000 per 4-dose course) | Infusion reactions (first dose: 12%-77%, mostly mild-moderate; decreases with subsequent infusions) | Likely increases relapse-free survival | Consequences of immunosuppression especially with retreatment |
| HBV reactivation (2%) | Upfront for acute iTTP (10% vs 57% at 5 y) | Risk of progressive multifocal leukoencephalopathy (never reported in iTTP) | |
| Preemptively for ADAMTS13 deficiency in remission (15% vs 74%) | |||
| Caplacizumab (cost, ∼$270 000 for a 4-wk course) | Mucosal bleeding (65%, mostly mild to moderate) | Reduction in mortality (0% vs 4%) | Real-world safety/efficacy outside of expert centers |
| Reduction in refractoriness (0% vs 4%) | Effect of cost on utilization | ||
| Reduction in median hospital days (9 vs 12 d) | Adherence in nonclinical trial | ||
| Reduction in PEX days (5 vs 7 d) | Optimal duration of therapy | ||
| (all vs placebo) | |||
| Bortezomib (cost, ∼$5200 for 4 doses) | Thrombocytopenia (16%-52%) | Targets plasma cells | Identifying patients likely to benefit |
| Drug-induced TMA (rare) | Extensive experience with drug (from use in plasma cell dyscrasias) | Durability of response | |
| Peripheral neuropathy (37% with subcutaneous dosing) | Optimal delivery and schedule | ||
| Varicella zoster reactivation (6%-11%) | Utility of combining with other immunosuppressive agents | ||
| Recombinant ADAMTS13 (cost unknown) | None reported | Raises ADAMTS13 activity in congenital TTP | Currently no evidence on use in iTTP |
HBV, hepatitis B virus; TMA, thrombotic microangiopathy.
Acknowledgments
This work was supported by a Mentored Research Award from the Hemostasis and Thrombosis Research Society (S.C.). The funders had no role in preparation of the manuscript, or decision to publish.
Authorship
Contribution: S.C., C.M., and M.A.M. reviewed the literature and drafted and approved the final draft of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Shruti Chaturvedi, Division of Hematology, Department of Medicine, Johns Hopkins University School of Medicine, 720 Rutland Ave, Ross Research Building, Room 1025, Baltimore, MD 21205; e-mail: schatur3@jhmi.edu.
REFERENCES
Author notes
M.A.M. and C.M. contributed equally to this manuscript.