Key Points
Among lymphoid malignancy patients treated with ibrutinib, the subsequent incidence of new hypertension is nearly 72%.
Development of new or worsened HTN after ibrutinib initiation associates with a more than twofold increased risk of other cardiac events.
Abstract
Ibrutinib is associated with dramatic efficacy against B-cell malignancies. Yet, it has been linked with potentially limiting cardiotoxicity, including emerging reports of profound hypertension (HTN). The long-term incidence, severity, and impact of HTN development with ibrutinib are unknown. Therefore, in 562 consecutive patients treated with ibrutinib for B-cell malignancies from 2009 through 2016, we assessed the new/incident or worsened HTN (systolic blood pressure [BP] cutoff, 130 mm Hg). Observed incident HTN rates were compared with Framingham-heart–predicted incident HTN rates. We also evaluated the relationship of HTN to the development of other major adverse cardiovascular events (MACEs), including arrhythmia, myocardial infarction, stroke, heart failure, and cardiovascular death. Further, we assessed the effects of different antihypertensive classes on ibrutinib-related HTN. Overall, 78.3% of ibrutinib users developed new or worsened HTN over a median of 30 months. New HTN developed in 71.6% of ibrutinib users, with a time to 50% cumulative incidence of 4.2 months. Among those without preceding HTN, 17.7% developed high-grade HTN (BP >160/100 mm Hg). In multivariate regression, new or worsened HTN was associated with increased MACEs (hazard ratio [HR], 2.17; 95% confidence interval [CI], 1.08-4.38). No single antihypertensive class was associated with prevention or control of ibrutinib-related HTN. However, antihypertensive initiation was associated with a lower risk of a MACE (HR, 0.40; 95% CI, 0.24-0.66). Collectively, these data suggest that ibrutinib is associated with a substantial increase in the incidence and severity of HTN, and that HTN development carries a higher risk of subsequent cardiotoxic events.
Introduction
Ibrutinib is an oral Bruton’s tyrosine kinase inhibitor, with dramatic efficacy against many lymphoid malignancies.1-5 It also targets several alternative kinases, broadening its efficacy as an effective immune modulator. Accordingly, >150 additional trials testing ibrutinib’s efficacy against other cancers are ongoing.6-9 Despite ibrutinib’s benefits, cardiotoxicity has emerged as an increasingly important complication of this life-saving therapy.5,10-14 Available long-term data have suggested that nearly 20% of ibrutinib users go on to develop incident arryththmia.10-13 Although initial clinical trials had limited ability to describe these events over time, the profile of many of these events has been increasingly well defined.
However, emerging initial reports have suggested that ibrutinib is linked with an even higher prevalence of significant or profound hypertension (HTN), which had not been recognized.15,16 In an analysis of chronic lymphocytic leukemia (CLL) early-phase trial data, nearly 23% of ibrutinib users developed new or worsened HTN.15 Similarly, follow-up data from early clinical trials have suggested a potential continual rise in the incidence of HTN development over time.16,17 Despite this, available initial reports have not linked ibrutinib-related HTN with other cardiovascular events, including arrhythmias.16 Yet, whether this pattern holds over long-term follow-up and the degree to which ibrutinib-related HTN has ramifications on the development of other cardiovascular events remains unknown.
Methods
Study population
From consecutive patients treated with ibrutinib for lymphoid malignancies at The Ohio State University’s Comprehensive Cancer Center from 2009 through 2016, we evaluated the rates of incident or worsening HTN, after Institutional Review Board approval.11,13 We also assessed the incidence of other major cardiovascular events. Study patients included adults ≥18 years of age treated with ibrutinib for any lymphoid malignancy. Blood pressure and other traditional cardiovascular variables were collected across time. Patients with incomplete medical records for the cancer and cardiovascular variables of interest were excluded. Incident (new) HTN was defined as systolic blood pressure (SBP) ≥130 mm Hg on 2 separate visits within 3 months, accounting for contemporary HTN definitions after publication of the Systolic Blood Pressure Intervention Trial (SPRINT) and the stronger correlation between change in SBP (compared with diastolic blood pressure) and major cardiovascular events after 50 years of age.18,19 Worsened HTN was defined as an increase in HTN grade by Common Terminology Criteria for Adverse Events (CTCAE) or an increase in antihypertensive therapy (supplemental Table 1, available on the Blood Web site).20 The presence of baseline HTN was considered to be a documented SBP ≥130 mm Hg on 2 visits within 3 months before ibrutinib initiation or a reported history of HTN with the current use of at least 1 BP-lowering medication. Baseline antihypertensive therapy use by medication class was also recorded. Moreover, we manually searched all subject charts for incident major adverse cardiovascular events (MACEs), defined as stroke, myocardial infarction, heart failure, and cardiac arrhythmia including atrial fibrillation and ventricular arrhythmias, in addition to cardiovascular death.11
Outcomes
The primary outcome was the development of new or worsened HTN after ibrutinib initiation. The secondary outcome was the occurrence of MACEs during ibrutinib use. Follow-up began from the time of ibrutinib initiation. HTN severity and MACEs were graded using CTCAE v5, followed by adjudication by 2 independent cardiologists. A Naranjo Probability Score was also calculated for new or worsened HTN, as well as each MACE, to determine the likelihood of ibrutinib association, with a score of ≥6, suggestive of at least a probable association.21 Moreover, we assessed the avoidance of HTN by baseline antihypertensive class, as well as the relationship of ibrutinib dose (280, 420, 560, and 840 mg) to new or worsened HTN development across time.
Statistical analysis
Descriptive statistics were used to summarize patient characteristics, using the mean ± standard deviation (SD) or median (interquartile range) for continuous variables and frequency counts with percentages for categorical variables. Time-to-event analysis methods were used to summarize HTN and MACE outcomes and evaluate associations with these outcomes. In all analyses, death and ibrutinib discontinuation without the outcome event (HTN or MACE) were considered to be competing risks.11,13 Patients without the outcome of interest or competing risk events were considered censored at the last follow-up time point. Cumulative incidence estimates for primary outcomes were estimated, and cumulative incidence curves were created. From this, time to 50% cumulative incidence (analogous to Kaplan-Meier, but accounting for competing risks) was estimated. Events per 1000 person-years of follow-up were also assessed by HTN status. In order to further understand the relationship of HTN development or worsening to the occurrence of MACEs, we constructed cumulative incidence curves, stratified according to degree of change in BP (≤5, 5-10, and >10 mm Hg) after ibrutinib initiation.
For the assessment of new and worsened HTN, univariate models were fit by using potential risk factors followed by a multivariate model. Fine and Gray proportional subdistribution hazard regression was used, accounting for competing risks of death and ibrutinib discontinuation. In addition to factors significant on univariate modeling, traditional risk factors including age, body mass index (BMI), sex, African American race, diabetes mellitus (DM), chronic kidney disease (CKD), and smoking status were included in the multivariate model as control variables regardless of the univariate P value observed.18 All variables with P < .10 in univariate modeling were initially included in the multivariate models, and backward selection was used to sequentially (stepwise) remove variables with P > .05 from the final model. A similar modeling approach was applied to MACEs, with the primary comparison of patients who developed new or worsened HTN with patients who had no or stable HTN. Stable HTN was defined as patients who did not have an increase in CTCAE HTN grade and did not require additional antihypertensive therapy during follow-up. Covariates in the new/worsened HTN vs no or stable HTN models for the occurrence of postibrutinib initiation MACEs also included age, BMI, sex, DM, CKD, atrial fibrillation, coronary artery disease, cerebrovascular disease, and heart failure.22
Moreover, we evaluated the relationship of new or worsened HTN during ibrutinib use to arrhythmia development (atrial fibrillation or ventricular arrhythmias) alone. In sensitivity analyses, new or worsened HTN status was considered to be a time-varying covariate: patients were classified as no or stable HTN up to the time point of new or worsened HTN development and remained in the new or worsened HTN category from that point forward. We also assessed the effects of antihypertensive therapy initiation on the prevention of subsequent MACEs among those with new or worsened HTN during ibrutinib use. Within this analysis, patients starting a new antihypertensive drug only after a MACE were not classified as receiving antihypertensive therapy (using their follow-up time before MACE development), to minimize confounding reasons for drug initiation (eg, beta-blockers for atrial fibrillation). To assess the impacts of HTN on long-term survival, we performed an additional landmark analysis of time to disease progression or death, beginning at 12 months after ibrutinib initiation. This analysis included only those patients who survived to 12 months without disease progression and remained on ibrutinib beyond 12 months.
In order to better understand ibrutinib’s effect on BP modification, observed rates of new HTN were compared with available short-term (1-year), Framingham-predicted rates of new HTN, accounting for differences in HTN definition (rate of incident increase of BP to ≥140/90 mm Hg, as used within original validation).23,24 To provide a more vetted comparison with the Framingham predictions, only those patients aged 20 to 69 years without a diagnosis of diabetes were included, matching the inclusion criteria used for the prediction model. Observed rates of new HTN development rates were calculated as cumulative incidence of BP ≥140/90 mm Hg, accounting for available follow-up. The expected Framingham predicted rate of new HTN within 1 year for this study cohort was calculated by averaging the individual patient probabilities obtained after applying the individual patient-level risk factors. All analyses were performed with SAS Software, version 9.4, and Stata 15, and the statistical tests were 2 sided, with statistical significance evaluated at the α = 0.05 significance level.
Results
Overall, 562 patients treated with ibrutinib were identified. Demographics of the complete cohort have been previously described (Table 1).11,13 The patients had a mean age of 63.8 ± 10.8 years and a mean BMI of 28.0 ± 5.5 kg/m2; 70.6% were male, and 92.5% had an Eastern Cooperative Oncology Group performance status of 0 to 1; in addition, 73.8% of patients had CLL, and most were treated with ibrutinib for relapsed disease. Ibrutinib was initiated as part of combination therapy in 173 patients (30.8%). Sixty-two percent (347 patients) had preceding HTN at the time of ibrutinib initiation, among which 63% were on at least 1 antihypertensive medication.
Baseline characteristics
| Variable . | Total (n = 562) . | No HTN at baseline (n = 215) . | HTN present at baseline (n = 347) . |
|---|---|---|---|
| Age, mean y (SD) | 63.8 (10.8) | 60.8 (10.5) | 65.6 (10.7) |
| Sex, n (%) | |||
| Male | 397 (70.6) | 149 (69.3) | 248 (71.5) |
| Female | 165 (29.4) | 66 (30.7) | 99 (28.5) |
| Race, n (%) | |||
| White | 521 (92.7) | 206 (95.8) | 315 (90.8) |
| African American | 30 (5.3) | 4 (1.9) | 26 (7.5) |
| Other* | 2 (0.4) | 0 (0.0) | 2 (0.6) |
| BMI, mean (SD) | 28.0 (5.5) | 26.2 (4.8) | 29.1 (5.7) |
| BMI, n (%) | |||
| <25 | 180 (32.0) | 96 (44.7) | 84 (24.2) |
| 25-29.9 | 228 (40.6) | 88 (40.9) | 140 (40.4) |
| ≥30 | 154 (27.4) | 31 (14.4) | 123 (35.5) |
| Other baseline traditional HTN risk factors | |||
| DM | 78 (13.9) | 15 (7.0) | 63 (18.2) |
| MI | 37 (6.6) | 9 (4.2) | 28 (8.1) |
| CAD | 65 (11.6) | 15 (7.0) | 50 (14.4) |
| CKD | 130 (23.1) | 40 (18.6) | 90 (25.9) |
| CHF | 15 (2.7) | 3 (1.4) | 12 (3.5) |
| AF | 25 (4.5) | 10 (4.7) | 15 (4.3) |
| Smoking status | |||
| Never | 306 (54.5) | 125 (58.1) | 181 (52.2) |
| Previous | 232 (41.3) | 82 (38.1) | 150 (43.2) |
| Current | 24 (4.3) | 8 (3.7) | 16 (4.6) |
| Primary malignancy, n (%) | |||
| CLL | 415 (73.8) | 168 (78.1) | 247 (71.2) |
| MCL | 58 (10.3) | 17 (7.9) | 41 (11.8) |
| WM | 10 (1.8) | 2 (0.9) | 8 (2.3) |
| Other† | 79 (14.1) | 28 (13.0) | 51 (14.7) |
| Rai stage, n (%)‡ | |||
| 0 | 2 (0.5) | 1 (0.6) | 1 (0.4) |
| 1 | 36 (8.7) | 17 (10.1) | 19 (7.7) |
| 2 | 80 (19.3) | 20 (11.9) | 60 (24.3) |
| 3 | 95 (22.9) | 41 (24.4) | 54 (21.9) |
| 4 | 201 (48.4) | 89 (53.0) | 112 (45.3) |
| Unknown | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| CLL, risk stratification, n (%) | |||
| Del17p13 | 155 (37.4) | 73 (43.5) | 82 (33.2) |
| Del11q22 | 98 (23.6) | 35 (20.8) | 63 (25.5) |
| Trisomy 12 | 37 (8.9) | 12 (7.1) | 25 (10.1) |
| Del13q14 | 58 (14.0) | 26 (15.5) | 32 (13.0) |
| None of the above | 67 (16.1) | 22 (13.1) | 45 (18.2) |
| Baseline ECOG performance status, n (%) | |||
| 0 | 196 (34.9) | 78 (36.3) | 118 (34.0) |
| 1 | 321 (57.1) | 119 (55.3) | 202 (58.2) |
| 2 | 34 (6.0) | 11 (5.1) | 23 (6.6) |
| 3 | 2 (0.4) | 1 (0.5) | 1 (0.3) |
| 4 | 2 (0.4) | 1 (0.5) | 1 (0.3) |
| Unknown | 7 (1.2) | 5 (2.3) | 2 (0.6) |
| Treatment history, n (%) | |||
| Number of prior anticancer therapies, median (IQR) | 2 (0-5) | 2 (0-5) | 2 (0-5) |
| Prior anthracycline | 131 (23.3) | 51 (23.7) | 80 (23.1) |
| Concurrent anthracycline | 9 (1.6) | 3 (1.4) | 6 (1.7) |
| Prior chemotherapy | 469 (83.5) | 184 (85.6) | 285 (82.1) |
| Prior monoclonal antibody | 500 (89.0) | 199 (92.6) | 301 (86.7) |
| Prior targeted therapy | 105 (18.7) | 36 (16.7) | 69 (19.9) |
| Prior immunomodulatory | 76 (13.5) | 27 (12.6) | 49 (14.1) |
| CYP3A4 inhibitor | 33 (5.9) | 18 (8.4) | 15 (4.3) |
| Cyclosporine during ibrutinib use | 5 (0.9) | 1 (0.5) | 4 (1.2) |
| No prior anticancer therapies, n (%) | 35 (6.2) | 11 (5.1) | 24 (6.9) |
| Baseline mm Hg SBP, n (%) | |||
| <100 | 18 (3.2) | 17 (7.9) | 1 (0.3) |
| 100-119 | 136 (24.2) | 94 (43.7) | 42 (12.1) |
| 120-129 | 129 (23.0) | 85 (39.5) | 44 (12.7) |
| 130-139 | 142 (25.3) | 14 (6.5) | 128 (36.9) |
| 140-179 | 136 (24.2) | 5 (2.3) | 131 (37.8) |
| 180+ | 1 (0.2) | 0 (0.0) | 1 (0.3) |
| Baseline mm Hg DBP, n (%) | |||
| <70 | 256 (45.6) | 133 (61.9) | 123 (35.4) |
| 70-79 | 192 (34.2) | 64 (29.8) | 128 (36.9) |
| 80-89 | 101 (18.0) | 18 (8.4) | 83 (23.9) |
| 90-119 | 13 (2.3) | 0 (0.0) | 13 (3.7) |
| Baseline anti-HTN medications, n (%) | |||
| Beta-blocker | 131 (23.1) | 22 (10.2) | 109 (31.4) |
| ACE inhibitor/ARB | 118 (21.0) | 6 (2.8) | 112 (32.3) |
| Calcium channel blocker | 69 (12.3) | 3 (1.4) | 66 (19.0) |
| Diuretic§ | 89 (15.8) | 11 (5.1) | 78 (22.5) |
| Other¶ | 20 (3.6) | 2 (0.9) | 18 (5.2) |
| Variable . | Total (n = 562) . | No HTN at baseline (n = 215) . | HTN present at baseline (n = 347) . |
|---|---|---|---|
| Age, mean y (SD) | 63.8 (10.8) | 60.8 (10.5) | 65.6 (10.7) |
| Sex, n (%) | |||
| Male | 397 (70.6) | 149 (69.3) | 248 (71.5) |
| Female | 165 (29.4) | 66 (30.7) | 99 (28.5) |
| Race, n (%) | |||
| White | 521 (92.7) | 206 (95.8) | 315 (90.8) |
| African American | 30 (5.3) | 4 (1.9) | 26 (7.5) |
| Other* | 2 (0.4) | 0 (0.0) | 2 (0.6) |
| BMI, mean (SD) | 28.0 (5.5) | 26.2 (4.8) | 29.1 (5.7) |
| BMI, n (%) | |||
| <25 | 180 (32.0) | 96 (44.7) | 84 (24.2) |
| 25-29.9 | 228 (40.6) | 88 (40.9) | 140 (40.4) |
| ≥30 | 154 (27.4) | 31 (14.4) | 123 (35.5) |
| Other baseline traditional HTN risk factors | |||
| DM | 78 (13.9) | 15 (7.0) | 63 (18.2) |
| MI | 37 (6.6) | 9 (4.2) | 28 (8.1) |
| CAD | 65 (11.6) | 15 (7.0) | 50 (14.4) |
| CKD | 130 (23.1) | 40 (18.6) | 90 (25.9) |
| CHF | 15 (2.7) | 3 (1.4) | 12 (3.5) |
| AF | 25 (4.5) | 10 (4.7) | 15 (4.3) |
| Smoking status | |||
| Never | 306 (54.5) | 125 (58.1) | 181 (52.2) |
| Previous | 232 (41.3) | 82 (38.1) | 150 (43.2) |
| Current | 24 (4.3) | 8 (3.7) | 16 (4.6) |
| Primary malignancy, n (%) | |||
| CLL | 415 (73.8) | 168 (78.1) | 247 (71.2) |
| MCL | 58 (10.3) | 17 (7.9) | 41 (11.8) |
| WM | 10 (1.8) | 2 (0.9) | 8 (2.3) |
| Other† | 79 (14.1) | 28 (13.0) | 51 (14.7) |
| Rai stage, n (%)‡ | |||
| 0 | 2 (0.5) | 1 (0.6) | 1 (0.4) |
| 1 | 36 (8.7) | 17 (10.1) | 19 (7.7) |
| 2 | 80 (19.3) | 20 (11.9) | 60 (24.3) |
| 3 | 95 (22.9) | 41 (24.4) | 54 (21.9) |
| 4 | 201 (48.4) | 89 (53.0) | 112 (45.3) |
| Unknown | 1 (0.2) | 0 (0.0) | 1 (0.4) |
| CLL, risk stratification, n (%) | |||
| Del17p13 | 155 (37.4) | 73 (43.5) | 82 (33.2) |
| Del11q22 | 98 (23.6) | 35 (20.8) | 63 (25.5) |
| Trisomy 12 | 37 (8.9) | 12 (7.1) | 25 (10.1) |
| Del13q14 | 58 (14.0) | 26 (15.5) | 32 (13.0) |
| None of the above | 67 (16.1) | 22 (13.1) | 45 (18.2) |
| Baseline ECOG performance status, n (%) | |||
| 0 | 196 (34.9) | 78 (36.3) | 118 (34.0) |
| 1 | 321 (57.1) | 119 (55.3) | 202 (58.2) |
| 2 | 34 (6.0) | 11 (5.1) | 23 (6.6) |
| 3 | 2 (0.4) | 1 (0.5) | 1 (0.3) |
| 4 | 2 (0.4) | 1 (0.5) | 1 (0.3) |
| Unknown | 7 (1.2) | 5 (2.3) | 2 (0.6) |
| Treatment history, n (%) | |||
| Number of prior anticancer therapies, median (IQR) | 2 (0-5) | 2 (0-5) | 2 (0-5) |
| Prior anthracycline | 131 (23.3) | 51 (23.7) | 80 (23.1) |
| Concurrent anthracycline | 9 (1.6) | 3 (1.4) | 6 (1.7) |
| Prior chemotherapy | 469 (83.5) | 184 (85.6) | 285 (82.1) |
| Prior monoclonal antibody | 500 (89.0) | 199 (92.6) | 301 (86.7) |
| Prior targeted therapy | 105 (18.7) | 36 (16.7) | 69 (19.9) |
| Prior immunomodulatory | 76 (13.5) | 27 (12.6) | 49 (14.1) |
| CYP3A4 inhibitor | 33 (5.9) | 18 (8.4) | 15 (4.3) |
| Cyclosporine during ibrutinib use | 5 (0.9) | 1 (0.5) | 4 (1.2) |
| No prior anticancer therapies, n (%) | 35 (6.2) | 11 (5.1) | 24 (6.9) |
| Baseline mm Hg SBP, n (%) | |||
| <100 | 18 (3.2) | 17 (7.9) | 1 (0.3) |
| 100-119 | 136 (24.2) | 94 (43.7) | 42 (12.1) |
| 120-129 | 129 (23.0) | 85 (39.5) | 44 (12.7) |
| 130-139 | 142 (25.3) | 14 (6.5) | 128 (36.9) |
| 140-179 | 136 (24.2) | 5 (2.3) | 131 (37.8) |
| 180+ | 1 (0.2) | 0 (0.0) | 1 (0.3) |
| Baseline mm Hg DBP, n (%) | |||
| <70 | 256 (45.6) | 133 (61.9) | 123 (35.4) |
| 70-79 | 192 (34.2) | 64 (29.8) | 128 (36.9) |
| 80-89 | 101 (18.0) | 18 (8.4) | 83 (23.9) |
| 90-119 | 13 (2.3) | 0 (0.0) | 13 (3.7) |
| Baseline anti-HTN medications, n (%) | |||
| Beta-blocker | 131 (23.1) | 22 (10.2) | 109 (31.4) |
| ACE inhibitor/ARB | 118 (21.0) | 6 (2.8) | 112 (32.3) |
| Calcium channel blocker | 69 (12.3) | 3 (1.4) | 66 (19.0) |
| Diuretic§ | 89 (15.8) | 11 (5.1) | 78 (22.5) |
| Other¶ | 20 (3.6) | 2 (0.9) | 18 (5.2) |
ACE, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin receptor blocker; CYP3A4, cytochrome P450, family 3, subfamily A; DBP, diastolic blood pressure; ECOG, Eastern Cooperative Oncology Group; IQR, interquartile range; MCL, mantle cell lymphoma; WM, Waldenström’s macroglobulinemia.
Hispanic, Asian, multiracial, and unknown race.
CLL alone.
Diffuse large B-cell lymphoma, follicular lymphoma, hairy cell leukemia, graft-versus-host disease, and marginal zone lymphoma.
Includes loop, thiazide, and potassium-sparing diuretics.
Clonidine, hydralazine, nitrates, and α-1 antagonists.
Incidence, severity, and risk factors for HTN
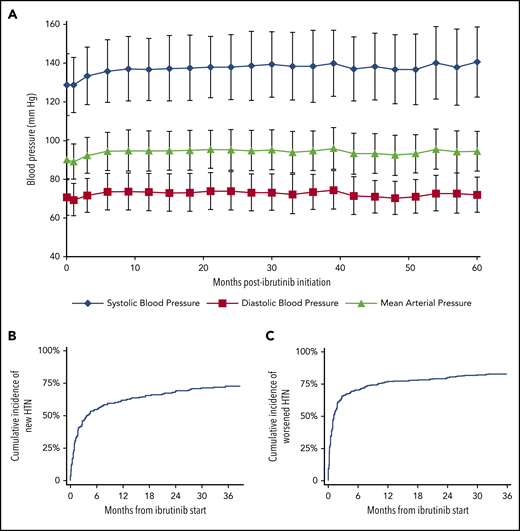
Over a median follow-up of 30 months (interquartile range, 8-48 months), 440 (78.3%) patients developed new or worsening HTN, according to an SBP cutoff of 130 mm Hg (Figure 1; supplemental Table 1), of which 84.8% had at least probable association with ibrutinib. The mean increase in SBP (SD) was 5.2 (20.7) mm Hg, with 1.8 months to 50% cumulative incidence of new or worsened HTN (Figure 1). Examining maximum SBP readings during ibrutinib therapy, at least a 10-mm Hg increase from baseline was observed in more than 80% of patients, and a 50-mm Hg increase was observed in more than 10% (supplemental Table 2). Among those without baseline HTN (215 patients), 71.6% developed new HTN while on ibrutinib, with a mean increase in SBP (SD) of 13.4 (20.1) mm Hg (Figure 1; supplemental Figure 1). This included 54.1% who reached HTN thresholds within 6 months of ibrutinib initiation (4.2 months to 50% cumulative incidence). In those with baseline HTN (347 patients), worsening of HTN was noted in 286 (82.4%), including 77.2% with increase in CTCAE HTN grade.
Effect of ibrutinib on BP over the 60-month study period. (A) Change in mean BP. Error bars represent SD. The cumulative incidence of new (B) or worsened (C) HTN across time after initiation of ibrutinib therapy is also shown. (B) Time to 50% cumulative incidence, 4.2 months; median follow-up, 31.0 months. (C) Time to 40% cumulative incidence, 1.1 months; median follow-up, 29.3 months.
Effect of ibrutinib on BP over the 60-month study period. (A) Change in mean BP. Error bars represent SD. The cumulative incidence of new (B) or worsened (C) HTN across time after initiation of ibrutinib therapy is also shown. (B) Time to 50% cumulative incidence, 4.2 months; median follow-up, 31.0 months. (C) Time to 40% cumulative incidence, 1.1 months; median follow-up, 29.3 months.
Overall, 205 (37.6%, excluding the 17 with high-grade HTN at baseline) developed high-grade (3 or 4) HTN while on ibrutinib, including 17.7% of those without baseline HTN. Nineteen (3.4%) subjects were hospitalized for hypertensive events (ie, grade 4), and 3 subjects required ibrutinib dose reduction or discontinuation related to uncontrolled HTN. Among those needing ibrutinib modification, 2 specifically saw dose reductions (including 1 patient who developed subsequent AF), whereas 1 patient discontinued therapy because of severe, difficult-to-control HTN.
In univariate analysis, age, increased BMI, history of diabetes, CLL, concurrent anthracycline use, and increased baseline SBP were associated with new or worsened HTN (supplemental Table 3). There was no association between ibrutinib dose and the development of new or worsened HTN (supplemental Figure 2). However, in a multivariate model, only CLL vs other malignancy (non-CLL, nonmantle cell lymphoma [MCL]; hazard ratio [HR], 1.64; 95% confidence interval [CI], 1.17-2.28; P = .004), CYP3A4 inhibitor (HR, 1.80; 95% CI, 1.25-2.59; P = .02), and baseline SBP remained associated with HTN development among ibrutinib users (Table 2). There was a significant interaction between baseline SBP and baseline HTN status: among patients not having HTN at baseline, greater baseline SBP was associated with HTN development (HRs ≥2.9 across baseline SBP strata vs SBP <100; P ≤ 0.01); among patients with precedent HTN baseline SBP > 139 was associated with a lower risk of developing worsened HTN compared with baseline SBP <120 (HR, 0.44; 95% CI, 0.29-0.65; P < .001). Moreover, among those without baseline HTN, only diabetes (HR, 1.73; 95% CI, 1.03-2.93; P = .04) and baseline SBP (HRs ≥3.0 across baseline SBP strata vs SBP <100; P < 0.01) were associated with HTN development (supplemental Table 3). In those with precedent HTN, increased age, BMI, CLL or MCL, CYP3A4 inhibitor, and lower baseline SBP were significantly associated with worsened HTN (supplemental Table 3).
Multivariate predictors for the development of new or worsened hypertension
| Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age* | 1.01 | (1.00-1.02) | .06 |
| BMI* | 1.02 | (1.00-1.04) | .052 |
| Male | 1.01 | (0.82-1.24) | .95 |
| African American race | 0.81 | (0.47-1.39) | .44 |
| Prior DM | 1.13 | (0.84-1.52) | .41 |
| Prior CKD | 0.93 | (0.72-1.20) | .58 |
| Smoking status: current/previous vs never | 0.92 | (0.75-1.12) | .40 |
| Primary malignancy | .01† | ||
| CLL vs MCL | 1.13 | (0.76-1.69) | .53 |
| CLL vs other, including WM‡ | 1.64 | (1.17-2.28) | .004 |
| MCL vs other, including WM‡ | 1.44 | (0.88-2.36) | .15 |
| Concurrent anthracycline | 1.39 | (0.73-2.66) | .31 |
| CYP3A4 inhibitor | 1.80 | (1.25-2.59) | .002 |
| Baseline SBP by baseline HTN status interaction | <.001† | ||
| Baseline SBP if no baseline HTN, mm Hg | |||
| <100 | Reference | Reference | |
| 100-119 | 2.94 | (1.38-6.26) | .01 |
| 120-129 | 3.20 | (1.47-6.98) | .003 |
| 130-139 | 3.84 | (1.58-9.36) | .003 |
| Baseline SBP if precedent baseline HTN, mm Hg | |||
| <120 | Reference | Reference | |
| 120-129 | 0.62 | (0.37-1.05) | .07 |
| 130-139 | 0.95 | (0.62-1.44) | .80 |
| >139 | 0.44 | (0.29-0.65) | <.001 |
| Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| Age* | 1.01 | (1.00-1.02) | .06 |
| BMI* | 1.02 | (1.00-1.04) | .052 |
| Male | 1.01 | (0.82-1.24) | .95 |
| African American race | 0.81 | (0.47-1.39) | .44 |
| Prior DM | 1.13 | (0.84-1.52) | .41 |
| Prior CKD | 0.93 | (0.72-1.20) | .58 |
| Smoking status: current/previous vs never | 0.92 | (0.75-1.12) | .40 |
| Primary malignancy | .01† | ||
| CLL vs MCL | 1.13 | (0.76-1.69) | .53 |
| CLL vs other, including WM‡ | 1.64 | (1.17-2.28) | .004 |
| MCL vs other, including WM‡ | 1.44 | (0.88-2.36) | .15 |
| Concurrent anthracycline | 1.39 | (0.73-2.66) | .31 |
| CYP3A4 inhibitor | 1.80 | (1.25-2.59) | .002 |
| Baseline SBP by baseline HTN status interaction | <.001† | ||
| Baseline SBP if no baseline HTN, mm Hg | |||
| <100 | Reference | Reference | |
| 100-119 | 2.94 | (1.38-6.26) | .01 |
| 120-129 | 3.20 | (1.47-6.98) | .003 |
| 130-139 | 3.84 | (1.58-9.36) | .003 |
| Baseline SBP if precedent baseline HTN, mm Hg | |||
| <120 | Reference | Reference | |
| 120-129 | 0.62 | (0.37-1.05) | .07 |
| 130-139 | 0.95 | (0.62-1.44) | .80 |
| >139 | 0.44 | (0.29-0.65) | <.001 |
n = 562. Bold P values indicate statistically significant results.
WM, Waldenström’s macroglobulinemia.
Considered a continuous variable.
Omnibus P value (reflects overall variable effect).
Diffuse large B-cell lymphoma, follicular lymphoma, hairy cell leukemia, graft-versus-host disease, and marginal-zone lymphoma.
Efficacy of standard antihypertensive therapies
Initiation or increase in the number of antihypertensives prescribed was observed among 209 (37.2%) of the ibrutinib users, with 18% reaching resistant HTN levels, necessitating the use of 3 or more drugs. HTN requiring the initiation of more than 1 agent was noted in 101 patients (18.0%). Treatment of HTN varied, with diuretics (22.4%), employed most frequently, followed by angiotensin blocking agents (18%), beta-blockers (15.4%), calcium channel blockers (12.4%), and all others (3.6%; eg, clonidine). Among those patients in whom a single antihypertensive drug was initiated or added, there was no difference in subsequent control of SBP across time (supplemental Table 4); there was a nonsignificant trend toward reduction in those managed with the addition of ≥2 antihypertensives. Similarly, in those with precedent HTN requiring antihypertensive monotherapy before ibrutinib initiation (21.9%), no antihypertensive class was associated with the prevention of subsequent worsening of HTN.
Effect of new or worsened HTN on major cardiovascular events
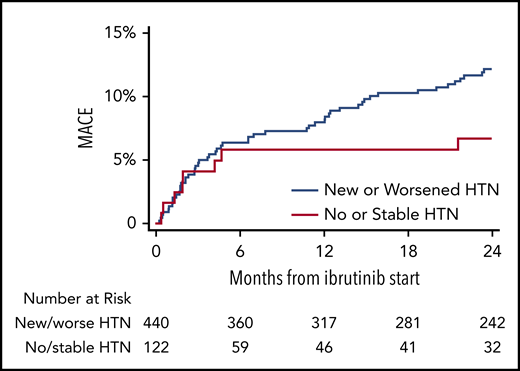
MACEs were observed among 93 patients (16.5%), including 84 (19.1%) in those with new or worsened HTN, compared with 9 (8.2%) in those with stable or no HTN (P = .03 in multivariate model; Figure 2; supplemental Tables 5 and 6). Most events were of at least probable ibrutinib association. Atrial fibrillation was the most common cardiovascular complication during ibrutinib use, occurring in 73 patients (13%), followed by new heart failure (3.7%), cerebrovascular event (2.1%), myocardial infarction (1.4%), and ventricular arrhythmia or sudden cardiac death (1.1%). Furthermore, in a multivariate model containing known predictors of MACE, new or worsened HTN was associated with increased MACEs (HR, 2.17; 95% CI, 1.08-4.38; Table 3). In addition, new or worsened HTN was associated with development of arrhythmia (atrial fibrillation or ventricular arrhythmia; HR, 3.18; CI, 1.37-7.37; supplemental Table 5), with 66 vs 41 events per 1000 person-years (new/worsened HTN vs no/stable HTN). However, when stratified by the degree of ibrutinib-related change in SBP, the magnitude of increase in SBP after ibrutinib initiation did not correspond to the subsequent development of a MACE (supplemental Figure 3). The maximum number of concurrent anti-HTN medications also was not associated with a MACE. When atrial fibrillation was not included in the MACE definition, MACEs were observed in only 31 patients (5.5%), and the association between new or worsened HTN and MACEs was not statistically significant (HR, 1.34; 95% CI, 0.47-3.84).
Cumulative incidence of MACEs, stratified by HTN status during ibrutinib use. Shown is overall incidence of grade 3 or more HTN occurrence at baseline, 3, 6, 9, and 12 months after ibrutinib initiation. Data reflect the JNC-8 HTN cutoff of 140/90 mm Hg for comparison with established HTN prediction models (includes only subjects aged 20-69 years without diabetes). Subjects without known discussion of parenteral history of HTN, were assigned a value of 1 (ie, 1 of 2 parents with HTN) in the Framingham model.18 JNC-8, Joint National Committee-8.
Cumulative incidence of MACEs, stratified by HTN status during ibrutinib use. Shown is overall incidence of grade 3 or more HTN occurrence at baseline, 3, 6, 9, and 12 months after ibrutinib initiation. Data reflect the JNC-8 HTN cutoff of 140/90 mm Hg for comparison with established HTN prediction models (includes only subjects aged 20-69 years without diabetes). Subjects without known discussion of parenteral history of HTN, were assigned a value of 1 (ie, 1 of 2 parents with HTN) in the Framingham model.18 JNC-8, Joint National Committee-8.
Multivariate predictors of MACEs during ibrutinib use
| Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| New/worsened HTN vs no/stable HTN* | 2.17 | (1.08-4.38) | .03 |
| Age ≥ 60 | 1.78 | (1.06-2.99) | .03 |
| BMI ≥30 | 1.30 | (0.84-2.02) | .24 |
| Male | 1.80 | (1.05-3.09) | .03 |
| Prior AF | 0.72 | (0.24-2.21) | .57 |
| Prior CAD | 1.38 | (0.77-2.48) | .27 |
| Prior CHF | 2.71 | (1.36-5.39) | .01 |
| Prior CVE | 0.91 | (0.34-2.39) | .85 |
| Prior DM | 1.29 | (0.74-2.25) | .37 |
| Prior CKD | 1.22 | (0.75-1.99) | .43 |
| Variable . | HR . | 95% CI . | P . |
|---|---|---|---|
| New/worsened HTN vs no/stable HTN* | 2.17 | (1.08-4.38) | .03 |
| Age ≥ 60 | 1.78 | (1.06-2.99) | .03 |
| BMI ≥30 | 1.30 | (0.84-2.02) | .24 |
| Male | 1.80 | (1.05-3.09) | .03 |
| Prior AF | 0.72 | (0.24-2.21) | .57 |
| Prior CAD | 1.38 | (0.77-2.48) | .27 |
| Prior CHF | 2.71 | (1.36-5.39) | .01 |
| Prior CVE | 0.91 | (0.34-2.39) | .85 |
| Prior DM | 1.29 | (0.74-2.25) | .37 |
| Prior CKD | 1.22 | (0.75-1.99) | .43 |
n = 562. MACE includes the combined outcome of AF, CHF, CVE, myocardial infarction, ventricular fibrillation/ventricular tachycardia, and cardiovascular death during ibrutinib use. Bold P values indicate statistically significant results.
AF, atrial fibrillation; CAD, coronary artery disease; CHF, congestive heart failure; CVE, cerebrovascular event; TIA, transient ischemic attack.
Nontime-varying HTN.
Among those with new or worsened HTN (440 patients; 78.3%), the initiation of a new antihypertensive agent was associated with a reduced risk of subsequent MACE (HR, 0.40; 95% CI, 0.24-0.66; supplemental Table 7). The effect was just under significance, after accounting for beta-blocker and calcium channel blocker use (HR, 0.52; 95% CI, 0.27-1.01).
Moreover, in a landmark analysis restricted to patients who survived progression free and remained on ibrutinib beyond 1 year, the development of new or worsened HTN was not associated with increased mortality or progression (supplemental Figure 4).
Comparative incidence of HTN with ibrutinib
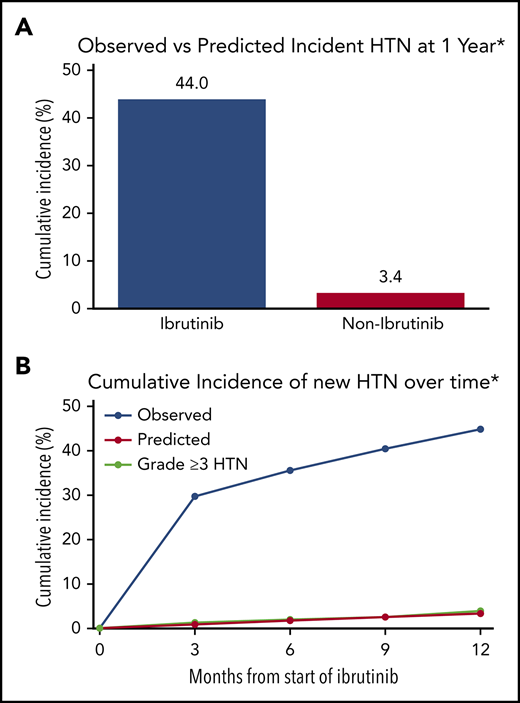
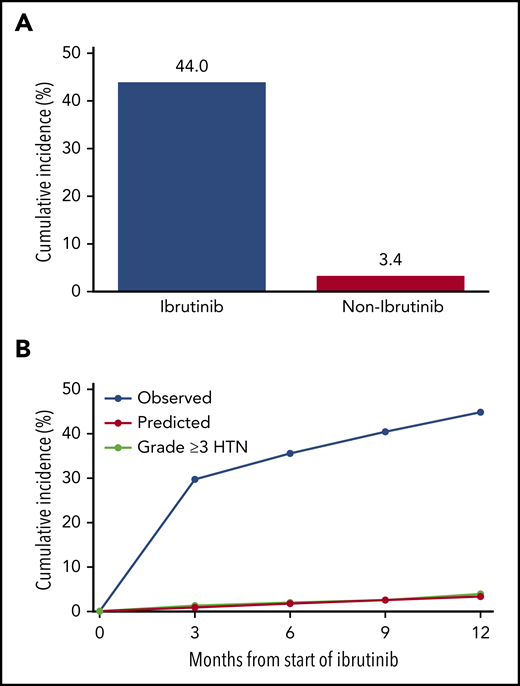
When the previously used JNC-8 cutoffs for HTN classification were applied, the incidence of new HTN was 49.5% among ibrutinib users.24 This translated into an observed new HTN cumulative incidence at 1 year after ibrutinib initiation of 467 per 1000 person-years. Restricted to patients aged 20 to 69 years without a diagnosis of diabetes (n = 157), the cumulative incidence at 1 year was 442 per 1000 person-years, a value 12.9-fold higher than the Framingham risk-predicted rate of 34 per 1000 person-years (Figure 3; supplemental Table 8).
Cumulative incidence of MACEs, stratified by the presence or absence of new or worsened HTN development among patients treated with ibrutinib. Observed vs predicted cumulative new HTN at 1 year (A) along with the incidence of grade 3 or higher HTN during ibrutinib use in patients without preceding HTN at baseline, 3, 6, 9, and 12 months (B). Data reflect JNC-8 HTN cutoffs of 140/90 mm Hg for comparison to established HTN models.18
Cumulative incidence of MACEs, stratified by the presence or absence of new or worsened HTN development among patients treated with ibrutinib. Observed vs predicted cumulative new HTN at 1 year (A) along with the incidence of grade 3 or higher HTN during ibrutinib use in patients without preceding HTN at baseline, 3, 6, 9, and 12 months (B). Data reflect JNC-8 HTN cutoffs of 140/90 mm Hg for comparison to established HTN models.18
Discussion
In this study of the incidence and cardiovascular ramifications of ibrutinib-associated HTN, more than 75% of patients developed new or worsened HTN during therapy. This relationship remained, even after adjustment for ibrutinib dose and was not attenuated by the use of any specific antihypertensive class. Moreover, among those with HTN development, the rate of MACE, including atrial fibrillation and ventricular arrhythmia, during ibrutinib use was disproportionately elevated, in line with prior similar observations in persons treated with other nonspecific or multitargeted tyrosine kinase inhibitors for cancer control.25-27 Yet, we also noted that the initiation of an antihypertensive after HTN development was associated with a lower risk of a subsequent MACE. These observations are of particular importance, given the rapidly increasing number of ibrutinib users and the lack of available tools or early markers to guide the assessment of long-term cardiotoxicity risk.
The observation of increase in BP after ibrutinib initiation adds to a growing body of evidence of linking ibrutinib with adverse cardiovascular outcomes. In a recent review of available published randomized trial data, the use of ibrutinib appeared to associate with a nearly threefold increase in the incidence of grade 3 or 4 HTN.16,17 Similarly, focused follow-up analysis from the early phase PCYC-1102/1103 study of high-risk CLL subgroups (www.clinical trials.gov # NCT01105247) revealed an incidence of grade 3 or more HTN development of 26%.28 Within these, the reported incidence of HTN was significant, yet trailed the 38% development of grade 3 to 4 HTN rates observed in the present examination. Although unclear, the reasons behind these differences may be explained by the more critical and systematic nature of present examination, the inclusion of nontrial ibrutinib users with more comorbidities, as well as the use of contemporary SBP cutoff of 130 mm Hg to define HTN, as opposed to 140 mm Hg from prior guidelines.24-27,29-31 Yet, even when accounting for proceeding guidelines and less stringent HTN cutoffs, the incidence of HTN development among ibrutinib users still exceeds previously reported rates, although by a narrower margin.20,28 Specifically, the rate of new HTN by prior definition was ∼44%, which compares to the 40% within 12 months observed in a recent preliminary report.32 The implications of these findings may bear weight on the understanding of the cardiovascular effects of long-term ibrutinib use.
The predictors of MACEs, including arrhythmia, during ibrutinib use are not well understood. Data from prior studies and smaller prospective investigations have suggested that traditional factors, including age, prior atrial fibrillation, diabetes, myocardial infarction, and heart failure, may underlie, at least in part, a significant burden of the observed risk.10,11 However, many of these events are not explained by these factors alone, with many patients appearing to have no underlying risk factors.13 Although within this analysis the presence of new or worsened HTN did not remain associated with MACE development across SBP strata, the elevated adjusted association, coupled with the high prevalence of new and early HTN after ibrutinib use, may lend strong credence to the consideration of HTN in the assessment of subsequent MACE risk. Akin to patients treated with antiandrogenic and anti–vascular endothelial growth factor (VEGF)–inhibiting multitargeted tyrosine kinase inhibitors, ibrutinib’s broad activity with observed HTN may serve as a critical marker for understanding its off-target cardiotoxic effects.25,26 Further, the treatment of the HTN may allow for the reduction in long-term risk of subsequent MACE events. However, additional prospective studies are needed.
Although the exact reasons behind ibrutinib’s HTN association are unknown, several plausible mechanisms may underlie these findings. Ibrutinib has been shown to inhibit the phosphoinositide 3-kinase (PI3K)/Akt pathway, including indirect downregulation of PI3K-p110α.33,34 Inhibition of PI3K-p110α has also been linked with consequent vascular tissue fibrosis and cellular remodeling (factors well established to underlie HTN development) in histologic cardiovascular tissue from subjects with arrhythmogenic cardiotoxicity.35 However, increased rates of HTN and MACE have not been consistently observed with more potent inhibition of PI3K-p110α and other p110 isoforms.36-40 Downregulation of nitric oxide formation and endothelial dysfunction has also been postulated.41 This mechanism remains a focus of study in anti-VEGF inhibitor–associated HTN, and in vivo analyses have suggested at least some VEGF downregulation with Bruton’s tyrosine kinase inhibition,42 but the specific relation to ibrutinib remains unknown.39,43 With increasing data-linking alterations in inflammatory pathways with cardiac incident events, including HTN, arrhythmia, and other MACEs, it may be reasonable to postulate that the systemic inflammatory alterations seen among ibrutinib users factors into these events by an as yet unexplored mechanism.44-47 It is also plausible that the reduction in MACE among those treated with antihypertensives may have been linked to the suppressive nature of many antihypertensives in arrhythmia treatment. This notion is supported by the loss of statistical significance, after accounting for atrioventricular node blockade use (ie, beta-blockers and calcium channel blockers). Yet, in conjunction with the observation that no specific antihypertensive class appears to control or prevent HTN development during ibrutinib use, additional mechanistic and prospective studies focused on the determination of optimal management strategies for ibrutinib-related HTN are needed.
Several limitations should be acknowledged. Given the retrospective nature of the study, available follow-up within the cohort was nonuniform, which may have limited the ability to capture additional events. Despite accounting for variation in follow-up via extensive modeling, it is possible that variable adherence or other unmeasured factors were present (and may in part explain the observed association between CLL and HTN). Observed rates from comparator populations were not included, as this was not routinely available. The decision to initiate antihypertensive therapy was at the discretion of the treating clinicians and reflected routine practice patterns. Similarly, the selected class and dose of therapy was not predetermined. However, the low incidence of ibrutinib discontinuation suggests at least some efficacy of combinational standard antihypertensive care.28 Consistent adjudication of cause of death was difficult, as many patients did not return after stopping anticancer care. Further, the exclusion of antihypertensive therapy initiated only after an arrhythmic event, may have excluded some patients in whom HTN was recognized and treatment was started only after a MACE. Finally, given that the risk of HTN development was not known during early experience with ibrutinib, it is possible that despite extensive chart reviews, some HTN and MACEs, including sudden death, went uncaptured in medical records.
In summary, ibrutinib is associated with a significantly elevated risk of severe early-onset HTN, even after accounting for traditional baseline risk factors. The occurrence of this HTN appears to associate with long-term risk for the development of other major cardiac events, including incident arrhythmia. Given the expected continued increase in ibrutinib use, further studies characterizing the mechanisms, treatment, and implications of HTN during therapy are needed.
Data related to this study may be requested by e-mail to the corresponding author.
The online version of this article has a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients and their families treated at The Ohio State University Comprehensive Cancer Center.
This work was supported, in part, by National Institutes of Health (NIH), National Cancer Institute grants K23-CA178183 (J.A.W.), R01-CA197870 (J.C.B. and J.A.W.), R35-CA197734 (J.C.B.), and K12-CA133250 (D.A. and J.C.B.). Support was also received from the D. Warren Brown Foundation, the Four Winds Foundations, and the Connie Brown CLL Foundation.
The article’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: T.D., T.W., A.W., A.G., F.A., and D.A. were responsible for the concept and design of the study; T.D., J.P., K.P., D.H., A.G., F.A., and D.A. acquired, analyzed, or interpreted the data; T.D., T.W., K.P., and D.A. drafted the manuscript; K.P. performed the statistical analyses; T.D., A.W., J.C.B., J.A.W., and D.A. provided administrative, technical, and material support; T.W., F.A., and D.A. supervised the work; and S.B., K.A.R, and all other authors were involved in the critical revision of the manuscript for important intellectual content, had full access to all the data in the study, reviewed drafts of the manuscript, and approved the final version.
Conflict-of-interest disclosure: J.C.B. received research funding and consulted for Acerta Pharma and Pharmacyclics. F.A. received research funding from Innate Pharma and Pharmacyclics; has provided consulting services to Gilead Sciences, Pharmacyclics, Janssen, Abbvie, Sunesis, AstraZeneca, Genentech, and Novartis Oncology; and has served on the speakers’ bureau of Abbvie and AstraZeneca. K.A.R. received research funding from Genentech and served on an advisory board for Acerta Pharma. J.A.W. received research funding from Abbvie, Pharmacyclics, Janssen, Acerta, Loxo, Karyopharm, and Morphosys and has consulted for Janssen and Pharmacyclics. The remaining authors declare no competing financial interests.
Correspondence: Daniel Addison, Division of Cardiovascular Medicine, Davis Heart & Lung Research Institute, 473 West 12th Ave, Suite 200, Columbus, OH, 43210; e-mail: daniel.addison@osumc.edu.
REFERENCES
Author notes
J.A.W., F.A., and D.A. contributed equally to this study.