TO THE EDITOR:
Tisagenlecleucel (CTL019; Kymriah) is an anti-CD19 genetically modified autologous T-cell therapy that is approved by the US Food and Drug Administration for treatment of relapsed/refractory pediatric and young adult B-cell acute lymphocytic leukemia (ALL) and adult diffuse large B-cell lymphoma (DLBCL).1,2 Before commercially manufactured CTL019 products can be labeled as tisagenlecleucel and released for administration to patients, a cryopreserved sentinel vial of manufactured cell product is thawed and tested for viability, using either Trypan blue exclusion or dual-fluorescence automated cell counting. For commercially manufactured CTL019, the viability specification for release of manufactured cells is at least 80% viability. In our and others’ experience, there is a proportion of CTL019 products that have viabilities lower than 80%. In the United States, CTL019 products with less than 80% viability currently require that patients enroll in an expanded access program or a single-patient Investigational New Drug application to receive their “out of specification” chimeric antigen receptor (CAR) T cell product. Because the initial clinical trials of CTL019 set the viability specification for CTL019 product release at 70% or more viable cells, we aimed to assess whether there were differences in clinical outcomes (complete response [CR] rate, peak in vivo CTL019 expansion, progression-free survival [PFS], overall survival [OS]) between patients who received CTL019 products with viabilities lower than 80% vs 80% or higher.
Data used in the current analyses include 123 relapsed/refractory pediatric patients with ALL treated at the Children’s Hospital of Philadelphia who were evaluable for response (NCT01626495 and NCT02906371); PFS and OS outcomes were also available for 62 patients on NCT01626495.3 Data from a clinical trial of 25 patients with relapsed/refractory DLBCL treated at the University of Pennsylvania (NCT02030834) were also analyzed.4 CTL019 was manufactured at the University of Pennsylvania. P < .05 was considered statistically significant.
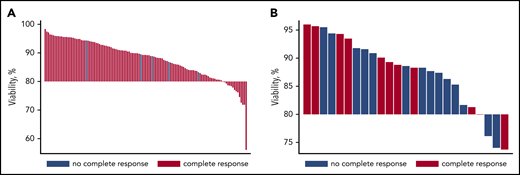
For patients with ALL, the median product viability was 89.3% (range, 56.0%-98.4%); 15 (12%) of 123 products had less than 80% viability, with a median viability of 77.0% (range, 56.0%-79.8%). For patients with DLBCL, the median product viability was 88.6% (range, 73.7%-96%); 4 (16%) of 25 products had less than 80% viability, with a median viability of 75.1% (range, 73.7%-79.9%; Figure 1).
Distribution of product viability and complete responses. (A) ALL. (B) DLBCL.
We found no statistically significant association between cell product viability and complete remission rate; product viability was 87.9% ± 7.02% (mean ± standard deviation) for 118 patients with ALL patients achieving CR vs 88.8% ± 4.05% for 5 non-CR patients with ALL (Student t test, P = .82) and 88.3% ± 7.17% for 11 patients with DLBCL achieving CR vs 87.1% ± 6.27% for 14 non-CR patients with DLBCL (Student t test, P = .67). CTL019 product viability was not predictive of CR (area under the curve, 0.52 [95% confidence interval (CI), 0.32-0.71] for ALL and area under the curve, 0.59 [95% CI, 0.34-0.83]) for DLBCL. Furthermore, Pearson correlation coefficients, r = −0.0986 for ALL (N = 62; P = .45) and r = 0.23 for DLBCL (N = 24; P = .28) suggested a very weak association between product viability and peak CTL019 expansion in vivo.
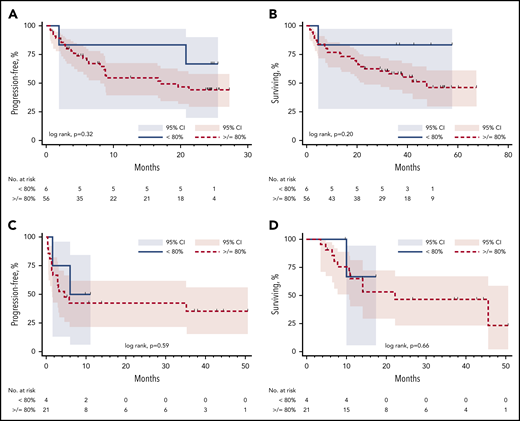
There was no significant difference in PFS or OS between patients with ALL who received products with less than 80% viability or products with at least 80% viability (log rank, PFS, P = .32 [Figure 2A] and OS, P = .20 [Figure 2B]). Similarly, there was no significant difference in PFS or OS between patients with DLBCL who received products with less than 80% viability or products with at least 80% viability (log rank, PFS, P = .59 [Figure 2C] and OS, P = .66 [Figure 2D]).
Progression-free survival and overall survival based on product viability. (A) ALL PFS. (B) ALL OS. (C) DLBCL PFS. (D) DLBCL OS.
Progression-free survival and overall survival based on product viability. (A) ALL PFS. (B) ALL OS. (C) DLBCL PFS. (D) DLBCL OS.
For patients with ALL with product viabilities lower than 80%, the estimated 24-month progression-free probability was 67% (95% CI, 19.5%-90.4%) vs 44% (95% CI, 29.2%-58.2%) for patients with viabilities of 80% or more. For patients with DLBCL with viabilities lower than 80%, the estimated 9-month progression-free probability was 50% (95% CI, 5.8%-84.5%) vs 42% (95% CI, 21.3%-62.0%) for patients with viabilities of at least 80%.
As far as we are aware, this is the first analysis of CAR T cell outcomes based on product viability test results. Although this is a retrospective analysis of products manufactured at our institution with relatively small numbers of lower-viability (<80%) products, our findings suggest no association between CAR T product viability lower than 80% or at least 80% viability and clinical outcomes for CTL019. There is no evidence to support differences in response, in vivo CAR T cell expansion, or survival between patients receiving products with lower than 80% viability compared with those receiving products with at least 80% viability. We suggest a CTL019 viability release criterion of at least 70% based on these clinical trial outcomes. Moreover, we urge more detailed study of release criteria for CAR T cell products and advocate that release criteria selection be driven by demonstrated effect on clinical outcomes.
Acknowledgments
This work was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32CA009615 from the National Cancer Institute and the Lymphoma Research Foundation under a Postdoctoral Fellowship Grant (E.A.C.).
Authorship
Contribution: E.A.C. and S.J.S. designed and performed the research, analyzed the data, and wrote the manuscript; B.L.L., M.M.D., and C.H.J. designed the research and wrote the manuscript; S.A.G., D.L.S., S.L.M., W.L.G., N.V.F., D.L.P., and E.R.C. performed research and wrote the manuscript; W.-T.H. confirmed the methodology and analyzed the data; and all authors approved of the final manuscript.
Conflict-of-interest disclosure: E.A.C. consults for Novartis and Tessa Therapeutics. B.L.L. reports grants from Novartis and Tmunity Therapeutics; consults for CRC Oncology, TerumoBCT, and Novartis; is on the scientific advisory boards for Avectas, Brammer Bio, Cure Genetics, Incysus, and Vycellix; has equity ownership in Tmunity Therapeutics; and has financial interest because of intellectual property and patents in the field of cell and gene therapy. S.A.G. consults for Novartis, Adaptimmune, and Jazz Pharmaceuticals; receives research funding from Novartis; and has patents with the University of Pennsylvania. M.M.D. reports patents and royalties from Novartis Institutes for Biomedical Research. D.L.S. reports royalties from Novartis. S.L.M. reports consulting for and membership on advisory boards for Novartis and Kite. N.V.F. reports consulting for Novartis and Servier Consultancy. W.L.G. has no conflicts of interest to disclose. D.L.P. reports participations on advisory boards for Kite, Incyte, Glenmark and Novartis; has patents/royalties and research funding from Novartis and salary/stock from Genentech (spouse). W.-T.H. reports research funding from Novartis and Tmunity Therapeutics. E.R.C. has no conflicts of interest to disclose. C.H.J. reports grants from Novartis and Tmunity Therapeutics; consults for Novartis, Immune Design, Celldex, Viracta, and Carisma; serves on advisory committees for Immune Design, Tmunity Therapeutics, and Celldex; and is a stockholder in Tmunity Therapeutics. S.J.S. reports research grants from Acerta, AbbVie, Celgene, DTRM Bio Pharma, Genentech, Incyte, Novartis, Merck, Regeneron, and TG therapeutics; consults for Acerta, AstraZeneca, Celgene, Juno, LoxoOncology, Novartis, and Tessa Therapeutics; serves on steering committees for AbbVie, Celgene, Novartis, Nordic Nanovector, and Pfizer; and has a patent for Combination Therapies of CAR and PD-1 inhibitors with royalties paid to Novartis.
Correspondence: Stephen J. Schuster, 3400 Civic Center Blvd, PCAM South Pavilion, 12th Floor, Room 12-178, Philadelphia, PA 19104; e-mail: stephen.schuster@uphs.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal