Key Points
Low FV, accompanied by low fTFPI in F5F8D, has optimal procoagulant activity, and addition of FV to plasma only results in anticoagulation.
The use of DDAVP as a potential substitute for FVIII concentrates without FFP infusion is suggested in F5F8D patients with minor bleeding.
Abstract
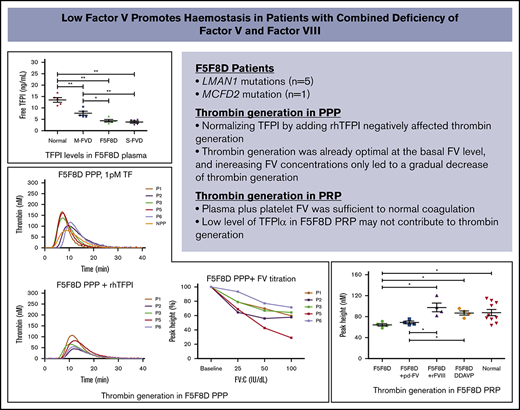
Combined factor V (FV) and FVIII deficiency (F5F8D) is a rare autosomal-recessive bleeding disorder caused by mutations in lectin mannose binding-1 (LMAN1) and multiple coagulation factor deficiency-2 (MCFD2). Six causative homozygous mutations (5 in LMAN1 and 1 in MCFD2) were identified in 6 patients with F5F8D. A thrombin-generation assay, triggered with tissue factor (1 pM) in F5F8D plasma, paradoxically exhibited enhanced thrombin generation compared with normal plasma. Significantly lower free tissue factor pathway inhibitor (fTFPI) was found in F5F8D patients compared with healthy controls (P < .01). Normalizing tissue factor pathway inhibitor α (TFPIα) in F5F8D plasma greatly delayed and reduced thrombin generation. Increasing FV concentrations by adding plasma FV to F5F8D plasma only caused a gradual decrease in thrombin generation, suggesting that low levels of TFPIα and FV cocontributed to the elevated thrombin generation by reducing anticoagulant effects. On the contrary, thrombin generation in F5F8D platelet-rich plasma (PRP) was significantly lower than in normal controls (P < .05); however, it was fully corrected by normalizing FVIII or after 1-deamino-8-d-arginine vasopressin (DDAVP) infusion, indicating that the hypocoagulable state of F5F8D patients is associated with low FVIII levels. In addition, plasma and platelet FV in F5F8D PRP were sufficient to support normal thrombin generation, and low TFPIα may have no effect on thrombin generation. DDAVP infusion induced a complete response in 5 F5F8D patients and a partial response in the remaining patient. Based on our findings, we suggest that DDAVP may be considered a potential substitute for FVIII concentrates, and fresh-frozen plasma (FFP) infusion may not be necessary for F5F8D patients with minor bleeding challenges.
Introduction
Combined factor V (FV) and FVIII deficiency (F5F8D; Online Mendelian Inheritance in Man #227300) is a rare autosomal-recessive disorder with a frequency of 1 in 1 000 000. F5F8D is characterized by the concomitant reduction of FV and FVIII levels in plasma1 ; FV and FVIII activities are generally in the range of 5% to 30% of normal (typically 10-20%). Different from FV or FVIII deficiency, the disease involves gene mutations in lectin mannose binding-1 (LMAN1) or multiple coagulation factor deficiency-2 (MCFD2), causing defects in the secretion of FV and FVIII.2-4 Mild to moderate bleeding is manifested in patients with F5F8D.5-9 Bleeding episodes are usually treated on demand, requiring a source of FV and FVIII. Currently, replacement of FV can only be achieved through use of fresh-frozen plasma (FFP), whereas a large number of products are available for the treatment of FVIII deficiency.10,11 The use of 1-deamino-8-d-arginine vasopressin (DDAVP) in the treatment of F5F8D has recently been reported in a few cases, with its efficacy being attributed to an increase in FVIII levels rather than FV levels.12,13
Duckers et al14 initially reported that FV deficiency is associated with reduced levels of natural anticoagulant tissue factor pathway inhibitor (TFPI). They later showed that severe FV-deficient patients may have residual platelet FV, which, in combination with the low TFPIα level, supports sufficient thrombin generation.15 Recently, it has been demonstrated that FV can function as a synergistic TFPIα cofactor with protein S (PS) in the inhibition of activated factor X (FXa), and this TFPIα-dependent anticoagulant effect of FV increases in parallel with the physiological range of FV levels.16-18 Therefore, the low FV in F5F8D patients may actually be beneficial to their overall coagulation status and mitigate bleeding diatheses imposed by low levels of FVIII. To test this hypothesis, we analyzed hemostatic roles of a low level of FV in F5F8D plasma and platelets using thrombin-generation assays (TGAs). In addition, the efficacy of DDAVP infusion for minor bleeding challenges was further evaluated in F5F8D patients.
Materials and methods
Subjects
Six unrelated patients from different parts of China were invited to participate in the study. All patients, with the exception of 1 (P6) whose parents came from the same region, were the offspring of consanguineous marriages. Clinical data, including demographics, bleeding manifestations, and treatment of patients and related family members, were collected by 2 independent clinicians, and the consensus information was determined. The consensus International Society for Thrombosis and Hemostasis Bleeding Assessment Tool was used to evaluate the bleeding diathesis.19 Patients with congenital FV deficiency and healthy individuals were also included in the study as controls.
This study was approved by the Ethics Committee of Ruijin Hospital. All related individuals gave their informed consent to participate.
Blood sampling
Peripheral blood was collected from participants. Platelet-poor plasma (PPP) was collected following a double centrifugation at 3000g for 15 minutes at room temperature. Normal pooled plasma (NPP) was prepared from 30 healthy donors. Platelet-rich plasma (PRP) was prepared by centrifugation at 100g for 10 minutes at room temperature. Platelet counts were measured and adjusted to 150 × 109 platelets per liter by the addition of autologous PPP. PRP was used within 90 minutes of venipuncture, and PPP was aliquoted and stored at −80°C until use.
Platelets were isolated from washed PRP from 5 F5F8D patients and age- and sex-matched healthy controls, as previously described.20,21 The pellet was resuspended and adjusted to 150 × 109 platelets per liter in FV-depleted plasma (Instrumentation Laboratory, Bedford, MA).
In addition, artificially reconstituted PRPs were obtained by supplementing platelets of patient P5 with NPP and adding normal pooled platelets to patient P5 PPP for cross-reconstitution TGA studies.
Hemostatic assays
FV clotting activity (FV:C) and FVIII clotting activity (FVIII:C) of 6 F5F8D patients were reassayed on an ACL TOP coagulometer (Instrumentation Laboratory). Thrombophilia screening tests, including activities of antithrombin, protein C, and PS and antigens of free PS and total PS, were performed as previously reported.22,23 von Willebrand factor antigen and von Willebrand factor activity were also measured, as previously described.24
Thrombin-generation assay
The kinetics of thrombin generation were assessed according to methods described by Hemker et al25 using a microtiter plate fluorometer (Thrombinoscope BV, Maastricht, The Netherlands) equipped with a 390/460-nm filter set for excitation/emission. TGA (CAT; Diagnostica Stago, Asnières-sur Seine, France) was performed in F5F8D PRP/PPP with a 1-pM TF trigger (5-pM TF was used only in F5F8D plain plasma), in accordance with the manufacturer’s recommendations. Thrombin-generation curves were plotted with Thrombinoscope Software, version 5.0.0.742 (Thrombinoscope BV). Lag time (LT; minutes) and peak height (PH; nM) were used as main read-out parameters.
In some TGAs, recombinant FVIII (rFVIII; Kogenate; Bayer), recombinant human TFPI (rhTFPI; 10 ng/mL; BioLegend, San Diego, CA), or plasma-derived FV (pd-FV) (Haematologic Technologies, Essex Junction, VT) was added to the plasma. TGAs in F5F8D were performed using FV titration by adding pd-FV in the absence and presence of a polyclonal anti-TFPI antibody with a final concentration of 10 μg/mL (PAHTFPI; Haematologic Technologies).17,26 Neutralizing antibodies against TFPI (16 μg/mL; equimolar mixture of anti–Kunitz-1, anti–Kunitz-2, anti–Kunitz-3, and anti–C terminus antibodies; Sanquin, Amsterdam, The Netherlands) were alternatively adopted.
In addition, a neutralizing anti-FV antibody (Haematologic Technologies) was titrated in F5F8D PPP supplemented with pd-FV up to 100 IU/dL as an alternative way to get varying FV.
TFPI antigen measurement
Levels of total TFPI (tTFPI) and free TFPI (fTFPI) antigens of related plasma were determined with commercial enzyme-linked immunosorbent assay kits (Diagnostica Stago).
DDAVP infusion
DDAVP (Zhonghe Pharmaceutical Co. Ltd., Hainan, China) was administered IV to all 6 F5F8D individuals, at a dose of 0.3 μg/kg with 100 mL of 0.9% sodium chloride, for 30 minutes. Blood samples were obtained at 0 hours (before the injection) and at 0.5, 1, 2, and 4 hours after the injection for FV:C and FVIII:C assays. Depending on the peak FVIII:C level after DDAVP infusion, the DDAVP response was defined as complete (FVIII:C ≥ 50 IU/dL), partial (FVIII:C < 50 IU/dL but increased at least threefold), or no response (neither criterion).27 The effect of DDAVP infusion on thrombin generation was evaluated in F5F8D PPP and PRP acquired 0.5 hour after DDAVP injection.
Genetic analysis
Genomic DNA was extracted from peripheral whole blood using a QIAamp DNA Blood Mini Kit (QIAGEN, Hilden, Germany). Genetic analysis of LMAN1 and MCFD2 was carried out by directly sequencing on an ABI 3700 sequencer (Applied Biosystems, Foster City, CA).
Statistical analysis
TFPI levels and TGA parameters were analyzed using the nonparametric Mann-Whitney U test because of the limited sample number per group. Statistical analysis was performed with SPSS 18.0 (SPSS Inc., Chicago, IL), and P < .05 was considered significant.
Results
Clinical and genetic characteristics of F5F8D patients
Among the 6 patients investigated, gingival bleeding was the most common problem in 4 of those patients. Half of the patients experienced prolonged bleeding after dental extraction, which could be controlled by gauze pads. Surgery-related bleeding was only mentioned by patient P3, who experienced excessive bleeding after transcervical resection of myoma and was managed with combined FFP and FVIII concentrates at a local hospital. Heavy menorrhagia after menstruation had occurred in patient P6, and an oral contraceptive was adopted subsequent to her diagnosis. Notably, patient P4 was complicated with hyperlipidemia and hyperuricemia, which were found to be associated with high TFPI levels.28,29
Direct sequencing revealed 6 homozygous mutations (5 in LMAN1 and 1 in MCFD2). Four were novel mutations: c.790dupA and c.1150-2A>G in LMAN1 and c.149+1G>C in MCFD2 were null mutations, and the remaining (V147I in LMAN1) was a novel missense mutation; it was located at a site that is highly conserved across different species, and in silico analysis predicted that it might be pathogenic (Table 1).
Clinical and laboratory findings for 6 F5F8D patients
| Patients . | Gender/age, y . | Clinical manifestations (bleeding score) . | FV:C (IU/dL)† . | FVIII:C (IU/dL)† . | Mutations . | |
|---|---|---|---|---|---|---|
| LMAN1 . | MCFD2 . | |||||
| P1 | F/27 | No bleeding diatheses (0) | 18.1 | 21.2 | c.439G>A, V147I‡ | |
| P2 | M/60 | Bleeding after dental extraction, gingival bleeding (3) | 12.3 | 18.8 | c.790dupA, T264Nfs*6‡ | |
| P3 | F/36 | Bleeding after dental extraction/surgery, gingival bleeding (7) | 11.3 | 13.9 | c.912dupA, E305Rfs*20 | |
| P4 | M/33 | Gingival bleeding (4) | 14.2 | 17.4 | c.949C>T, Q317* | |
| P5 | F/27 | Bleeding after dental extraction (3) | 12.9 | 16.0 | c.1150-2A>G‡ | |
| P6 | F/22 | Gingival bleeding, menorrhagia (4) | 6.6 | 8.1 | c.149+1G>C‡ | |
| Patients . | Gender/age, y . | Clinical manifestations (bleeding score) . | FV:C (IU/dL)† . | FVIII:C (IU/dL)† . | Mutations . | |
|---|---|---|---|---|---|---|
| LMAN1 . | MCFD2 . | |||||
| P1 | F/27 | No bleeding diatheses (0) | 18.1 | 21.2 | c.439G>A, V147I‡ | |
| P2 | M/60 | Bleeding after dental extraction, gingival bleeding (3) | 12.3 | 18.8 | c.790dupA, T264Nfs*6‡ | |
| P3 | F/36 | Bleeding after dental extraction/surgery, gingival bleeding (7) | 11.3 | 13.9 | c.912dupA, E305Rfs*20 | |
| P4 | M/33 | Gingival bleeding (4) | 14.2 | 17.4 | c.949C>T, Q317* | |
| P5 | F/27 | Bleeding after dental extraction (3) | 12.9 | 16.0 | c.1150-2A>G‡ | |
| P6 | F/22 | Gingival bleeding, menorrhagia (4) | 6.6 | 8.1 | c.149+1G>C‡ | |
F, female; M, male.
Reference ranges are 50 to 150 IU/dL.
Novel mutation.
Hemostatic assays
A decrease in FV:C and FVIII:C, ranging from 10 IU/dL to 25 IU/dL, was observed in all cases, with the exception of 1 patient (P6) who had factor levels <10 IU/dL (Table 1). Other coinherited hemorrhagic or thrombophilic defects were not detected (data not shown). The coagulation factor activities of patient P4 were also measured on a STart analyzer (Diagnostica Stago), using a viscosity-based system to eliminate possible interference from lipemic blood samples.
Plasma TFPI levels
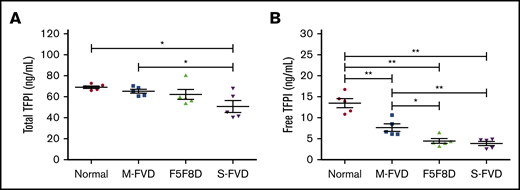
Plasma levels of fTFPI and tTFPI antigens were determined in 5 F5F8D patients (P4 demonstrated high TFPI levels and was not included in this analysis or in the following PPP/PRP TGA studies). Age- and sex-matched healthy controls, mild FV-deficient patients (FV:C ∼ 50 IU/dL), and severe FV-deficient patients (FV:C < 2 IU/dL) were also included for comparison. Compared with healthy donors (69.2 ± 2.8 ng/mL), patients exhibited a gradual decrease in tTFPI levels that ranged from 65.4 ± 4.3 ng/mL (P > .05) in mild FV-deficient patients and 62.2 ± 10.6 ng/mL (P > .05) in F5F8D patients to 50.8 ± 12.4 ng/mL (P < .05) in severe FV-deficient patients. A more pronounced trend was observed for fTFPI levels; the average fTFPI level in healthy controls was 13.5 ± 2.4 ng/mL, which was significantly higher than that in mild FV-deficient patients (7.6 ± 2.0 ng/mL, P < .01), F5F8D patients (4.4 ± 1.2 ng/mL, P < .01), and severe FV-deficient patients (3.8 ± 1.1 ng/mL, P < .01) (Figure 1). Notably, there were no significant differences in fTFPI levels between F5F8D and severe FV-deficient patients (P > .05).
Plasma TFPI levels in 5 F5F8D patients. Total and free TFPI antigen levels of F5F8D patients were measured and compared with groups of severe FVD patients, mild FVD patients, and normal controls. (A) Total TFPI antigen levels. (B) Free TFPI antigen levels. The horizontal lines represent the means of the respective groups. *P < .05, **P < .01. FVD, FV-deficient patients.
Plasma TFPI levels in 5 F5F8D patients. Total and free TFPI antigen levels of F5F8D patients were measured and compared with groups of severe FVD patients, mild FVD patients, and normal controls. (A) Total TFPI antigen levels. (B) Free TFPI antigen levels. The horizontal lines represent the means of the respective groups. *P < .05, **P < .01. FVD, FV-deficient patients.
Contributions of low levels of FV and TFPIα to thrombin generation in PPP
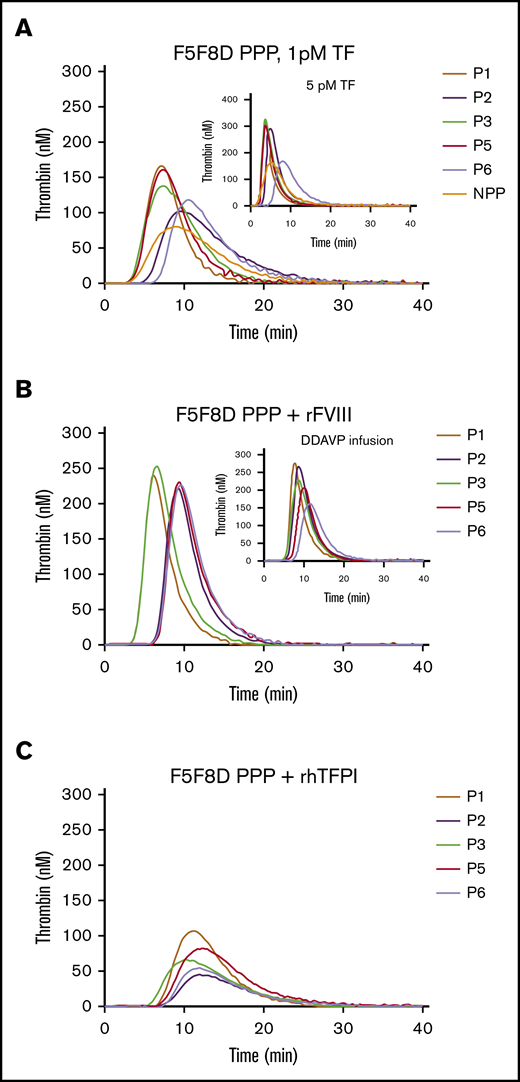
Despite low levels of FV, FVIII, and TFPIα in F5F8D plasma, TGAs, triggered with 1-pM TF, demonstrated a delayed, but higher, thrombin-generation peak (PH values, 103.1-166.0 nM; LT values, 4.0-7.3 minutes) in 5 F5F8D PPP samples compared with that in NPP (81.4 nM, 4.7 minutes; Figure 2A). A similar trend of higher PH (170.6-324.5 nM) and shorter LT (2.2-5.3 minutes) values was observed with 5-pM TF (Figure 2A, inset). FV, FVIII, and TFPIα were normalized to determine the contributing factor(s) responsible for the elevated thrombin generation in F5F8D PPP. Normalizing FVIII, by adding rFVIII up to 100 IU/dL in F5F8D PPP or after DDAVP infusion, resulted in a more robust thrombin generation, with PH values increasing to 220.7 to 253.2 nM or 162.0 to 277.7 nM, respectively; however, this had little effect on LTs (Figure 2B). However, normalizing TFPI by adding rhTFPI negatively affected thrombin generation, leading to markedly decreased PHs (44.7-107.4 nM) and prolonged LTs (6.17-8.33 minutes), indicating that low TFPI levels dramatically contribute to elevated thrombin generation in F5F8D plasma (Figure 2C). To carefully determine the effect of FV levels in F5F8D plasma on thrombin generation, F5F8D plasma was titrated with different concentrations of pd-FV (basal level, 25 IU/dL, 50 IU/dL, and 100 IU/dL). Interestingly, thrombin generation reached its optimum at the basal FV level, and further FV titrations only led to a gradual decrease in thrombin generation, with PH values decreasing to 29.1% to 71.6% of the optimal maximum at 100 IU/dL FV (Figure 3A); this indicates that addition of FV to plasma only causes a paradoxical anticoagulation. A similar pattern was observed when FV:C was varied by a neutralizing anti-FV antibody, confirming that the anticoagulant effect was FV dependent (data not shown). In contrast to PH, LT values were largely shortened when FV was increased to 25 IU/dL, yet further FV supplementation to increase its concentration from 25 IU/dL to 100 IU/dL minimally affected the LT values in the plasma samples measured (Figure 3B; supplemental Table 1, available on the Blood Web site). These results, which are consistent with the literature,30,31 suggest that the initiation phase of coagulation is under the influence of FV levels (<25 IU/dL).
Effect of normalized FVIII or TFPIα on thrombin generation in 5 F5F8D plasmas. (A) Thrombin generation in F5F8D plasma triggered at 1-pM TF and 5-pM TF (inset). (B) Thrombin generation in 5 F5F8D plasmas with the addition of rFVIII to bring FVIII level to 100 IU/dL or plasmas received 0.5 hours after DDAVP infusion (inset). (C) Thrombin generation in 5 F5F8D plasmas added to with final concentration of 10 ng/mL TFPIα.
Effect of normalized FVIII or TFPIα on thrombin generation in 5 F5F8D plasmas. (A) Thrombin generation in F5F8D plasma triggered at 1-pM TF and 5-pM TF (inset). (B) Thrombin generation in 5 F5F8D plasmas with the addition of rFVIII to bring FVIII level to 100 IU/dL or plasmas received 0.5 hours after DDAVP infusion (inset). (C) Thrombin generation in 5 F5F8D plasmas added to with final concentration of 10 ng/mL TFPIα.
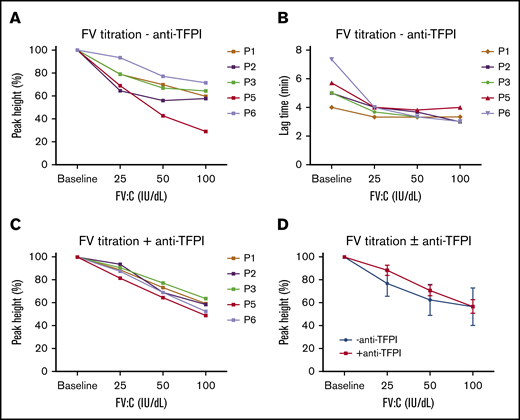
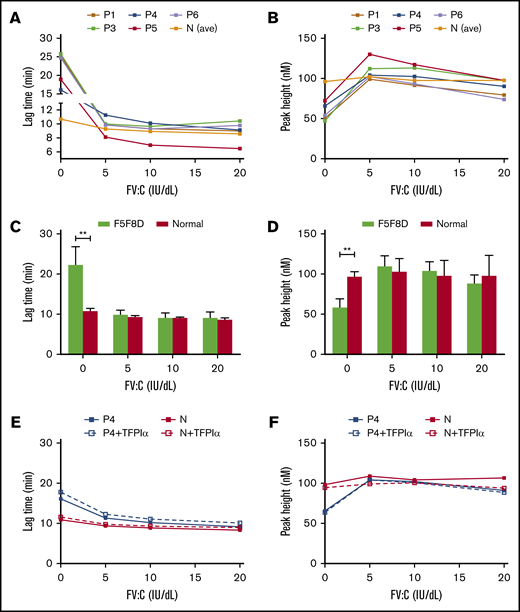
Effect of increasing FV concentrations on thrombin generation in 5 F5F8D plasmas. F5F8D plasma was titrated with increasing concentrations of FV (baseline FV, 25 IU/dL, 50 IU/dL, or 100 IU/dL), and PH (A) and LT (B) values of thrombin generation were plotted as a function of FV in the absence of anti-TFPI antibody. PH values were expressed as percentages of the maximal PH (PH %). (C) Effect of increasing FV concentrations on PH values in the presence of anti-TFPI antibody. (D) Comparison of average PH values in the absence and presence of the anti-TFPI antibody. Average PH values are presented as mean ± standard deviation.
Effect of increasing FV concentrations on thrombin generation in 5 F5F8D plasmas. F5F8D plasma was titrated with increasing concentrations of FV (baseline FV, 25 IU/dL, 50 IU/dL, or 100 IU/dL), and PH (A) and LT (B) values of thrombin generation were plotted as a function of FV in the absence of anti-TFPI antibody. PH values were expressed as percentages of the maximal PH (PH %). (C) Effect of increasing FV concentrations on PH values in the presence of anti-TFPI antibody. (D) Comparison of average PH values in the absence and presence of the anti-TFPI antibody. Average PH values are presented as mean ± standard deviation.
In light of a significantly low TFPIα level in F5F8D PPP, TGA with FV titration was repeated in the presence of an anti-TFPI antibody to further explore whether the anticoagulant effect of FV was TFPIα dependent. At lower FV concentrations, the anticoagulant effect of FV was largely abolished in the presence of the anti-TFPI antibody (Figure 3A,C,D). However, this effect became less pronounced at increasing FV concentrations and, eventually, the PH values were virtually superimposable at 100 IU/dL FV (Figure 3D; supplemental Table 1), suggesting that, at lower FV levels (probably from baseline to 50 IU/dL), the anticoagulant effect of FV was primarily TFPI dependent. The existence of anti-TFPI antibody minimally altered LT values (supplemental Table 1), indicating that the low TFPIα level has little effect on the initiation of coagulation. Similar results were obtained using antibodies from Sanquin, confirming the efficacy of the anti-TFPI antibody used in the assays (data not shown). It should be noted that a gradual decrease in the PH values with increasing FV concentrations in the presence of the anti-TFPI antibody was also observed (Figure 3D; supplemental Table 1). It is possible that this TFPIα-independent anticoagulant effect of FV, which has also been observed by other investigators,17,18 may be due to the active form of FV (FVa), as reported by Al Dieri et al.32
Interestingly, it was also observed that the anticoagulant effect of added FV was quite different among 5 F5F8D PPP samples (Figure 3A), although the heterogeneity was largely overcome in the presence of the anti-TFPI antibody (Figure 3C). We hypothesize that the basis for the differences in the extent of this anticoagulant effect relates to variations in TFPIα and PS levels in F5F8D patients (supplemental Table 2), which is consistent with recent findings that FV and PS function as synergistic cofactors for TFPIα.16,18
Contributions of FV and TFPIα to thrombin generation in PRP
The thrombin-generation potential in PRP was evaluated in 4 F5F8D patients and 10 healthy donors. In contrast to F5F8D PPP, thrombin generation in F5F8D PRP was significantly decreased compared with healthy donors (PH values, 64.2 ± 5.2 nM vs 87.6 ± 18.9 nM; P < .05; Figure 4A,E). Normalizing FV had a minimal effect on thrombin generation (PH value, 68.7 ± 4.9 nM; Figure 4B), yet normalizing FVIII in PRP to 100 IU/dL or analyzing PRP from F5F8D patients after 0.5 hours of DDAVP infusion greatly improved thrombin generation, with PH values increasing to 97.5 ± 16.5 nM and 86.8 ± 8.1 nM, respectively. These values were significantly higher than those in F5F8D PRP (P < .05; Figure 4C-E) but were comparable to normal controls. These results, combined with the results of cross-reconstituted PRP studies between patient P5 and normal controls (supplemental Figure 1), suggested that the reduced thrombin generation is caused by a low FVIII level in F5F8D PRP, whereas platelet FV in plasma was sufficient to support normal coagulation. No significant differences were noted in the LT values of thrombin generation in PRP between the patient and control groups or among patient groups with normalized FV/FVIII levels (data not shown).
Effect of normalized FV or FVIII on thrombin generation in 4 F5F8D PRPs. (A) Thrombin generation in PRP of 4 F5F8D patients. (B) Thrombin generation in 4 F5F8D PRPs with addition of FV to bring the FV level to 100 IU/dL. (C) Thrombin generation in 4 F5F8D PRPs with addition of FVIII to bring the FVIII level to 100 IU/dL. (D) Thrombin generation in PRP of 4 F5F8D patients received 0.5 hour after DDAVP infusion. The average TGA curve of 10 normal PRPs is presented for reference. (E) PH values of thrombin generation were compared among the different groups mentioned above. *P < .05.
Effect of normalized FV or FVIII on thrombin generation in 4 F5F8D PRPs. (A) Thrombin generation in PRP of 4 F5F8D patients. (B) Thrombin generation in 4 F5F8D PRPs with addition of FV to bring the FV level to 100 IU/dL. (C) Thrombin generation in 4 F5F8D PRPs with addition of FVIII to bring the FVIII level to 100 IU/dL. (D) Thrombin generation in PRP of 4 F5F8D patients received 0.5 hour after DDAVP infusion. The average TGA curve of 10 normal PRPs is presented for reference. (E) PH values of thrombin generation were compared among the different groups mentioned above. *P < .05.
To further evaluate the relative contribution of plasma and platelet FV to thrombin generation in F5F8D PRP, FV-depleted plasma was reconstituted with platelets isolated from 5 F5F8D patients or from age- and sex-matched normal controls and then titrated with different concentrations of FV (0-20 IU/dL). Thrombin generation in normal platelets was already optimal in the absence of plasma FV and did not increase further when FV concentration was increased up to 20 IU/dL. In contrast to normal platelets, F5F8D platelets with no added FV demonstrated a significantly delay in the initiation of coagulation (LT values, 22.3 ± 4.4 minutes vs 10.7 ± 1.5 minutes, P < .01; Figure 5A,C) and reduced thrombin generation (PH values, 58.1 ± 10.8 nM vs 96.5 ± 6.6 nM, P < .01; Figure 5B,D). Increasing plasma FV up to 5 IU/dL normalized thrombin generation with markedly shortened LT (9.8 ± 1.1 minutes, P > .05) and increased PH values (109.8 ± 12.7 nM, P > .05). Thrombin generation remained stable following the subsequent increase in plasma FV to 20 IU/dL. These results suggested that plasma plus platelet FV are sufficient to support normal thrombin generation in F5F8D. This is because FV levels in all F5F8D patients were higher than 5 IU/dL. To verify whether the low TFPIα also improved thrombin generation in F5F8D PRP, as demonstrated in F5F8D PPP, we compensated for the low TFPIα level by adding rhTFPI (10 ng/mL) to the PRP of patient P4 and a normal control. Compared with the control sample without supplemental TFPIα, thrombin generation minimally changed at increasing plasma FV levels in both reconstituted PRPs (Figure 5E-F). Thus, anticoagulant activity for TFPIα or a TFPIα-dependent anticoagulant effect for FV could not be detected, indicating that the low level of TFPIα in F5F8D PRP may not contribute to thrombin generation.
Respective contribution of plasma and platelet FV, as well as TFPIα, to thrombin generation in 5 F5F8D PRPs. FV-depleted plasma was reconstituted with isolated platelets of 5 F5F8D patients, as well as age- and sex-matched normal controls. LT (A) and PH (B) values for thrombin generation were plotted against functions of FV (0-20 IU/dL). Statistical difference in LT (C) and PH (D) values were calculated between F5F8D patients and normal controls. (E-F) Reconstituted PRPs of patient P4 and a normal control were supplemented with 10 ng/mL TFPIα at each FV titration. LT (E) and PH (F) values were plotted against functions of FV (0-20 IU/dL) with and without the supplemental TFPIα. **P < .01.
Respective contribution of plasma and platelet FV, as well as TFPIα, to thrombin generation in 5 F5F8D PRPs. FV-depleted plasma was reconstituted with isolated platelets of 5 F5F8D patients, as well as age- and sex-matched normal controls. LT (A) and PH (B) values for thrombin generation were plotted against functions of FV (0-20 IU/dL). Statistical difference in LT (C) and PH (D) values were calculated between F5F8D patients and normal controls. (E-F) Reconstituted PRPs of patient P4 and a normal control were supplemented with 10 ng/mL TFPIα at each FV titration. LT (E) and PH (F) values were plotted against functions of FV (0-20 IU/dL) with and without the supplemental TFPIα. **P < .01.
DDAVP-infusion trials
The efficacy of DDAVP infusion was analyzed for all 6 F5F8D patients. After administration of DDAVP, FVIII levels were increased at the indicated time points and peaked at 0.5 hours (46.5–90.5 IU/dL), yielding a median 4.4-fold increase (range, 3.3-fold to 5.7-fold). A complete response was observed for 5 F5F8D patients, whereas only a partial response was noted for the remaining patient. This patient had the lowest baseline FVIII:C (8.1 IU/dL) but exhibited a maximal increase of 5.7-fold. DDAVP infusion did not have any effect on FV levels in any of the 6 F5F8D patients (Table 2).
The efficacy of DDAVP infusion in 6 F5F8D patients
| Patients . | 0 h . | DDAVP infusion . | Outcome . | ||||
|---|---|---|---|---|---|---|---|
| 0.5 h . | 1 h . | 2 h . | 4 h . | ||||
| FVIII:C (IU/dL) . | FVIII:C (IU/dL) . | Increase (fold) . | FVIII:C (IU/dL) . | FVIII:C (IU/dL) . | FVIII:C (IU/dL) . | ||
| P1 | 21.2 | 77.0 | 3.6 | 75.8 | 74.2 | 65.5 | CR |
| P2 | 18.8 | 75.4 | 4.0 | 70.1 | 61.0 | 52.9 | CR |
| P3 | 13.9 | 66.1 | 4.8 | 60.2 | 53.6 | 46.2 | CR |
| P4 | 17.4 | 90.5 | 5.2 | 78.7 | 61.5 | 47.8 | CR |
| P5 | 16.0 | 52.1 | 3.3 | 50.1 | 46.1 | 43.0 | CR |
| P6 | 8.1 | 46.5 | 5.7 | 43.3 | 39.7 | 28.4 | PR |
| Patients . | 0 h . | DDAVP infusion . | Outcome . | ||||
|---|---|---|---|---|---|---|---|
| 0.5 h . | 1 h . | 2 h . | 4 h . | ||||
| FVIII:C (IU/dL) . | FVIII:C (IU/dL) . | Increase (fold) . | FVIII:C (IU/dL) . | FVIII:C (IU/dL) . | FVIII:C (IU/dL) . | ||
| P1 | 21.2 | 77.0 | 3.6 | 75.8 | 74.2 | 65.5 | CR |
| P2 | 18.8 | 75.4 | 4.0 | 70.1 | 61.0 | 52.9 | CR |
| P3 | 13.9 | 66.1 | 4.8 | 60.2 | 53.6 | 46.2 | CR |
| P4 | 17.4 | 90.5 | 5.2 | 78.7 | 61.5 | 47.8 | CR |
| P5 | 16.0 | 52.1 | 3.3 | 50.1 | 46.1 | 43.0 | CR |
| P6 | 8.1 | 46.5 | 5.7 | 43.3 | 39.7 | 28.4 | PR |
CR, complete responder; PR, partial responder.
Discussion
F5F8D is characterized by mild to moderate bleeding diathesis. Despite the concomitant reduction in FV and FVIII levels, the combined cofactor deficiency fails to lead to more severe bleeding episodes than the single FVIII defect of similar degree. In the present study, we have provided a possible explanation for this clinical manifestation by evaluating the hemostatic roles of low FV levels in F5F8D patients using an in vitro thrombin-generation assay.
Unexpectedly, we discovered a paradoxical hypercoagulable state in F5F8D PPP. Further studies revealed that a low FV level in PPP may be responsible for this observation. These results can be best explained in the context of a pioneering study by Duckers et al14 and subsequent studies by other investigators who demonstrated that severe FV deficiency is associated with reduced levels of TFPIα and that FV circulates in plasma as a carrier of TFPIα.33,34 In agreement with these results, we found that the TFPIα level in F5F8D plasma was significantly lower than in the normal control but similar to that in severe FV deficiency (Figure 1B). TFPIα exerts its anticoagulant activity by inhibiting TF/FVIIa and FXa, as well as by inhibiting early forms of the prothrombinase.35,36 It has recently been reported that TFPIα can also inhibit thrombin generation by delaying FV activation, particularly if the coagulation was triggered by a low concentration of TF.37 Normalizing TFPIα in F5F8D PPP greatly delayed the initiation of coagulation and reduced thrombin generation, indicating that lower TFPIα levels in PPP significantly promote coagulation and dramatically contribute to enhanced thrombin generation (Figure 2C).
Noting that FV functions as a cofactor for TFPIα in the inhibition of FXa, as recently reported,16,38 the effect of the FV level in F5F8D PPP on thrombin generation was evaluated by supplementing increasing concentrations of FV. Thrombin generation reached optimum levels at the basal level of FV, and any further addition of FV paradoxically caused an anticoagulant effect. Despite the significantly low TFPIα level in F5F8D PPP, we found that the anticoagulant effect of FV was still TFPIα dependent (Figure 3D), suggesting that the low FV level, combined with the low TFPIα level, in F5F8D PPP contributes to more thrombin generation by reducing the TFPIα-dependent anticoagulant effect of FV. After comparing the results of thrombin generation in FV-deficient PPP, as reported by Duckers et al,14 with our results in F5F8D PPP, we propose that the hemostatic contributions of low TFPIα in 2 plasmas are mediated by different mechanisms. In severe FV deficiency (<1%), FV plays only a procoagulant role, and low TFPIα decreases the amount of FV required for minimal thrombin generation.14 By contrast, in F5F8D plasma, in which the FV level is sufficient to support normal thrombin generation, the addition of FV only causes a TFPIα-dependent anticoagulant effect, whereas the low TFPIα level by itself in the absence of added FV improves thrombin generation in F5F8D plasma.
Platelets contain 20% of the FV in whole blood, which originates from endocytosis of plasma FV by megakaryocytes.39 Platelet FV is partially catalyzed into FVa (FV/FVa), which exhibits a more procoagulant potential at the site of injury.40-42 It has been reported that platelet FV levels in F5F8D patients are reduced to the same extent as plasma levels (13% of normal platelets in LMAN1 deficiency and 8% in MCFD2 deficiency).43 In vitro thrombin generation and in vivo observation of a patient with acquired FV deficiency and undetectable plasma FV demonstrated that normal platelet FV was sufficient to maintain hemostasis15,44 ; however, it is unknown to what extent platelet FV of F5F8D contributes to thrombin generation. In contrast to F5F8D plasma with hypercoagulable state, F5F8D PRP generated significantly less thrombin compared with normal controls (P < .05). The downregulation of thrombin generation in F5F8D PRP was exclusively associated with the low FVIII level. To determine the relative contribution of plasma and platelet FV, as well as the low TFPIα level, to thrombin generation, F5F8D platelets were reconstituted with different concentrations of FV in FV-depleted plasma, with or without normalizing the TFPIα level. TGAs revealed that the low plasma FV level (<5 IU/dL) plus platelet FV were sufficient to support normal thrombin generation. However, the anticoagulant activity of TFPIα and the TFPIα-dependent anticoagulant effect of FV were not observed in the reconstituted PRP. It has been reported that the low TFPIα in plasma improved thrombin generation in severe FV-deficient PRP,15 indicating that the procoagulant activity of trace levels of platelet and plasma FV in severe FV-deficient PRP is sensitive to the inhibitory effect of TFPIα. We predict that the difference in the hemostatic role of low TFPIα between F5F8D and severe FV-deficient patients may be caused by different amounts of FV in the respective PRP samples.
The striking discrepancy in the hemostatic state between F5F8D PPP and PRP observed in this study may be attributable to different responses to pro- and anticoagulant reactions on synthetic phospholipids in plasma and platelet surface in PRP. Activated platelets expose phosphatidylserine, as well as release FV/FVa in PRP, which can be activated by FXa/thrombin and binds FXa to form prothrombinase complexes on the platelet surface that are resistant to the action of TFPIα.45 Synthetic phospholipids added in plasma may provide surfaces for equal binding of procoagulant and anticoagulant proteins. Therefore, the reduced anticoagulant activities of TFPIα and FV overcompensated for the hypocoagulable state that resulted from the low FVIII level in F5F8D PPP, whereas this compensation was abolished and only exerted FVIII deficiency as mild hemophilia A in F5F8D PRP.
Despite the completely different nature of the molecular defects in hemophilia A and F5F8D, the efficacy of DDAVP infusion has been confirmed in F5F8D patients by only a few studies.12,13 In this study, DDAVP infusion induced a complete response in 5 F5F8D patients harboring mutations in LMAN1 and a partial response in the 1 remaining patient who had the lowest FVIII level caused by a null mutation in MCFD2, suggesting that baseline FVIII activities and F5F8D genotypes are predicting factors for the extent of the DDAVP response. The increased FVIII level after DDAVP infusion improved the thrombin-generation potential that was comparable to the normal control, supporting its beneficial use in the management of F5F8D patients for bleeding episodes.
There are some limitations to this study. First, the small sample size reduces the statistical power of our conclusions. Second, a low TF concentration was adopted in this study. Although a low TF is required to detect the anticoagulant effect associated with TFPI, FV, and PS in the initiation phase of coagulation, different TF concentrations should be considered to evaluate the overall hemostatic state of F5F8D patients in future studies. Third, more F5F8D patients need to be recruited to verify the efficacy of DDAVP infusion. Based on this study, DDAVP infusion was administered to patient P5 twice before and 8 hours after her dental extraction without FFP transfusion, and no excessive bleeding occurred during the surgery. Finally, considering that infused FV by FFP transfusion can be internalized by megakaryocytes, which may eventually end up replenishing the platelet FV pool for long-term protection from bleeding,46 it should be further evaluated whether FFP transfusion benefits F5F8D patients experiencing major surgery or trauma.
In summary, we have presented in vitro data showing that a low level of FV accompanied by a low TFPI level in F5F8D patients exerts an optimal procoagulant activity. Thus, the addition of FV to plasma in these patients does not contribute to hemostasis but actually plays a paradoxical anticoagulant role. DDAVP infusion could be a potential substitute for FVIII concentrates, whereas FFP transfusion may not be suggested in F5F8D patients with minor bleeding challenges.
For original data, please contact roger3212@126.com.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Audrey Rezaie and Alireza Rezaie (Oklahoma Medical Research Foundation and the Department of Biochemistry and Molecular Biology, University of Oklahoma Health Sciences Center) for editorial work, expert comments, and revision of the manuscript. They are also grateful to all patients for their enthusiastic participation in this study.
This work was supported by grants from the General Program of the National Natural Science Foundation of China (81570114, 81570115, 81770135, and 81770136), and the National Key Research and Development Program of China (2016YFC0905100).
Authorship
Contribution: Y.S. designed experiments, performed research, analyzed data. and wrote the manuscript; W.W analyzed the data and reviewed the manuscript; G.X performed experiments; X.W. supervised the studies conducted in Ruijin Hospital, recruited the patients, and collected clinical histories; and Q.D recruited patients, collected clinical histories, analyzed data, and critically reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Qiulan Ding, Department of Laboratory Medicine, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, No. 197 Ruijin Second Rd, Shanghai 200025, China; e-mail: qiulan_ding@126.com; and Xuefeng Wang, Department of Laboratory Medicine, Ruijin Hospital, Shanghai Jiaotong University School of Medicine, No. 197 Ruijin Second Rd, Shanghai 200025, China; e-mail: wangxuefeng6336@hotmail.com.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal