Key Points
L-selectin shedding amplifies integrin outside-in signaling and is needed for bacterial clearance in K pneumoniae–induced pneumonia.
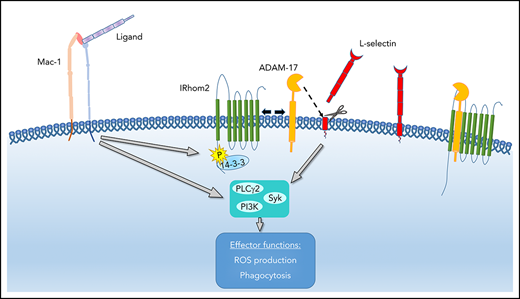
IRhom2-dependent ADAM17 activation leads to L-selectin shedding and subsequently amplifies neutrophil effector functions.
Abstract
Pneumonia induced by Gram-negative bacteria is a common and serious disease associated with high morbidity and mortality. Elimination of bacterial pathogens relies on the recruitment and functions of neutrophils. The adhesion molecule L-selectin has recently been implicated in integrin activation in neutrophils (inside-out signaling). However, the molecular mechanism by which L-selectin participates in host defense against Klebsiella pneumoniae–induced pulmonary inflammation is unknown. We demonstrate that L-selectin–deficient mice are prone to pulmonary infection compared with wild-type controls. Mechanistically, L-selectin cleavage from the neutrophil surface triggered by integrin engagement is involved in neutrophil recruitment into the lung and bacterial clearance. Downstream of integrin ligation, the metalloproteinase A disintegrin and metalloproteinase 17 (ADAM17) sheds L-selectin from the neutrophil surface in an IRhom2-dependent manner. L-selectin cleavage enhances integrin-mediated outside-in signaling, resulting in increased neutrophil effector functions. Thus, we identify a novel regulatory mechanism in neutrophils required for an adequate immune response triggered by integrin engagement during K pneumoniae–induced pulmonary inflammation.
Introduction
Pneumonia is a leading cause of hospitalization in the United States and is the most common infectious cause of death.1,2 Gram-negative bacteria, like Klebsiella pneumoniae, are the most common cause of health care–associated pneumonia, and the mortality from Gram-negative pneumonia ranges from 30% to 60%.3 Therapy aimed at augmenting the host response has the potential to enhance bacterial clearance and improve outcome and may provide a new avenue for therapeutic advances in combating Gram-negative pneumonia.
Inflammatory responses require efficient recruitment of leukocytes.4 Neutrophils are particularly important in the early inflammatory responses to bacterial infection.5 They rapidly migrate into sites of inflammation and deploy antimicrobial effectors, which often results in collateral tissue damage.4 Thus, uncontrolled accumulation and activation of neutrophils may lead to profound immunopathology.6 This is exemplified in acute inflammatory insults of the lung, including pneumonia.
During neutrophil recruitment, several cell surface receptors are involved in sensing the inflammatory microenvironment and mediating neutrophil responses. The adhesion molecule L-selectin is expressed on the microvilli tips of neutrophils7,8 and plays an important role in neutrophil capturing from the bloodstream to the endothelial wall during the recruitment cascade.9 In addition, L-selectin contributes to selectin-mediated integrin activation, which is important for neutrophil recruitment into inflamed tissue.10 Furthermore, an in vivo study suggests that L-selectin is also involved in neutrophil migration within inflamed tissue,11 but the mechanism of this observation is unknown. During neutrophil recruitment and activation, L-selectin is rapidly shed from the cell surface. Several studies describe this process as a mechanism to reduce adhesion receptor density to allow neutrophil migration following extravasation.12 However, little is known about possible downstream signaling mechanisms following L-selectin cleavage.13,14
A disintegrin and metalloproteinase 17 (ADAM17) is a transmembrane protein specialized in the cleavage (ectodomain shedding) of distinct substrates like L-selectin, CXCR2, ICAM-1, or tumor necrosis factor-α (TNF-α) at extracellular cleavage sites proximal to the cell membrane in a cis manner from neutrophils or endothelial cells.15 Several studies demonstrated that shedding processes can downregulate the concentration of adhesion molecules and receptors on activated cells or increase the release of soluble inflammatory mediators or receptors, thereby influencing the immune response.16-18 A recent report by Cavadas et al demonstrated that IRhom2 phosphorylation following cellular stimulation with various agents induces a translocation of IRhom2, enabling the increase in ADAM17 sheddase activity.19
Integrins are a class of adhesion molecules on the neutrophil surface able to signal bidirectionally across the plasma membrane. Following integrin engagement, integrin outside-in signaling is involved in different cellular processes, including firm adhesion, spreading, migration, and degranulation.20-22 Neutrophils express β2-family integrins (CD11/CD18), consisting of an α-subunit and a common β-subunit (CD18).23 Lymphocyte-function–associated antigen 1 (LFA-1; αLβ2, CD11a) and macrophage antigen-1 (Mac-1; αMβ2, CD11b) are the most important β2-integrins involved in neutrophil recruitment.
Downstream of integrin-mediated outside-in signaling, major neutrophil effector functions are elicited. Neutrophils kill pathogens via phagocytosis and the production of reactive oxygen species (ROS). Both processes are essential for the defense against bacterial and fungal infections. The specialized acidic and hydrolytic milieu within the phagolysosomes mediates degradation of the phagocytosed particle. ROS are produced via the NADPH oxidase complex either extracellularly, releasing ROS into the environment at sites of infection, or intracellularly in phagolysosomes.24 Defects in ROS production result in bacterial dissemination following infections and increased bacterial colonization in different tissues and organs.25,26 In addition to ROS, other functions in neutrophils can be elicited, including degranulation, cytokine production, and neutrophil extracellular trap formation.27-29 Overall, the combination of these neutrophil effector mechanisms provides a powerful antimicrobial weapon within the innate immune defense.
The current study was designed to determine the role of L-selectin in integrin-mediated outside-in signaling during K pneumoniae–induced pneumonia. Using functional ex vivo, in vivo, and in vitro assays, we found that L-selectin cleavage from the neutrophil surface triggered by integrin engagement is involved in neutrophil recruitment and bacterial clearance in the lung. L-selectin cleavage amplifies integrin-mediated outside-in signaling in neutrophils, resulting in increased migration capacity, elevated production of ROS, and improved phagocytosis. ADAM17, which cleaves L-selectin, is activated in an IRhom2-dependent pathway following integrin engagement. In conclusion, we demonstrate that the cleavage of L-selectin triggered by integrin engagement is indispensable for an adequate immune response during K pneumoniae–induced pneumonia.
Methods
Lung infection with K pneumoniae
Overnight cultures (37°C) of K pneumoniae (American Type Culture Collection strain 13883) were grown in Tryptic Soy medium, washed, and resuspended in sterile saline (0.9%). Mice were anesthetized by intraperitoneal injection of ketamine (125 μg/g body weight; Pfizer, New York, NY) and xylazine (12.5 μg/g body weight; Bayer, Leverkusen, Germany). Subsequently, the trachea was exposed, and a 50-µL inoculum of bacterial suspension was administered via a 30-gauge needle. Animals were challenged with 4 × 107 viable K pneumoniae per mouse. At this inoculation dose, all mice survived the 24-hour observation period. After 24 hours, the mice were euthanized, and the lungs were lavaged 4 times with 0.7 mL physiologic saline solution. The number of neutrophils in the bronchoalveolar lavage fluid (BALF) was counted using Kimura staining. Neutrophils were counted and further discriminated by flow cytometry. Colony-forming units in the BALF, lung, blood, and spleen were counted by serial plating on Tryptic Soy agar plates. One half of the lung was fixed in 4% formaldehyde, embedded in paraffin, and sectioned at 5 µm for hematoxylin and eosin staining.
Polymorphonuclear cell migration ex vivo
The migration of polymorphonuclear cells (PMNs) in lung tissue ex vivo was investigated using a technique published by Hasenberg et al, with some modifications.30 Mice were injected intratracheally with K pneumoniae and were injected IV with Alexa Fluor 488–coupled anti-Gr1 antibody (5 μg per mouse; clone RB6-8C5) and Alexa Fluor 568–coupled anti-PECAM antibody (50 μg per mouse; clone 390, BD Biosciences) to stain neutrophils and endothelial cells, respectively. After 4 hours, mice were euthanized, and lungs were filled with 1 mL of low-melting agarose. After removal, lungs were cut using a vibratome. Lungs were fixed in a cell culture dish and submersed in phosphate-buffered saline (PBS), and time-lapse z-stacks were recorded using a spinning disc confocal microscope (Cell Observer SD; Zeiss, Göttingen, Germany) equipped with a 20×/1.0 NA objective. Neutrophil migration velocity and displacement were analyzed using ImageJ.31
In vitro chemotaxis assay
In vitro chemotaxis assay was performed as described previously.32 Following isolation, bone marrow–derived murine or whole-blood human neutrophils were seeded on fibronectin-coated (from bovine plasma, 50 µg/mL, 37°C, overnight; Sigma-Aldrich,) chemotaxis μ-slides (Ibidi). Within the chemotaxis slide, a CXCL1 (or interleukin-8 [IL-8] for human neutrophils) gradient was applied by diffusion of a Patent Blue colored CXCL1 (or IL-8 for human neutrophils) solution (1 µg/mL) in 1 reservoir of the slide, according to the manufacturer’s instructions. Cell movement was recorded with a microscope platform (37°C, 5% CO2; Axio Observer, Zeiss) over a period of 30 minutes using time-lapse microscopy (3 frames per minute). For analysis, cells were tracked with Manual Tracking (ImageJ) and analyzed with Chemotaxis plug-in (Ibidi). We analyzed the distance, velocity, and forward migration index of the cells.32
See supplemental Methods (available on the Blood Web site) for further information on methods and statistics.
Results
L-selectin is required for host defense during K pneumoniae–induced pneumonia
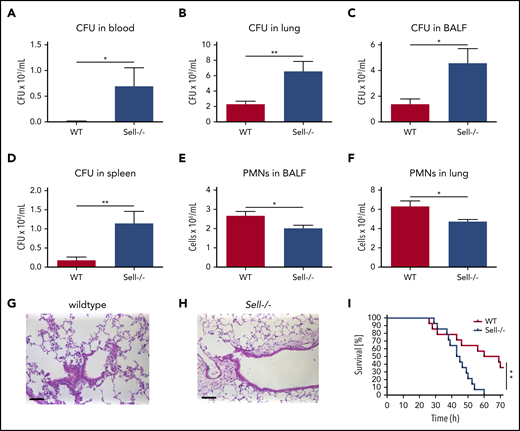
Several lines of evidence demonstrated that L-selectin may trigger different signaling pathways leading to tyrosine phosphorylation, integrin activation, and chemokine release.9,12,33 First, we investigated whether L-selectin is involved in effective bacterial clearance in a murine model of K pneumoniae–induced pneumonia. Intratracheal instillation of the clinically relevant pathogen K pneumoniae led to a highly increased bacterial burden, as determined by colony forming units (CFU), in blood (Figure 1A), lung tissue (Figure 1B), BAL fluid (Figure 1C), and spleen (Figure 1D) in L-selectin–deficient mice compared with wild-type (WT) mice. The number of neutrophils in the bronchoalveolar space (Figure 1E) and lung tissue (Figure 1F) in Sell−/− mice was slightly, but also significantly, decreased compared with WT mice. Histological analysis of lung tissue following K pneumoniae–induced pneumonia also showed a reduced number of neutrophils in the lung tissue of L-selectin–deficient mice (Figure 1G-H). The survival of Sell−/− mice following K pneumoniae–induced pneumonia was also significantly reduced in comparison with WT mice (Figure 1I).
L-selectin is required for host defense during K pneumoniae–induced pneumonia. (A-I) WT and Sell−/− mice were subjected to K pneumoniae intratracheal injection. Bacterial burden in terms of CFU in blood (A), lung (B), BALF (C), and spleen (D), as well as neutrophil recruitment into the alveoli (E) and lung tissue (F), were determined 24 hours after Kpneumoniae injection. Representative hematoxylin and eosin staining of the lung of WT (G) and Sell−/− (H) mice 24 h after intratracheal instillation with K pneumoniae (scale bars, 50 µm). (I) Survival of WT and Sell−/− mice after K pneumoniae intratracheal injection. n = 8 mice per genotype. Data are mean ± standard error of the mean. *P = .05, **P = .01, Student t test and log-rank (Mantel-Cox) test.
L-selectin is required for host defense during K pneumoniae–induced pneumonia. (A-I) WT and Sell−/− mice were subjected to K pneumoniae intratracheal injection. Bacterial burden in terms of CFU in blood (A), lung (B), BALF (C), and spleen (D), as well as neutrophil recruitment into the alveoli (E) and lung tissue (F), were determined 24 hours after Kpneumoniae injection. Representative hematoxylin and eosin staining of the lung of WT (G) and Sell−/− (H) mice 24 h after intratracheal instillation with K pneumoniae (scale bars, 50 µm). (I) Survival of WT and Sell−/− mice after K pneumoniae intratracheal injection. n = 8 mice per genotype. Data are mean ± standard error of the mean. *P = .05, **P = .01, Student t test and log-rank (Mantel-Cox) test.
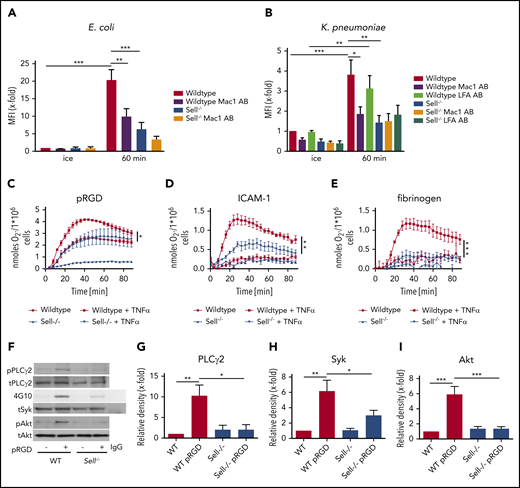
We were able to confirm these data in an Escherichia coli–induced pneumonia model (supplemental Figure 1), demonstrating that the observed function of L-selectin is not specific to K pneumoniae–induced pneumonia.
It is unlikely that the severe phenotype observed in the bacterial burden of Sell−/− mice is due to the slightly decreased neutrophil recruitment. These data indicate an additional defect in neutrophil effector functions following recruitment to the site of infection.
L-selectin affects integrin-dependent neutrophil migration
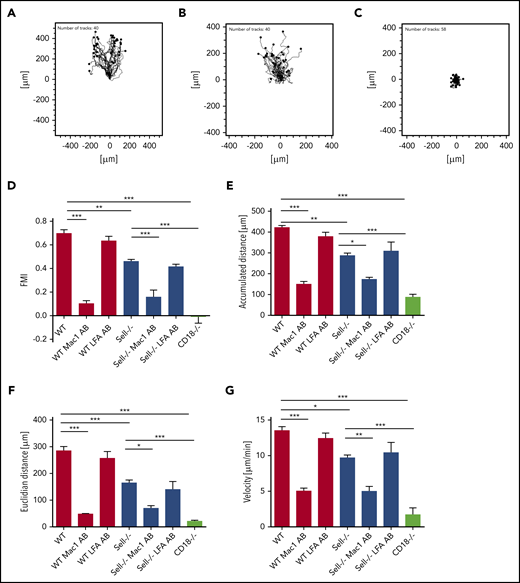
Neutrophil recruitment into the lung is dependent on chemotaxis.32 To investigate whether L-selectin is involved in this process, we investigated chemotaxis toward the chemokine CXCL1 using an in vitro assay (Figure 2). WT neutrophils, adherent on fibronectin, were able to migrate along a CXCL1 gradient (Figure 2A,D). In contrast, L-selectin–deficient neutrophils migrated less efficiently along the chemotactic gradient (Figure 2B,D). Forward migration index (Figure 2D), migration distance (Figure 2E-F), and migration velocity (Figure 2G) of L-selectin–deficient neutrophils were significantly reduced compared with WT neutrophils. To test whether the observed migration is dependent on β2 integrin signaling in neutrophils, we performed the same experiments with CD18−/− neutrophils. The migration ability of CD18−/− neutrophils was almost completely abolished (Figure 2C-G). Additionally, we blocked LFA-1 or Mac-1 using blocking antibodies in WT and L-selectin–deficient neutrophils to investigate the importance of the 2 β2-integrins for chemotaxis. Blocking LFA-1 did not affect migration, whereas blocking Mac-1 in WT and L-selectin–deficient neutrophils completely blocked migration (Figure 2D-G), suggesting that Mac-1–dependent migration is affected by, but not completely dependent on, L-selectin–dependent processes.
L-selectin plays a pivotal role in integrin-dependent neutrophil migration. (A-G) Chemotaxis of WT, Sell−/−, and CD18−/− neutrophils on fibronectin in response to a soluble CXCL1 gradient in vitro. Representative trajectory plots of WT (A), Sell−/− (B), and CD18−/− (C) neutrophils. Forward migration index (D), accumulated (E) and Euclidian (F) distance, and migration velocity (G) of chemotaxing neutrophils that were left untreated or were pretreated with LFA-1– or Mac-1–blocking antibody. A total of 40 cells was analyzed per experiment. n = 4. Data are mean ± standard error of the mean. *P = .05,**P = .01, ***P = .001, 1-way analysis of variance (ANOVA).
L-selectin plays a pivotal role in integrin-dependent neutrophil migration. (A-G) Chemotaxis of WT, Sell−/−, and CD18−/− neutrophils on fibronectin in response to a soluble CXCL1 gradient in vitro. Representative trajectory plots of WT (A), Sell−/− (B), and CD18−/− (C) neutrophils. Forward migration index (D), accumulated (E) and Euclidian (F) distance, and migration velocity (G) of chemotaxing neutrophils that were left untreated or were pretreated with LFA-1– or Mac-1–blocking antibody. A total of 40 cells was analyzed per experiment. n = 4. Data are mean ± standard error of the mean. *P = .05,**P = .01, ***P = .001, 1-way analysis of variance (ANOVA).
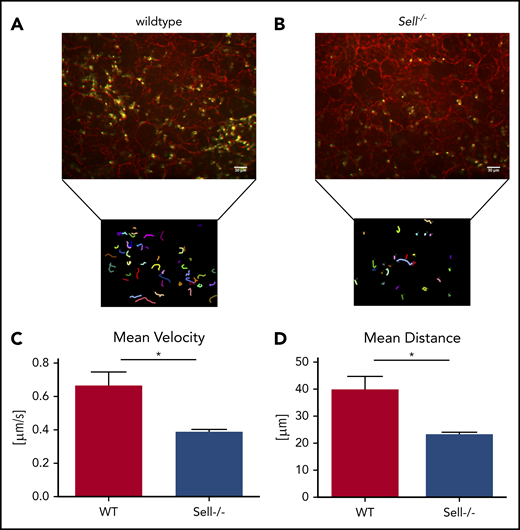
To investigate the integrin-dependent migration in the lung following infection with K pneumoniae, we performed confocal microscopy of viable ex vivo lung sections of WT mice (Figure 3A; supplemental Video 1) and Sell−/− mice (Figure 3B; supplemental Video 2). The migration velocity (Figure 3C), as well as the total migration distance within 60 minutes of observation (Figure 3D), of Sell−/− neutrophils within the lung was significantly decreased in comparison with WT neutrophils.
Neutrophil migration in a mouse model of pneumonia is L-selectin–dependent. (A-D) WT and Sell-/- mice were injected intratracheally with Kpneumoniae. (A-B) Representative confocal images and neutrophil migratory tracks in agarose-filled lung slices of wildtype (A) and Sell−/− (B) mice over a 60 minute timeframe 4 hours after intratracheal instillation of Kpneumoniae. The CellObserver SD (Zeiss, Göttingen, Germany) equipped with a 20 ×/1.0 NA objective was used, white scale bars represent 30 µm. Neutrophil migration velocity (C) and distance (D). n = at least 3 mice/genotype, mean ± standard error of the mean, rank-sum test *P = .05.
Neutrophil migration in a mouse model of pneumonia is L-selectin–dependent. (A-D) WT and Sell-/- mice were injected intratracheally with Kpneumoniae. (A-B) Representative confocal images and neutrophil migratory tracks in agarose-filled lung slices of wildtype (A) and Sell−/− (B) mice over a 60 minute timeframe 4 hours after intratracheal instillation of Kpneumoniae. The CellObserver SD (Zeiss, Göttingen, Germany) equipped with a 20 ×/1.0 NA objective was used, white scale bars represent 30 µm. Neutrophil migration velocity (C) and distance (D). n = at least 3 mice/genotype, mean ± standard error of the mean, rank-sum test *P = .05.
Importantly, L-selectin–deficient neutrophils showed no significant differences from WT cells in surface CD11a, CD11b, CD18, CD44, PSGL-1, or CXCR2 levels, as determined by fluorescence-activated cell sorting analysis (data not shown).
L-selectin regulates integrin-mediated outside-in signaling
Once neutrophils are at the site of infection, different neutrophil effector functions are required for bacterial clearance.34 To investigate whether L-selectin is also involved in integrin-mediated outside-in signaling leading to activation of neutrophil effector functions, we used WT and Sell−/− neutrophils to perform phagocytosis assays of E coli (Figure 4A) and K pneumoniae particles (Figure 4B). L-selectin–deficient neutrophils phagocytosed significantly fewer particles than did WT neutrophils after 60 minutes. Also, neutrophils pretreated with a blocking Mac-1 antibody phagocytosed fewer microbial particles; however, LFA-1–blocking antibody does not affect phagocytosis in this model. These data demonstrate that Mac-1, and not LFA-1, is involved in neutrophil phagocytosis of different pathogens (Figure 4A-B).
L-selectin regulates integrin outside-in signaling in neutrophils. Phagocytosis of pHrodo E coli (A) or K pneumoniae (B) particles by WT and Sell−/− neutrophils that were left untreated or were pretreated with Mac-1– or LFA-1–blocking antibody. Adhesion-dependent oxidative burst of WT and Sell−/− neutrophils plated on pRGD (C), ICAM-1 (D), or fibrinogen (E), alone or in the presence of TNF-α. WT and Sell−/− neutrophils were plated on pRGD for 10 minutes, and lysates were immunoblotted with anti–phosphorylated (p)-PLCγ2 or anti-PLCγ2 (F-G) or anti-phosphotyrosine (4G10) or total Syk (tSyk) (F,H) after immunoprecipitation for Syk or immunoprecipitation using an IgG control antibody and anti–p-Akt and anti-Akt (F,I). n = 3. Data are mean ± standard error of the mean. *P = .05, **P = .01, ***P = .001, 1-way or 2-way ANOVA.
L-selectin regulates integrin outside-in signaling in neutrophils. Phagocytosis of pHrodo E coli (A) or K pneumoniae (B) particles by WT and Sell−/− neutrophils that were left untreated or were pretreated with Mac-1– or LFA-1–blocking antibody. Adhesion-dependent oxidative burst of WT and Sell−/− neutrophils plated on pRGD (C), ICAM-1 (D), or fibrinogen (E), alone or in the presence of TNF-α. WT and Sell−/− neutrophils were plated on pRGD for 10 minutes, and lysates were immunoblotted with anti–phosphorylated (p)-PLCγ2 or anti-PLCγ2 (F-G) or anti-phosphotyrosine (4G10) or total Syk (tSyk) (F,H) after immunoprecipitation for Syk or immunoprecipitation using an IgG control antibody and anti–p-Akt and anti-Akt (F,I). n = 3. Data are mean ± standard error of the mean. *P = .05, **P = .01, ***P = .001, 1-way or 2-way ANOVA.
Superoxide production is another neutrophil effector function that is required for efficient bacterial clearance.34 To characterize whether L-selectin is involved in integrin-mediated superoxide production, we analyzed this function in WT and Sell−/− neutrophils after stimulating the cells with different integrin ligands (Figure 4C-E). The oxidative burst was significantly reduced in Sell−/− neutrophils when plated on multivalent integrin ligand tripeptide Arg-Gly-Asp (pRGD) in the absence of TNF-α (Figure 4C), as well as on pRGD (Figure 4C), ICAM-1 (Figure 4D), and fibrinogen (Figure 4E) in the presence of TNF-α, suggesting that L-selectin amplifies integrin-mediated superoxide production.
Integrin engagement induces a signaling pathway that relies on the tyrosine phosphorylation of different signaling molecules, including Src-family kinases, Syk, phospholipase Cγ2, and other molecules.35 When plated on pRGD (10 minutes, rom temperature), Sell−/− neutrophils exhibit reduced phosphorylation of PLCγ2 (Figure 4F-G), Syk (Figure 4F,H), and Akt (Figure 4F,I) compared with WT neutrophils, indicating that integrin-mediated outside-in signaling is dependent on L-selectin.
L-selectin shedding from the neutrophil surface increases integrin-mediated outside-in signaling
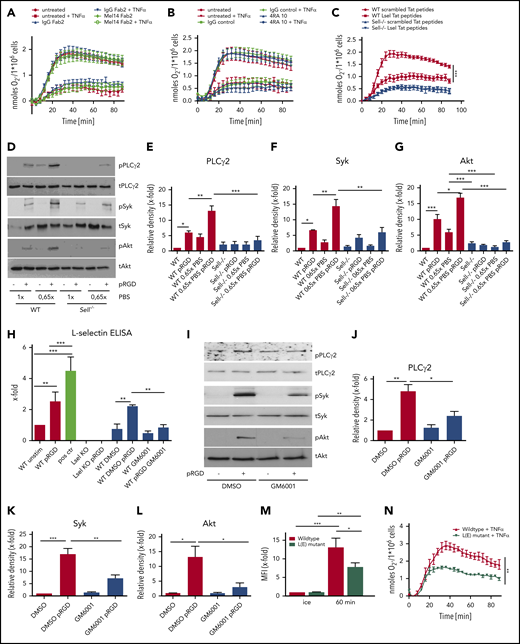
To investigate the molecular mechanism for the role of L-selectin in integrin-mediated outside-in signaling in neutrophils, we performed several blocking experiments. To block the extracellular part of L-selectin, we incubated isolated WT neutrophils with F(ab)2 fragments of a blocking anti–L-selectin antibody (Mel14) or an isotype-control antibody (rat immunoglobulin G2a [IgG2a]) prior to plating on fibrinogen, with or without TNF-α stimulation (Figure 5A). The oxidative burst on fibrinogen plus TNF-α was not affected in antibody-treated cells, indicating that the ligation of the extracellular L-selectin domain is not involved in integrin-mediated outside-in signaling. To investigate whether L-selectin binding to PSGL-1 amplifies superoxide production, isolated WT neutrophils were left untreated or were treated with IgG antibody or a blocking anti–PSGL-1 antibody (4RA10) prior to measurement of the oxidative burst, following the stimulation with fibrinogen, with or without TNF-α (Figure 5B). Blocking PSGL-1 did not influence the oxidative burst of WT neutrophils following fibrinogen stimulation.
Shedding of L-selectin from the neutrophil surface is responsible for its role in outside-in signaling. Adhesion-dependent oxidative burst of WT neutrophils and WT neutrophils pretreated with IgG F(ab)2 or Mel14 F(ab)2 (A), WT neutrophils pretreated with IgG or 4RA10 antibody (B), or WT and Sell−/− neutrophils pretreated with scrambled or L-selectin Tat peptides (C) prior to plating on fibrinogen alone or in the presence of TNF-α. WT and Sell−/− neutrophils preincubated for 2 hours in 1× or 0.65× PBS were plated on pRGD for 10 minutes and lysates were immunoblotted with anti–phosphorylated (p)-PLCγ2 and total PLCγ2 (D-E), p-Syk and total Syk (D-F), or anti–p-Akt or total Akt (D,G). (H) Levels of soluble L-selectin in the supernatant of unstimulated or pRGD-stimulated (10 minutes) WT and Sell−/− neutrophils and WT neutrophils that were preincubated for 2 hours with 0.65× PBS or for 30 minutes with dimethyl sulfoxide (DMSO) or the metalloproteinase inhibitor GM6001. (I-L) WT neutrophils pretreated with DMSO or GM6001 were plated on pRGD for 10 minutes, and lysates were immunoblotted with anti–p-PLCγ2 or total PLCγ2 (I-J), anti–p-Syk or total Syk (tSyk) (I,K), or anti–p-Akt or total Akt (I-L). (M) Phagocytosis of pHrodo-labeled K pneumoniae particles by isolated WT neutrophils and neutrophils of L(E) mutant mice. (N) Adhesion-dependent oxidative burst of isolated WT and L(E) mutant neutrophils plated on fibrinogen in the presence of TNF-α. n = 3. Data are mean ± standard error of the mean. *P = .05, **P = .01, ***P = .001, 1-way or 2-way ANOVA.
Shedding of L-selectin from the neutrophil surface is responsible for its role in outside-in signaling. Adhesion-dependent oxidative burst of WT neutrophils and WT neutrophils pretreated with IgG F(ab)2 or Mel14 F(ab)2 (A), WT neutrophils pretreated with IgG or 4RA10 antibody (B), or WT and Sell−/− neutrophils pretreated with scrambled or L-selectin Tat peptides (C) prior to plating on fibrinogen alone or in the presence of TNF-α. WT and Sell−/− neutrophils preincubated for 2 hours in 1× or 0.65× PBS were plated on pRGD for 10 minutes and lysates were immunoblotted with anti–phosphorylated (p)-PLCγ2 and total PLCγ2 (D-E), p-Syk and total Syk (D-F), or anti–p-Akt or total Akt (D,G). (H) Levels of soluble L-selectin in the supernatant of unstimulated or pRGD-stimulated (10 minutes) WT and Sell−/− neutrophils and WT neutrophils that were preincubated for 2 hours with 0.65× PBS or for 30 minutes with dimethyl sulfoxide (DMSO) or the metalloproteinase inhibitor GM6001. (I-L) WT neutrophils pretreated with DMSO or GM6001 were plated on pRGD for 10 minutes, and lysates were immunoblotted with anti–p-PLCγ2 or total PLCγ2 (I-J), anti–p-Syk or total Syk (tSyk) (I,K), or anti–p-Akt or total Akt (I-L). (M) Phagocytosis of pHrodo-labeled K pneumoniae particles by isolated WT neutrophils and neutrophils of L(E) mutant mice. (N) Adhesion-dependent oxidative burst of isolated WT and L(E) mutant neutrophils plated on fibrinogen in the presence of TNF-α. n = 3. Data are mean ± standard error of the mean. *P = .05, **P = .01, ***P = .001, 1-way or 2-way ANOVA.
To investigate whether L-selectin–mediated signaling is involved in integrin-mediated outside-in signaling, we blocked L-selectin signaling using Tat peptides that have been shown to block L-selectin–triggered downstream signaling9 (Figure 5C). The oxidative burst on fibrinogen plus TNF-α was significantly reduced in WT neutrophils pretreated with L-selectin Tat peptides in comparison with scrambled Tat peptides. In Sell−/− neutrophils, scrambled and L-selectin Tat peptides had no effect on the production of ROS (Figure 5C). These data indicate that L-selectin–mediated signaling plays an important role in amplifying integrin-mediated outside-in signaling.
Shedding of L-selectin from the cell surface might activate signaling pathways.11,13 L-selectin shedding can be induced by incubating neutrophils in hypotonic PBS (0.65×). Using fluorescence-activated cell sorting analysis, we demonstrated that incubating WT neutrophils in 0.65× PBS reduced the amount of L-selectin molecules on the cell surface to ∼20% (data not shown). To investigate whether L-selectin shedding activates the phosphorylation of different signaling molecules, WT neutrophils were incubated in 1× or 0.65× PBS under unstimulated or pRGD-stimulated conditions (Figure 5D-G). Incubation of WT neutrophils in 0.65× PBS led to rapid phosphorylation of PLCγ2, Syk, and Akt, which was increased further when cells were plated on pRGD (Figure 5D-G). By contrast, incubation of L-selectin–deficient neutrophils with 0.65× PBS did not induce phosphorylation of these signaling molecules, in unstimulated or pRGD-plated conditions, suggesting that the effect of 0.65× PBS is mediated through L-selectin shedding, which, in turn, augments integrin signaling.
Using an enzyme-linked immunosorbent assay for soluble L-selectin, we demonstrated that the amount of soluble L-selectin molecules in the supernatant was increased following plating neutrophils on pRGD-coated dishes compared with uncoated dishes (Figure 5H); this indicates that integrin engagement induces shedding of L-selectin molecules from the neutrophil surface. As a positive control, neutrophils were incubated in 0.65× PBS prior to the enzyme-linked immunosorbent assay (Figure 5H), leading to significant L-selectin shedding from the neutrophil surface. A metalloproteinase inhibitor (GM6001) that inhibits L-selectin shedding36 was able to decrease the amount of soluble L-selectin following pRGD treatment (Figure 5H). Inhibition of metalloproteinases also inhibited phosphorylation of PLCγ2, Akt, and Syk in neutrophils plated on pRGD (Figure 5I-L).
We also confirmed the importance of L-selectin shedding for neutrophil effector functions in the human system (supplemental Figure 2). Following inhibition of L-selectin shedding via GM6001, migration (supplemental Figure 2A-D), phagocytosis (supplemental Figure 2E), production of ROS (supplemental Figure 2F), and activation of distinct signaling molecules (supplemental Figure 2G-J) in human neutrophils were significantly reduced in comparison with DMSO-treated control groups.
To prove that the observed phenotype of L-selectin–knockout mice in neutrophil effector functions is mediated by the lack of L-selectin shedding during integrin outside-in signaling in neutrophils, we used isolated neutrophils from L(E) mutant mice and performed phagocytosis assays (Figure 5M) and induced the production of ROS (Figure 5N). L(E) neutrophils, which express a mutated form of L-selectin that cannot be shed from the surface, show significantly reduced phagocytosis of K pneumoniae particles (Figure 5M) and produce significantly less ROS in response to a combined stimulation with fibrinogen and TNF-α (Figure 5N). The reduction in these integrin-mediated effector functions was roughly equivalent to that seen in L-selectin–deficient cells.
Altogether, these data indicate that L-selectin shedding by a metalloproteinase triggers downstream signaling via the intracellular tail of L-selectin, which amplifies integrin-mediated outside-in signaling in neutrophils.
Shedding of L-selectin is required for bacterial clearance in the tissue during K pneumoniae–induced pneumonia
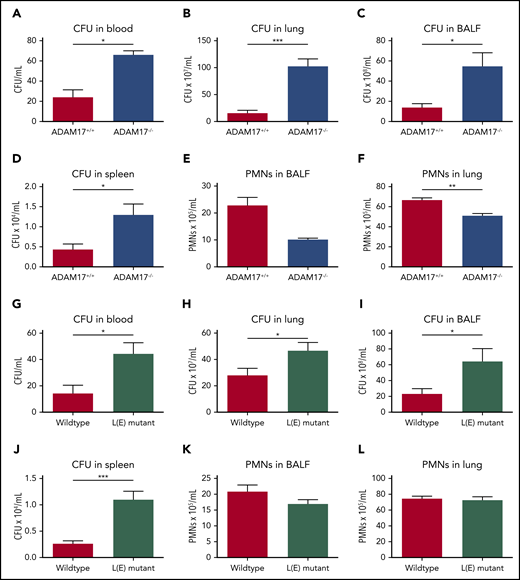
To further investigate the in vivo relevance of L-selectin shedding, we induced K pneumoniae–mediated pneumonia in ADAM17−/− and L(E) mutant mice (Figure 6). As observed in Sell−/− mice, the bacterial burden in the blood (Figure 6A,G), lung (Figure 6B,H), BALF (Figure 6C,I) and spleen (Figure 6D,J) of ADAM17−/− and L(E) mutant mice was significantly elevated in comparison with the respective control mice. Neutrophil recruitment into the lung and BALF in ADAM17−/− mice was significantly reduced compared with the control group (Figure 6E-F).
Shedding of L-selectin is required for bacterial clearance in the tissue during K pneumoniae–induced pneumonia. (A-L) ADAM17+/+ and ADAM17−/− mice, as well as WT and L(E) mutant mice, were subjected to K pneumoniae intratracheal injection. Bacterial burden, as reflected by CFU in blood (A,G), lung (B,H), BALF (C,I), and spleen (D,J), as well as neutrophil recruitment into the alveoli (E,K) and lung tissue (F,L), were determined 24 hours after K pneumoniae injection. n = 7 mice per genotype. Data are mean ± standard error of the mean. *P = .05, **P = .01, ***P = .001, Student t test.
Shedding of L-selectin is required for bacterial clearance in the tissue during K pneumoniae–induced pneumonia. (A-L) ADAM17+/+ and ADAM17−/− mice, as well as WT and L(E) mutant mice, were subjected to K pneumoniae intratracheal injection. Bacterial burden, as reflected by CFU in blood (A,G), lung (B,H), BALF (C,I), and spleen (D,J), as well as neutrophil recruitment into the alveoli (E,K) and lung tissue (F,L), were determined 24 hours after K pneumoniae injection. n = 7 mice per genotype. Data are mean ± standard error of the mean. *P = .05, **P = .01, ***P = .001, Student t test.
However, in contrast to Sell−/− mice, the recruitment of neutrophils into the lung and BALF after infection with K pneumoniae was not affected in L(E) mutant mice (Figure 6K-L). These data clearly demonstrate an important role for L-selectin shedding from the neutrophil surface in the bacterial clearance via neutrophil effector functions.
IRhom2-dependent ADAM17 activation leads to L-selectin shedding and subsequently amplifies superoxide production
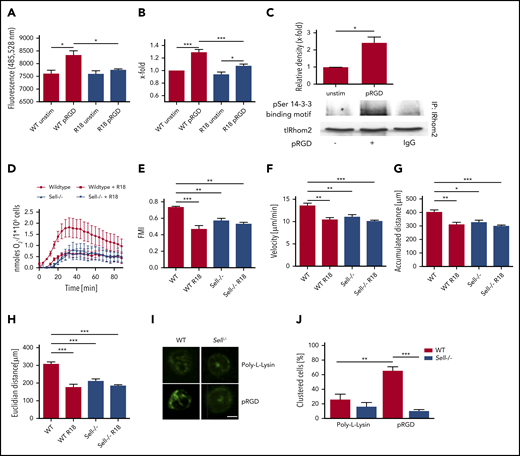
To investigate whether upregulated ADAM17 activity following integrin engagement is responsible for L-selectin shedding, we performed an ADAM17 activity assay (Figure 7A). Following pRGD stimulation of isolated neutrophils, ADAM17 activity was significantly increased in comparison with unstimulated neutrophils. This activation can be blocked by inhibiting IRhom2 activation with the inhibitor R18. As a consequence, the amount of L-selectin shed from the neutrophil surface in response to pRGD stimulation was significantly reduced after IRhom2 inhibition (Figure 7B). To confirm that IRhom2 is activated downstream of integrin ligation in neutrophils, we investigated IRhom2 phosphorylation in lysates of unstimulated and pRGD-stimulated neutrophils (Figure 7C). Immunoprecipitations for IRhom2 in lysates of unstimulated and pRGD-stimulated HL-60 cells were performed and subsequently immunoblotted with an anti–phospho-serine antibody specific for 14-3-3 ligand–binding motifs (Figure 7C). pRGD stimulation induced an increase in IRhom2 phosphorylation in comparison with unstimulated cells.
ADAM17 sheds L-selectin during integrin outside-in signaling in an IRhom2-dependent manner. ADAM17 activity (A) and levels of shed L-selectin (B) in unstimulated WT neutrophils or pRGD-stimulated (10 minutes) WT neutrophils that were left untreated or were pretreated with R18 inhibitor. (C) Immunoprecipitation of IRhom2 and Western blots of phosphorylated (p)-Ser 14-3-3 binding motif and total IRhom2 (tlRhom2) in lysates of HL-60 cells that were left unstimulated or were stimulated with pRGD (10 minutes). (D) Adhesion-dependent oxidative burst of WT and Sell−/− neutrophils, which were left untreated or were pretreated with R18 inhibitor, following plating on fibrinogen in the presence of TNF-α. (E-H) Chemotaxis of WT and Sell−/− neutrophils, which were left untreated or were pretreated with R18 inhibitor, on fibronectin in response to a soluble CXCL1 gradient in vitro. Forward migration index (E), velocity (F), and accumulated (G) and Euclidian (H) distance for chemotaxing neutrophils. A total of 40 cells was analyzed per experiment. n = 4. Mac-1 clustering on WT and Sell−/− neutrophils. Representative microscopy images (scale bar, 4 µm) (I) and percentage of cells (J) showing Mac-1 clusters on neutrophils that were left unstimulated or were stimulated with pRGD. A total of 30 cells was analyzed per experiment. n = 3. Data are mean ± standard error of the mean. *P = .05, **P = .01, ***P = .001, 1-way ANOVA.
ADAM17 sheds L-selectin during integrin outside-in signaling in an IRhom2-dependent manner. ADAM17 activity (A) and levels of shed L-selectin (B) in unstimulated WT neutrophils or pRGD-stimulated (10 minutes) WT neutrophils that were left untreated or were pretreated with R18 inhibitor. (C) Immunoprecipitation of IRhom2 and Western blots of phosphorylated (p)-Ser 14-3-3 binding motif and total IRhom2 (tlRhom2) in lysates of HL-60 cells that were left unstimulated or were stimulated with pRGD (10 minutes). (D) Adhesion-dependent oxidative burst of WT and Sell−/− neutrophils, which were left untreated or were pretreated with R18 inhibitor, following plating on fibrinogen in the presence of TNF-α. (E-H) Chemotaxis of WT and Sell−/− neutrophils, which were left untreated or were pretreated with R18 inhibitor, on fibronectin in response to a soluble CXCL1 gradient in vitro. Forward migration index (E), velocity (F), and accumulated (G) and Euclidian (H) distance for chemotaxing neutrophils. A total of 40 cells was analyzed per experiment. n = 4. Mac-1 clustering on WT and Sell−/− neutrophils. Representative microscopy images (scale bar, 4 µm) (I) and percentage of cells (J) showing Mac-1 clusters on neutrophils that were left unstimulated or were stimulated with pRGD. A total of 30 cells was analyzed per experiment. n = 3. Data are mean ± standard error of the mean. *P = .05, **P = .01, ***P = .001, 1-way ANOVA.
To further investigate the effect of IRhom2 inhibition on neutrophil effector functions, we determined the production of ROS by untreated and R18-pretreated WT and Sell−/− neutrophils plated on fibrinogen plus TNF-α (Figure 7D). R18 treatment of WT neutrophils reduced the production of ROS in comparison with control WT neutrophils. In Sell−/− neutrophils, R18 treatment had no effect on ROS production (Figure 7D).
Additionally, we investigated the effect of IRhom2 inhibition on neutrophil migration on fibronectin along a CXCL1 gradient (Figure 7E-H). Forward migration index (Figure 7E), migration velocity (Figure 7F), and accumulated (Figure 7G) and Euclidian (Figure 7H) distance of WT neutrophils pretreated with R18 were significantly reduced in comparison with untreated cells. R18 treatment did not have an additional effect on the migration behavior of Sell−/− neutrophils (Figure 7E-H).
To further investigate the mechanism by which L-selectin is involved in integrin outside-in signaling, we determined the amount of Mac-1 clustering on unstimulated and pRGD-stimulated (10 minutes) WT and Sell−/− neutrophils (Figure 7I-J). After pRGD stimulation, the vast majority of WT neutrophils exhibited profound Mac-1 clusters, whereas the percentage of Sell−/− neutrophils with clusters was markedly decreased.
Discussion
Antibacterial host defense crucially relies on immune cell recruitment and competent functionality. The recruitment of neutrophils in case of infection or tissue injury is a complex process that has to be tightly regulated. In this process, an adequate immune response is necessary, because an overwhelming leukocyte recruitment and activation lead to tissue injury, whereas a reduced immune response causes a dissemination of bacteria. Numerous signaling molecules are involved in different cellular processes during inflammation. L-selectin expressed on neutrophils is involved in integrin activation via inside-out signaling and leukocyte recruitment.9 In this study, we demonstrated that L-selectin has additional functions in combating bacteria during K pneumoniae–induced pneumonia. In this clinically relevant disease model, neutrophil recruitment is significantly reduced in Sell−/− and ADAM17−/− mice. In contrast, in L(E) mutant mice, in which L-selectin is mutated and cannot be shed from the neutrophil surface, neutrophil recruitment is not affected; however, the ability to combat bacterial burden in different organs is significantly impaired in these mice. This difference clearly demonstrates that shedding of L-selectin is required for neutrophil effector functions in vivo. ADAM17−/− mice exhibit reduced neutrophil recruitment into the lung in this model. ADAM17 is a metalloproteinase involved in the shedding of a number of cell surface proteins in addition to L-selection, such as TNF-α, which could influence the inflammatory phenotype of ADAM17−/− mice.
In several in vitro experiments we further demonstrated that integrin-mediated outside-in signaling–dependent processes, including neutrophil migration, production of ROS, and phagocytosis, are augmented by the shedding of L-selectin from the neutrophil surface via ADAM17 in murine and human cells. IRhom2 is involved in integrin-mediated outside-in signaling–induced ADAM17 activation.
The predominant integrins expressed on the neutrophil surface and important for neutrophil recruitment to sites of infection are the β2 integrins LFA-1 and Mac-1, as well as the β1 integrin VLA-4. Fibronectin is a ligand for a large variety of integrins; however, it was demonstrated that neutrophil migration on fibronectin is primarily mediated by β2 integrins.37
Cross-linking or engagement of L-selectin induces intracellular signaling, including phosphorylation of signaling molecules and integrin activation.9,38,39 With this study, we demonstrate for the first time that L-selectin–induced signaling is involved in integrin-mediated induction of ROS production. Blocking the intracellular part of L-selectin via Tat peptides reduced neutrophil ROS production to a level seen in Sell−/− neutrophils. These data indicate that intracellular signaling downstream of L-selectin activation is responsible for the L-selectin–dependent effect on integrin outside-in signaling. However, antibody blockade of PSGL-1 (4RA10) or L-selectin [Mel-14 F(ab)2] did not alter the amount of ROS produced by WT neutrophils, demonstrating that L-selectin engagement alone is not sufficient to induce integrin-mediated outside-in signaling.
Shedding of L-selectin was described as a mechanism to allow a detachment of leukocytes from the inflamed endothelium to enter into the tissue.40 Corresponding to this theory, a downregulation of surface L-selectin on neutrophils following transmigration in different in vivo models was described.41 In vitro, neutrophils downregulate surface L-selectin expression up to 80% following adhesion to or transmigration through endothelial cells.42 Although this was primarily described to be a physiological mechanism necessary for appropriate tissue infiltration, our findings indicate that L-selectin shedding from the neutrophil surface is also an important mechanism for amplifying integrin-dependent outside-in signaling and, via promoting Mac-1 clustering on the neutrophil surface, the activation of neutrophil effector functions. The induction of L-selectin shedding with hypotonic PBS results in an amplification of distinct intracellular signaling molecules that are activated downstream of integrin ligation. The reduced activation of downstream molecules in Sell−/− neutrophils demonstrates that the induced activity is L-selectin dependent and is not general cellular activation due to osmotic cell swelling. The concentration of soluble L-selectin, as well as the activity of the L-selectin sheddase ADAM17, is significantly increased following stimulation of neutrophils with integrin ligands, demonstrating a link between integrin ligation and ADAM17-dependent L-selectin cleavage. By using L(E) mutant mice, we were able to demonstrate that the observed phenotype of Sell−/− neutrophils in effector functions (ie, reduced phagocytosis of K pneumoniae and reduced ROS production) is due to the lack of L-selectin shedding downstream of integrin ligation during integrin outside-in signaling. By contrast, L-selectin deficiency did not affect integrin inside-out activation stimulated by chemokines (CXCL1 or fMLF, as determined by soluble ICAM-1 binding) (data not shown).
In a recent article by Liu et al, L-selectinN138G mutant mice were investigated.43 In these mice, substituting Gly for Asn138 in L-selectin changes L-selectin mechanochemistry causing longer catch-bond lifetimes of L-selectin. L-selectin shedding from the cell surface of neutrophils was significantly increased in LselectinN138G mice.43 Chemokine-dependent inside-out signaling was unaffected in the LselectinN138G mice; interestingly, however, the amount of PMA-induced ROS production, as well as the phagocytosis of bacteria by peripheral blood neutrophils, was highly increased. The focus of that study was an investigation of the effects of changes in L-selectin mechanochemistry; however, together with the results of our present study, the results reported by Liu et al strongly support our observations that L-selectin shedding is important for the activation of integrin-induced neutrophil effector functions.
In a recently published study, Cavadas et al demonstrated that IRhom2 plays an important role in ADAM17 activation in macrophages.19 Using the IRhom2 inhibitor R18, we were able to demonstrate that, downstream of integrin ligation, the activation of ADAM17, as well as L-selectin shedding, is IRhom2 dependent. Following blockade of IRhom2, ROS production and migration of neutrophils on fibronectin are reduced. Future studies will investigate the exact pathway by which integrin ligation leads to activation of IRhom2 in neutrophils. In a response to different types of macrophage stimulation (PMA, Toll-like receptors, and G protein-coupled receptors), MAPKs and 14-3-3 are involved in IRhom2 phosphorylation and dissociation.19 We were able to demonstrate that, downstream of integrin ligation, the 14-3-3–binding motif on IRhom2 is phosphorylated, pointing toward the same pathway for IRhom2-dependent ADAM17 activation in neutrophils. IRhom2 is also involved in ADAM17 maturation and transport to the plasma membrane.44,45 Thus, in IRhom2-deficient cells, inactive ADAM17 is retained in the endoplasmic reticulum, resulting in decreased L-selectin shedding. IRhom2 deficiency in mice ameliorates lung injury and neutrophil infiltration following intestinal ischemia-reperfusion injury,46 supporting our observation that L-selectin shedding augments neutrophil recruitment and effector functions during inflammation. The identification of a new role for ADAM17-dependent L-selectin shedding involving IRhom2 activation opens up possible new approaches for therapeutic interventions.
In addition to the well-known function of L-selectin shedding to facilitate leukocyte migration, our study identifies a novel function of L-selectin shedding in supporting integrin-dependent outside-in signaling processes, resulting in increased neutrophil effector functions in murine and human cells. Further insights into how these processes are mechanistically regulated may offer new approaches to treat different types of infections or inflammatory diseases. Although systemic administration of inhibitors or inducers of L-selectin shedding might pose a detrimental risk for serious adverse events, future research will focus on the potential of organ-specific targeting of these defense mechanisms.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Thomas F. Tedder for providing the L(E) mutant mice and Pia Lindental for expert technical assistance.
This work was supported by grants from the German Research Foundation (ZA428/5-2, SFB1009_A05 and INST 211/604-2) (A.Z.).
Authorship
Contribution: A.C., A. Margraf, K.T., D.A.M., and B.B. performed experiments and analyzed data; V.V.M. analyzed data; A.C., A. Margraf, A. Mellmann, and A.Z. interpreted data; A.C. wrote the manuscript; A. Margraf, C.A.L., and A.Z. contributed to the writing of the manuscript; A.C. and A.Z. designed the study; and A.Z. supervised the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alexander Zarbock, Department of Anesthesiology, Intensive Care and Pain Medicine, University of Muenster, Albert-Schweitzer-Campus 1, Building A1, 48149 Muenster, Germany; e-mail: zarbock@uni-muenster.de