Key Points
VRD was effective and well tolerated before ASCT; 33.4% complete response/28.8% minimal residual disease–negative after 6 induction cycles.
Responses deepened with VRD throughout induction and over the course of treatment with few discontinuations due to toxicity.
Abstract
Achieving and maintaining a high-quality response is the treatment goal for patients with newly diagnosed multiple myeloma (NDMM). The phase 3 PETHEMA/GEM2012 study, in 458 patients aged ≤65 years with NDMM, is evaluating bortezomib (subcutaneous) + lenalidomide + dexamethasone (VRD) for 6 cycles followed by autologous stem cell transplant (ASCT) conditioned with IV busulfan + melphalan vs melphalan and posttransplant consolidation with 2 cycles of VRD. We present grouped response analysis of induction, transplant, and consolidation. Responses deepened over time; in patients who initiated cycle 6 of induction (n = 426), the rates of a very good partial response or better were 55.6% by cycle 3, 63.8% by cycle 4, 68.3% by cycle 5, and 70.4% after induction. The complete response rate of 33.4% after induction in the intent-to-treat (ITT) population, which was similar in the 92 patients with high-risk cytogenetics (34.8%), also deepened with further treatment (44.1% after ASCT and 50.2% after consolidation). Rates of undetectable minimal residual disease (median 3 × 10−6 sensitivity) in the ITT population also increased from induction (28.8%) to transplant (42.1%) and consolidation (45.2%). The most common grade ≥3 treatment-emergent adverse events during induction were neutropenia (12.9%) and infection (9.2%). Grade ≥2 peripheral neuropathy (grouped term) during induction was 17.0%, with a low frequency of grade 3 (3.7%) and grade 4 (0.2%) events. VRD is an effective and well-tolerated regimen for induction in NDMM with deepening response throughout induction and over the course of treatment. This trial was registered at www.clinicaltrials.gov as #NCT01916252 and EudraCT as #2012-005683-10.
Introduction
Multiple myeloma (MM) remains an incurable disease. To help prolong progression-free survival (PFS) and overall survival, one goal of frontline treatment is to maximize depth of tumor reduction.1-4 This is often pursued with autologous stem cell transplant (ASCT), a standard of care for eligible patients. MM is the most frequent indication for ASCT in the United States and Europe.5,6 Maximizing response and achieving a very good partial response (VGPR) or better at the time of ASCT are associated with improved long-term outcomes2,3,7,8 ; depth of response, particularly undetectable minimal residual disease (MRD), is being explored as a surrogate for survival outcomes.9,10
Multiple studies have shown the results of different induction regimens. The 3-drug combination bortezomib + thalidomide + dexamethasone (VTD) had superior outcomes compared with the 2-drug thalidomide + dexamethasone and bortezomib + dexamethasone regimens for induction.11-13 Furthermore, a meta-analysis showed that bortezomib-based induction regimens have improved outcomes compared with those lacking the proteasome inhibitor.14 However, not all combinations are equivalent. For example, VTD achieved deeper responses than bortezomib + cyclophosphamide + dexamethasone. VTD also reduced grade 3/4 hematologic treatment-emergent adverse events compared with bortezomib + cyclophosphamide + dexamethasone but resulted in higher rates of grade 3/4 peripheral neuropathy.15-17 Other combinations, including bortezomib + doxorubicin + dexamethasone, did not achieve the depth of response seen with VTD.18
Although thalidomide and lenalidomide are both immunomodulatory agents, the use of thalidomide, even as a component of relatively short-duration induction therapy, is limited by the occurrence of peripheral neuropathy.19 Bortezomib use is similarly limited by the occurrence of peripheral neuropathy, and the combination with thalidomide further exacerbates the rate and severity of this treatment-emergent adverse event (TEAE).11,12 Therefore, lenalidomide has been explored in combination with bortezomib and dexamethasone. A dose-escalation study found that bortezomib + lenalidomide + dexamethasone (VRD) as induction followed by ASCT and VRD maintenance was effective, with favorable tolerability.20 Furthermore, VRD induction followed by ASCT and VRD consolidation followed by lenalidomide maintenance demonstrated high rates of response and increased depth of response over the course of treatment in the IFM2008 and IFM2009 studies.21,22 Subcutaneous administration of bortezomib has noninferior efficacy and an improved safety profile vs IV administration, making regimens with bortezomib more tolerable.23,24 VRD is now considered a potential standard of care in newly diagnosed MM25,26 and was recently approved in the European Union for transplant-ineligible patients.
Although VRD has been studied in multiple clinical trials, the schedule and dosing are not identical (supplemental Table 1, available on the Blood Web site).20-22,27-32 Since dose intensity can have an impact on the depth of response and there are no significant overlapping toxicities between bortezomib and lenalidomide, a VRD regimen using 25 mg lenalidomide for 21 days in 4-week cycles (instead of the 14 days in 3-week cycles used in the IFM2009 and SWOG S0777 trials) was selected to maximize induction response and obtain a greater long-term benefit posttransplant. This VRD regimen was tested in the Spanish Myeloma Group’s phase 3 trial. The PETHEMA/GEM2012 study investigates induction therapy with VRD for six 4-week cycles followed by ASCT with 2 different conditioning regimens, IV busulfan + melphalan vs melphalan, and posttransplant consolidation with 2 cycles of VRD in patients aged ≤65 years with newly diagnosed MM. The efficacy and safety results of VRD induction are presented and placed in context with GEM2005, the study which used VTD induction that was previously reported by this group.12
Methods
Study design and patients
This ongoing, open-label, randomized, phase 3 study was designed to compare 2 transplant conditioning regimens (IV busulfan + melphalan vs melphalan) in patients who received VRD induction and consolidation. The study includes patients with newly diagnosed, symptomatic MM based on standard criteria who had not received any prior treatment of MM and were aged 18 to 65 years and eligible for ASCT. Additional eligibility criteria included Eastern Cooperative Oncology Group performance status of ≤2 (or 3 if the status was due to myeloma), platelet count of ≥100 × 109/L, absolute neutrophil count of ≥1.0 × 109/L, corrected serum calcium of <14 mg/dL, aspartate transaminase and alanine transaminase of ≤2.5 times the upper limit of normal, total bilirubin within normal limits, and serum creatinine of ≤2 mg/dL. For secretory MM, measurable disease was defined by any quantifiable value of serum M-protein (immunoglobulin G [IgG] ≥10 g/L or IgA ≥5 g/L) and/or, when applicable, an excretion of light chain in urine of ≥200 mg per 24 hours. Measurable disease for oligo- or nonsecretory MM was defined by the presence of soft tissue (not bone) plasmacytomas, determined by clinical examination or imaging techniques. Patients with nonsecretory MM without measurable plasmacytomas were excluded, as were those with peripheral neuropathy of grade ≥2 in the 21 days prior to inclusion or known hypersensitivity to bortezomib, boric acid, mannitol, or lenalidomide.
Informed consent was required prior to patient participation. Each study site’s independent ethics committee reviewed and approved the protocol, amendments, and informed consent forms. The study was designed and conducted per the ethical principles of the Declaration of Helsinki and the International Council for Harmonization Guidelines. The study was registered at www.clinicaltrials.gov as #NCT01916252 and EudraCT as #2012-005683-10. PETHEMA sponsored the study and was responsible for the design, overall conduct, and analysis. Celgene assisted in data analysis and supported medical writing assistance for publication of this manuscript. All authors had access to the study data.
Treatment
All patients received induction with VRD, which consisted of bortezomib 1.3 mg/m2 (subcutaneous) on days 1, 4, 8, and 11 of each cycle; lenalidomide 25 mg/d on days 1 to 21; and dexamethasone 40 mg on days 1 to 4 and 9 to 12 at 4-week intervals for 6 cycles. Mobilization was performed after the third induction cycle in the absence of progression or unacceptable toxicity. The minimum number of CD34-positive cells was determined at the discretion of each site, although ≥2 × 106/kg was recommended. Patients next received conditioning with IV busulfan 9.6 mg/kg + melphalan 140 mg/m2 vs melphalan 200 mg/m2, as previously described,33 with the notable exception that busulfan 3.2 mg/kg was administered by a 3-hour infusion on days −5, −4, and −3 (total accumulated dose, 9.6 mg/kg) and consolidation with 2 additional cycles of VRD 3 months after ASCT. Thromboprophylaxis with low-molecular-weight heparin was mandatory. Antiviral prophylaxis was also required. Antibacterial prophylaxis was administered at each site’s discretion.
Assessments
Investigator assessment of response, based on International Myeloma Working Group (IMWG) criteria,34,35 was based on all eligible patients in the intent-to-treat (ITT) population and further assessed by an external independent response adjudication committee. Response assessments were performed at the start of each cycle during induction and consolidation, as well as after induction, after ASCT, and after consolidation. Samples for MRD assessment were collected regardless of response after induction, after ASCT, and after consolidation. MRD (median limit of detection, 3 × 10−6) was analyzed using next-generation flow following EuroFlow standard operation protocols as defined by the IMWG. Grading of adverse events was per National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). When noted, a grouped term was used for peripheral neuropathy, which included events labeled as peripheral neuropathy, neuralgia, polyneuropathy, or sensory loss. Similarly, a grouped term was used for venous thromboembolism, which included pulmonary embolism, deep vein thrombosis, and thrombophlebitis.
Statistical methods
A sample size of 460 was calculated for assessment of the primary objective (prolongation of PFS by 1 year with busulfan + melphalan vs the 31-month PFS expected for melphalan).33 As of the 31 March 2017 data cutoff for this analysis (median follow up of 24.2 months), the median PFS has not been reached in either arm. The current analysis focuses on a principal secondary end point, depth of response (complete response [CR] and MRD) throughout induction, ASCT, and consolidation, and evaluates safety during induction.
Results
Baseline demographics and patient disposition
Between 18 September 2013 and 16 November 2015, 458 eligible patients were enrolled at 69 sites in Spain (Table 1). The median age was 58 years, the M-protein type was most commonly IgG (59.6%), and 75.3% of patients had International Staging System stage I or II disease. Soft-tissue plasmacytomas were observed in 102 patients (22%). High-risk cytogenetics, defined as t(4;14), t(14;16), and/or del(17p) (p53 deletion), were reported in 20.1% of patients.
Baseline characteristics
| . | Total (N = 458) . |
|---|---|
| Median age (range), y | 58 (31-65) |
| Sex, n (%) | |
| Male | 240 (52.4) |
| Female | 218 (47.6) |
| ECOG performance status, n (%) | |
| 0 | 195 (42.6) |
| 1 | 182 (39.7) |
| 2 | 62 (13.5) |
| 3 | 16 (3.5) |
| Missing | 3 (0.7) |
| M-protein type, n (%) | |
| IgG | 273 (59.6) |
| IgA | 107 (23.4) |
| Light chain | 69 (15.1) |
| IgD | 3 (0.7) |
| Nonsecretory | 6 (1.3) |
| ISS stage, n (%) | |
| I | 179 (39.1) |
| II | 166 (36.2) |
| III | 107 (23.4) |
| Missing | 6 (1.3) |
| Creatinine clearance, n (%) | |
| <60 mL/min | 70 (15.3) |
| ≥60 mL/min | 370 (80.8) |
| Missing | 18 (3.9) |
| Lactate dehydrogenase elevated, n (%) | |
| Yes | 65 (14.2) |
| No | 376 (82.1) |
| Missing | 17 (3.7) |
| High-risk cytogenetics, n (%)* | 92 (20.1) |
| . | Total (N = 458) . |
|---|---|
| Median age (range), y | 58 (31-65) |
| Sex, n (%) | |
| Male | 240 (52.4) |
| Female | 218 (47.6) |
| ECOG performance status, n (%) | |
| 0 | 195 (42.6) |
| 1 | 182 (39.7) |
| 2 | 62 (13.5) |
| 3 | 16 (3.5) |
| Missing | 3 (0.7) |
| M-protein type, n (%) | |
| IgG | 273 (59.6) |
| IgA | 107 (23.4) |
| Light chain | 69 (15.1) |
| IgD | 3 (0.7) |
| Nonsecretory | 6 (1.3) |
| ISS stage, n (%) | |
| I | 179 (39.1) |
| II | 166 (36.2) |
| III | 107 (23.4) |
| Missing | 6 (1.3) |
| Creatinine clearance, n (%) | |
| <60 mL/min | 70 (15.3) |
| ≥60 mL/min | 370 (80.8) |
| Missing | 18 (3.9) |
| Lactate dehydrogenase elevated, n (%) | |
| Yes | 65 (14.2) |
| No | 376 (82.1) |
| Missing | 17 (3.7) |
| High-risk cytogenetics, n (%)* | 92 (20.1) |
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System.
High-risk cytogenetics defined as del(17p), t(4;14), and/or t(14;16).
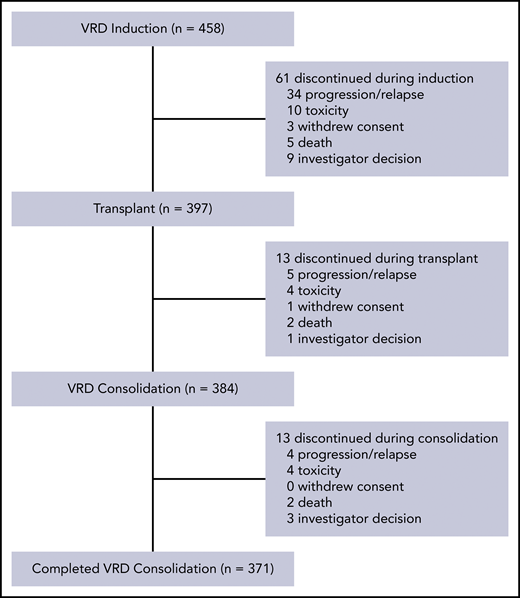
Stem cell mobilization was performed in 436 patients, with 86% (n = 373) of these patients requiring only 1 mobilization, 14% (n = 59) requiring 2, and 1% (n = 4) requiring 3. There were 2 stem cell mobilization failures (0.5%). Granulocyte colony-stimulating factor was used in 375 patients (86%), plerixafor in 53 patients (12%), and cyclophosphamide in 8 patients (2%). The median collected CD34-positive cell count was 4.66 × 106/kg.
Sixty-one patients (13.3%) discontinued during induction due to progressive disease (n = 34 [including 3 who did not have progressive disease per external adjudication]), toxicity (n = 10), investigator decision (n = 9), death (n = 5), or withdrawal of consent (n = 3). Among the 34 early progressors on VRD, 8 required bortezomib-dose reductions (6 to 1 mg/m2 and 2 to 0.7 mg/m2) and no patients discontinued bortezomib before progression was documented. Of note, 86.7% of patients who started induction went on to receive ASCT, and 81.0% completed all treatment phases (Figure 1).
Efficacy
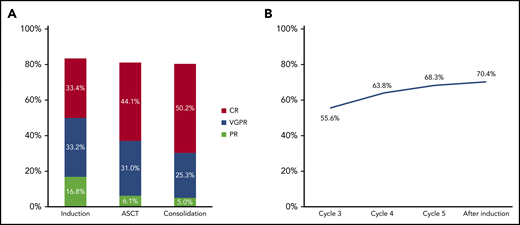
In the ITT population assessed by the external independent response adjudication committee, the CR rate at the end of induction therapy was 33.4% and the rate of VGPR or better was 66.6% (Table 2; Figure 2A). Progressive disease was reported in 6.8% of the patients at the end of induction. Analysis of response by investigator assessment indicated a stringent CR rate of 26.2%, with other response rates essentially similar to those of the external committee (supplemental Table 2). Similar rates were seen in patients with high-risk cytogenetics (CR rate, 34.8%; rate of VGPR or better, 70.7%). Progressive disease rates during induction were 20%, 13%, and 12% in patients with del(17p), t(4;14), and t(14;16), respectively. In patients with del(17p), 2, 3, and 3 patients experienced progression after cycle 2, after cycle 3, and beyond cycle 4, respectively.
Response rates after induction*
| . | VRD . | VTD . | ||
|---|---|---|---|---|
| GEM2012 . | GEM2005† . | |||
| . | All patients, n (%) . | High-risk cytogenetics, n (%) . | All patients, n (%) . | High-risk cytogenetics, n (%) . |
| (N = 458) . | (n = 92) . | (N = 130) . | (n = 23) . | |
| PR or better | 382 (83.4) | 75 (81.5) | 110 (84.6) | 18 (78.3) |
| CR | 153 (33.4) | 32 (34.8) | 46 (35.4) | 8 (34.8) |
| VGPR | 152 (33.2) | 33 (35.9) | 32 (24.6) | 5 (21.7) |
| PR | 77 (16.8) | 10 (10.9) | 32 (24.6) | 5 (21.7) |
| VGPR or better | 305 (66.6) | 65 (70.7) | 78 (60.0) | 13 (56.5) |
| Stable disease | 20 (4.4) | 0 | 7 (5.4) | 2 (8.7) |
| Progressive disease | 31 (6.8) | 14 (15.2) | 9 (6.9) | 1 (4.3) |
| Not evaluable | 25 (5.5) | 3 (3.3) | ||
| . | VRD . | VTD . | ||
|---|---|---|---|---|
| GEM2012 . | GEM2005† . | |||
| . | All patients, n (%) . | High-risk cytogenetics, n (%) . | All patients, n (%) . | High-risk cytogenetics, n (%) . |
| (N = 458) . | (n = 92) . | (N = 130) . | (n = 23) . | |
| PR or better | 382 (83.4) | 75 (81.5) | 110 (84.6) | 18 (78.3) |
| CR | 153 (33.4) | 32 (34.8) | 46 (35.4) | 8 (34.8) |
| VGPR | 152 (33.2) | 33 (35.9) | 32 (24.6) | 5 (21.7) |
| PR | 77 (16.8) | 10 (10.9) | 32 (24.6) | 5 (21.7) |
| VGPR or better | 305 (66.6) | 65 (70.7) | 78 (60.0) | 13 (56.5) |
| Stable disease | 20 (4.4) | 0 | 7 (5.4) | 2 (8.7) |
| Progressive disease | 31 (6.8) | 14 (15.2) | 9 (6.9) | 1 (4.3) |
| Not evaluable | 25 (5.5) | 3 (3.3) | ||
PR, partial response.
Efficacy data for GEM2012 was adjudicated by an independent response adjudication committee and IMWG criteria. The data for GEM2005 was based on investigator assessment with central review and European Society for Blood and Marrow Transplantation criteria with Uniform Response Criteria for VGPR categorization.
Data from Rosiñol at al.12
Response. (A) Response rates in the ITT population (N = 458). (B) Rates of VGPR or better throughout induction in the 426 patients who initiated cycle 6.
Response. (A) Response rates in the ITT population (N = 458). (B) Rates of VGPR or better throughout induction in the 426 patients who initiated cycle 6.
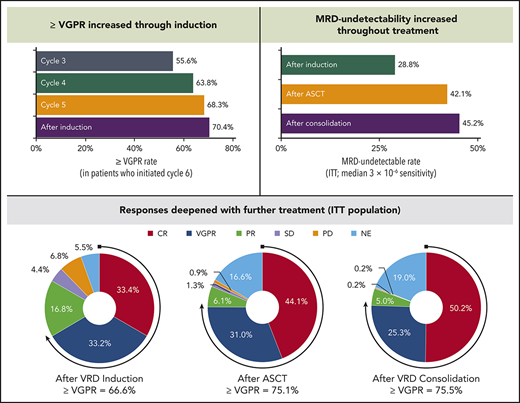
The depth of response increased by cycle during VRD induction (as measured by the rate of VGPR or better because a proportion of patients did not undergo bone marrow aspiration to confirm CR until the end of induction). In the 426 patients who initiated cycle 6, the rate of VGPR or better was 55.6% by cycle 3, 63.8% by cycle 4, 68.3% by cycle 5, and 70.4% after induction (Figure 2B). Responses also deepened across the treatment phases. In the ITT population, the CR rate increased from 33.4% after induction to 44.1% after ASCT and 50.2% after consolidation (Table 3).
Response rates after ASCT and consolidation based on independent response adjudication committee assessments in the ITT population (N = 458)
| . | After ASCT, n (%) . | After consolidation, n (%) . |
|---|---|---|
| PR or better | 372 (81.2) | 369 (80.6) |
| CR | 202 (44.1) | 230 (50.2) |
| VGPR | 142 (31.0) | 116 (25.3) |
| PR | 28 (6.1) | 23 (5.0) |
| VGPR or better | 344 (75.1) | 346 (75.5) |
| Stable disease | 6 (1.3) | 1 (0.2) |
| Progressive disease | 4 (0.9) | 1 (0.2) |
| Not evaluable | 76 (16.6) | 87 (19.0) |
| . | After ASCT, n (%) . | After consolidation, n (%) . |
|---|---|---|
| PR or better | 372 (81.2) | 369 (80.6) |
| CR | 202 (44.1) | 230 (50.2) |
| VGPR | 142 (31.0) | 116 (25.3) |
| PR | 28 (6.1) | 23 (5.0) |
| VGPR or better | 344 (75.1) | 346 (75.5) |
| Stable disease | 6 (1.3) | 1 (0.2) |
| Progressive disease | 4 (0.9) | 1 (0.2) |
| Not evaluable | 76 (16.6) | 87 (19.0) |
An analysis of MRD kinetics was conducted in the ITT population (which considered all patients without an assessment as MRD positive). In these patients, undetectable MRD at the median 3 × 10−6 threshold was 28.8% after induction, 42.1% after ASCT, and 45.2% after consolidation (Table 4).
MRD in the ITT population (N = 458)
| . | After induction . | After ASCT . | After consolidation . |
|---|---|---|---|
| Median 3 × 10−6sensitivity, n (%) | |||
| MRD undetectable | 132 (28.8) | 193 (42.1) | 207 (45.2) |
| MRD positive | 264 (57.6) | 167 (36.5) | 157 (34.3) |
| Missing* | 62 (13.5) | 98 (21.4) | 94 (20.5) |
| 10−4sensitivity, n (%) | |||
| MRD undetectable | 217 (47.4) | 287 (62.7) | 302 (65.9) |
| MRD positive | 179 (39.1) | 73 (15.9) | 62 (13.5) |
| Missing* | 62 (13.5) | 98 (21.4) | 94 (20.5) |
| . | After induction . | After ASCT . | After consolidation . |
|---|---|---|---|
| Median 3 × 10−6sensitivity, n (%) | |||
| MRD undetectable | 132 (28.8) | 193 (42.1) | 207 (45.2) |
| MRD positive | 264 (57.6) | 167 (36.5) | 157 (34.3) |
| Missing* | 62 (13.5) | 98 (21.4) | 94 (20.5) |
| 10−4sensitivity, n (%) | |||
| MRD undetectable | 217 (47.4) | 287 (62.7) | 302 (65.9) |
| MRD positive | 179 (39.1) | 73 (15.9) | 62 (13.5) |
| Missing* | 62 (13.5) | 98 (21.4) | 94 (20.5) |
The main cause of missing data was patient discontinuation (61 patients before ASCT, 13 patients before day 100 after ASCT, and 13 patients before the end of consolidation). Less frequent reasons were lack of appropriate sample at the central laboratory, test failure, and patient’s consent withdrawal.
Safety
Neutropenia was the most common hematologic TEAE during induction (Table 5). Peripheral neuropathy, a common side effect of bortezomib, was the most common nonhematologic TEAE during induction, although the frequency of grade ≥3 events was 3.9%. The frequency of grade ≥2 peripheral neuropathy (grouped term) during induction was 17.0%, with a low frequency of grade 3/4 events (13.1%, 3.7%, and 0.2% were grade 2, 3, and 4, respectively). The incidence of peripheral neuropathy was similar across induction cycles. In the 426 patients who initiated cycle 6, grade ≥2 peripheral neuropathy was reported in 1.4%, 2.3%, 4.5%, 5.6%, 4.0%, and 3.3% in cycles 1, 2, 3, 4, 5, and 6, respectively. The frequency of grade 3/4 venous thromboembolism (grouped term) was 1.3% in all patients. During induction, 14 patients (3.1%) had ≥1 TEAE leading to discontinuation (most commonly cardiac disorders [1.1%] and infections and infestations [0.9%]), and 9 patients (2.0%) died due to TEAEs.
Adverse events through induction
| . | Any grade . | Grade 3/4 . | |
|---|---|---|---|
| . | VRD GEM2012 (N = 458) . | VRD GEM2012 (n = 458) . | VTD GEM2005* (n = 130) . |
| Hematologic | |||
| Neutropenia | 146 (31.9)† | 59 (12.9) | 13 (10) |
| Thrombocytopenia | 116 (25.3) | 29 (6.3) | 10 (8) |
| Nonhematologic | |||
| Peripheral neuropathy | 174 (38.0)‡ | 18 (3.9)‡ | 17 (13) |
| Infection | 129 (28.2)† | 42 (9.2) | 27 (21) |
| Skin toxicity | 91 (19.9) | 14 (3.1) | |
| Pneumonia | 24 (5.2)† | 10 (2.2) | |
| . | Any grade . | Grade 3/4 . | |
|---|---|---|---|
| . | VRD GEM2012 (N = 458) . | VRD GEM2012 (n = 458) . | VTD GEM2005* (n = 130) . |
| Hematologic | |||
| Neutropenia | 146 (31.9)† | 59 (12.9) | 13 (10) |
| Thrombocytopenia | 116 (25.3) | 29 (6.3) | 10 (8) |
| Nonhematologic | |||
| Peripheral neuropathy | 174 (38.0)‡ | 18 (3.9)‡ | 17 (13) |
| Infection | 129 (28.2)† | 42 (9.2) | 27 (21) |
| Skin toxicity | 91 (19.9) | 14 (3.1) | |
| Pneumonia | 24 (5.2)† | 10 (2.2) | |
Data are n (%). Common Terminology Criteria for Adverse Events v4.03 was used for GEM2012, and v3.0 was used for GEM2012. Grade ≥3 adverse events were reported in ≥2% of patients in GEM2012.
Data from Rosiñol at al.12
Includes 1 patient who died due to neutropenia, 4 patients who died due to infection, and 2 patients who died due to pneumonia.
Grouped term; includes peripheral neuropathy, neuralgia, polyneuropathy, and sensory loss.
During induction, dose modifications (dose reductions and/or interruptions) were reported for bortezomib (n = 147 [32.1%]), lenalidomide (n = 121 [26.4%]), and dexamethasone (n = 52 [11.4%]). The modifications occurred most commonly in only 1 of the induction cycles and were most commonly due to nonhematologic toxicity for bortezomib and lenalidomide (supplemental Table 3).
Dose reductions in bortezomib and/or lenalidomide precipitated by ≥1 TEAE occurred in 99 patients (21.6%). Eighty-two (17.9%), 23 (5.0%), and 5 (1.1%) patients had ≥1 TEAE leading to a dose reduction in bortezomib, lenalidomide, and bortezomib and lenalidomide, respectively. Peripheral neuropathy was the most common TEAE leading to bortezomib dose reduction during induction, occurring in 67 patients (14.6%). Neutropenia and skin toxicity were the most common TEAEs during induction leading to lenalidomide dose reduction (n = 4 [0.9% each]).
Dose interruptions in bortezomib and/or lenalidomide precipitated by ≥1 TEAE occurred in 95 patients (20.7%). Thirty-one (6.8%), 52 (11.4%), and 31 (6.8%) patients had ≥1 TEAE leading to a dose interruption in bortezomib, lenalidomide, and bortezomib and lenalidomide, respectively. Peripheral neuropathy (n = 13[ 2.8%]) was the most common TEAE leading to bortezomib interruption, and skin toxicity (n = 18 [3.9%]) and infection (n = 15 [3.3%]) were the most common TEAEs during induction leading to lenalidomide interruption.
The most common hematologic TEAEs during consolidation were grade 3 neutropenia (10.2%) and thrombocytopenia (7.8%), while grade 4 events were 0.3% and 2.1%, respectively. Grade 3, 4, and 5 infections were observed in 2.3%, 0.3%, and 0.8% of patients, respectively. Peripheral neuropathy (grouped term) was 7.6% (all grades) and 0.3% (grade 3/4). During consolidation, dose modifications (dose reductions and/or interruptions) were reported for bortezomib (26.6%), lenalidomide (18.2%), and dexamethasone (6.8%).
Discussion
The results of the phase 3 PETHEMA/GEM2012 study show that six 28-day cycles of VRD using the full 25-mg dose of lenalidomide from days 1 to 21; subcutaneous bortezomib on days 1, 4, 8, and 11; and dexamethasone on days 1 to 4 and 9 to 12 is a highly effective pretransplant induction regimen, with a postinduction CR rate of 33.4% and undetectable MRD rates of 28.8% and 47.4% (median 3 × 10−6 and 10−4 thresholds, respectively). The regimen used is unique compared with other studies (supplemental Table 1). It uses 28-day cycles and includes lenalidomide for 21 of those days. Overall, the regimen has the highest lenalidomide and dexamethasone dose intensity per cycle and a lower bortezomib dose intensity per cycle than the 21-day regimens, which may offer high activity with low levels of toxicity, thereby enabling delivery of all planned induction cycles. In our previous GEM2005 trial using VTD, the CR rate after 3 cycles was 16% vs 35% after 6 cycles, and in the VTD arm of the Italian MMY-3006 trial, the CR rate after 3 induction cycles was 19%.11,12 Additionally, in the IFM2007-02 trial, the CR rate after 4 cycles of VTD using reduced doses of thalidomide and bortezomib was 13%.13 Thus, drug intensity and length of exposure can be critical to maximizing the induction response and the long-term benefit posttransplant. While cross-trial comparisons have limitations, the rate of VGPR or better after 3 cycles in our trial compares favorably with the rate in IFM2009 (55.6% vs 46%). In addition, although our study population (all transplant-eligible patients) is different, the rate of VGPR or better after 6 cycles in our trial was 66.6% vs 43% after the 8 induction cycles in SWOG S0777.30
The rate of VGPR or better and CR rates were also similar for the overall population and patients with high-risk cytogenetics, supporting the use of VRD induction regardless of cytogenetic risk. Of particular interest, this study assessed response throughout the 6 cycles of VRD induction and showed increasing rates of VGPR or better over all of the induction cycles, a likely reflection of the synergy between an immunomodulatory agent and a proteasome inhibitor. Furthermore, responses deepened throughout treatment as a whole, which was also seen with MRD measurements. Undetectable MRD at median 3 × 10−6 sensitivity increased from 28.8% after induction to 42.1% and 45.2% after ASCT and consolidation, respectively, in an ITT analysis. These data are supportive of other studies reporting undetectable MRD after induction, including a pattern of deepening response also seen in a phase 2 IFM study of VRD induction and consolidation.21,27 However, differences in methodology, sensitivity, and populations analyzed may limit MRD comparisons across trials.
The optimal number of induction cycles is a treatment aspect of particular interest. Although the rate of VGPR or better was lower after 3 cycles in IFM2009 vs GEM2012, the rates were similar after consolidation.22 Whether 3 induction cycles (plus 2 post-ASCT consolidation cycles) in IFM2009 are equivalent to the 6 induction cycles (plus 2 post-ASCT consolidation) in GEM2012 is not addressable, since different cycle lengths and/or dose intensities per cycle must be considered. A better comparison will need to await GEM2012 PFS maturity. While longer inductions could deepen response (thereby improving outcomes in a transplant-ineligible patient), it could also provide an option for transplant-eligible patients to potentially forego ASCT. Conversely, shorter inductions may result in fewer adverse events.
The broad VGPR or better category used in GEM2012 may also impact the ability to determine the optimal number of induction cycles based on depth of response. Thus, although the increase in the rate of VGPR or better after cycle 4 may seem incremental, the depth of those responses may be increasing in those patients who already achieved VGPR. Indeed, MRD would be a better measure, but the need for bone marrow limits the frequency at which this can be assessed.
In this study, the response improvement after 2 cycles of VRD consolidation is modest. It is interesting to note that the EMN02 trial showed a benefit of VRD consolidation after a suboptimal induction with 3 to 4 cycles of bortezomib + cyclophosphamide + dexamethasone, whereas STaMINA showed no benefit of consolidation after a variable but often long induction using VRD in 54% of the patients.36,37,38 This suggests that VRD consolidation may not offer additional benefit after a prolonged optimal induction with a lenalidomide-containing regimen.
Induction with VRD was feasible with no impact on stem cell mobilization. As per protocol, cells were collected only for a single transplant. The median collection was 4.66 × 106 CD34-positive cells/kg, and 86% of patients only required 1 mobilization. Additionally, 87% of patients went on to receive ASCT.
TEAEs, including grade ≥3 hematologic TEAEs, occurred at generally low rates during induction, which is of interest given that lenalidomide was administered on days 1 to 21 of the 28-day schedule. Dose reductions or interruptions, mostly due to nonhematological toxicity, were observed in approximately one-third and one-fourth of patients for bortezomib and lenalidomide, respectively. In >50% of the instances, the reductions were required in only 1 cycle. Of note, despite the dose of dexamethasone used, dose reduction/interruption was required in only 11% of patients. Although the general toxicity profile of VRD was similar to that of VTD reported in other studies, the rate of grade 3/4 peripheral neuropathy was lower with VRD.11,12,15,16 Furthermore, the discontinuation rate due to toxicity was lower with VRD in this study vs VTD in the GEM2005 study (2.2% vs 6.9%). These differences in toxicity may be influenced by the use of lenalidomide vs thalidomide, a 2-week break between courses of bortezomib here and in GEM2005 vs a 1-week break in other studies, and the subcutaneous use of bortezomib in this study, because the rate of peripheral neuropathy events in the VTD studies is similar to that in the VRD-alone arm of the IFM2009 study, when bortezomib was administered IV.22
VRD had a similar CR rate (33.4% vs 35%) and a trend toward a higher rate of VGPR or better (66.6% vs 60%) vs VTD in the GEM2005 study (Table 2).12 However, differences in study design and response assessment methodology limit cross-trial comparison. Since no randomized controlled trial of VRD vs VTD exists, an integrated analysis comparing GEM2012 and GEM2005 was recently conducted.39 The analysis used patient-level data and propensity-score–matched pairing to control for baseline characteristics, thus enabling comparisons across trials. Response was assessed in patients with nonmissing baseline characteristics via an independent response adjudication committee using IMWG criteria. The results showed that VRD induction had a statistically significant and clinically relevant improvement in the rate of VGPR or better vs VTD (66.3% vs 51.2%; odds ratio, 1.87; 95% confidence interval, 1.23-2.83; P = .00281) and a higher posttransplant rate of VGPR or better (74.4% vs 53.5%). Undetectable MRD at the 10−4 threshold was 46.7% vs 34.9% after induction and 62.4% vs 47.3% after ASCT for VRD vs VTD. These results suggest that VRD may maximize the value of initial therapy and ASCT vs VTD. Coupled with the peripheral neuropathy comparison in the integrated analysis population, which remained less frequent with VRD vs VTD (grade ≥2, 20.7% vs 44.6%; grade 3/4, 5.5% vs 15.4%), VRD has a favorable benefit-risk profile over VTD.39 As noted previously, however, IV vs subcutaneous administration of bortezomib may account for some, but perhaps not all, differences in the incidence of peripheral neuropathy between the 2 trials.
Combinations of immunomodulatory agents and proteasome inhibitors for induction remain an active area of investigation. Studies have shown that the activity of the lenalidomide and proteasome inhibitor combination is not limited to bortezomib, because lenalidomide + carfilzomib + dexamethasone has shown rates of VGPR or better of 70% to 78% and undetectable MRD (10−5 threshold) of 53% after induction.40-42 However, these results must be interpreted in light of the differences in trial design, dose, schedule, and patient population. There is also interest in building on the VRD regimen by adding agents with novel mechanisms of action. For example, monoclonal antibodies targeting CD38 and SLAMF7 have yielded encouraging early clinical results.43-45 Ongoing trials, such as the PERSEUS EMN study of VRD vs VRD + daratumumab (NCT03710603), will help elucidate the opportunity for VRD as part of quadruplet regimens.
VRD was recently approved in the European Union for transplant-ineligible patients. This approval did not extend to the transplant-eligible setting, which may impact its use for this patient population in the European Union.
Overall, the results presented here demonstrate that responses deepened with VRD over the 6-cycle course of induction and the treatment program, with few patients discontinuing due to toxicity. These results confirm that VRD is an effective pretransplant induction regimen and may be considered a new standard of care.
Presented in abstract form at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9 December 2017, and the 44th annual meeting of the European Society for Blood and Marrow Transplantation, Lisbon, Portugal, 20 March 2018.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Peter J. Simon for medical writing assistance, which was sponsored by Celgene Corporation.
The study was supported by unrestricted grants to PETHEMA by Celgene, Janssen, and Pierre Fabré and Spanish grants from Instituto de Salud Carlos III PI2012/0093. Lenalidomide, bortezomib, and busulfan were supplied by the above companies with no charge. The authors are fully responsible for all content and editorial decisions for this manuscript.
Authorship
Contribution: All authors contributed to the concept and design of the work, acquisition, analysis, or interpretation of data for the work; contributed to the drafting of the work; revised the manuscript critically for important intellectual content; approved the final version to be published; and agree to be accountable for all aspects of the work.
Conflict-of-interest disclosure: L.R. reports honoraria from Janssen, Celgene, Amgen, and Takeda. A.O. reports consultancy for and membership on board of directors, advisory committees, or speakers bureaus with Amgen, Celgene, Takeda, and Janssen. R.R. reports consultancy with Amgen, Celgene, Janssen, and Takeda. A.S. reports honoraria from Bristol-Myers Squibb, Takeda, Sanofi, Merck, and Roche; consultancy with BMS, Takeda, and Merck; and membership on speakers bureaus with Takeda. J. M. Moraleda reports membership on advisory committees with Gilead, Celgene, and Takeda. I.J. reports honoraria from Janssen, Celgene, Roche, Novartis, Amgen, and Takeda. F.d.A. reports honoraria from and consultancy with Celgene, Janssen, and Amgen. J. M. Martí reports membership on speakers bureau with Celgene and reimbursement of travel, accommodations, and/or expenses by Janssen and Celgene. J.M.A. reports honoraria from Celgene, Amgen, Janssen, Novartis, Takeda, and Roche. L.F.C. reports honoraria for lectures from and membership on advisory boards with Celgene, Janssen, Roche, Novartis, Bristol-Myers Squibb, Amgen, Takeda, Pfizer, Incyte, and AbbVie. B.P. reports honoraria for lectures from and membership on advisory boards with Amgen, Bristol-Myers Squibb, Celgene, Janssen, Merck, Novartis, Roche, and Sanofi; unrestricted grants from Celgene, EngMab, Sanofi, and Takeda; and consultancy for Celgene, Janssen, and Sanofi. M.-V.M. reports consultancy for, honoraria from, and membership on board of directors or advisory committees with Janssen, Celgene, GSK, Takeda, and Amgen. J.F.S.M. reports consultancy for Bristol-Myers Squibb, Celgene, Novartis, Takeda, Amgen, MSD, Janssen, and Sanofi and membership on board of directors or advisory committees with Takeda. J.-J.L. reports honoraria from and membership on board of directors or advisory committees with Takeda, Amgen, Celgene, and Janssen. J. Bladé reports honoraria from Celgene, Amgen, and Janssen. The remaining authors declare no competing financial interests.
Correspondence: Joan Bladé, Hospital Clínic de Barcelona, Hematology Department, Villarroel 170, 08036 Barcelona, Spain; e-mail: jblade@clinic.cat.