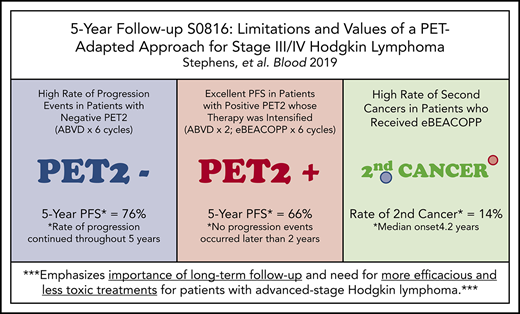
Key Points
Nearly 25% of PET2− patients relapsed, demonstrating limitations of frontline ABVD and low negative predictive value of PET2.
In patients with a positive PET2 who received eBEACOPP, PFS was favorable, but was associated with a high rate of second cancers.
Abstract
Patients with advanced-stage Hodgkin lymphoma (HL) demonstrated excellent 2-year progression-free survival (PFS) after receiving positron emission tomography (PET)–adapted therapy on SWOG S0816. Patients received 2 cycles of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD). Patients achieving complete response (CR) on PET scan following cycle 2 of ABVD (PET2) continued 4 additional cycles of ABVD. Patients not achieving CR on PET2 were switched to escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (eBEACOPP) for 6 cycles. After a median follow-up of 5.9 years, a subset of 331 eligible patients with central review of PET2 was analyzed. PET2 was negative in 82% and positive in 18%. For all patients, the estimated 5-year PFS and OS was 74% (95% confidence interval [CI], 69%-79%) and 94% (95% CI, 91%-96%), respectively. For PET2− and PET2+ patients, the 5-year PFS was 76% (95% CI, 70%-81%) and 66% (95% CI, 52%-76%), respectively. Seven (14%) and 6 (2%) patients reported second cancers after treatment with eBEACOPP and ABVD, respectively (P = .001). Long-term OS of HL patients treated on S0816 remains high. Nearly 25% of PET2− patients experienced relapse events, demonstrating limitations ABVD therapy and of the negative predictive value of PET2. In PET2+ patients who received eBEACOPP, PFS was favorable, but was associated with a high rate of second malignancies compared with historical controls. Our results emphasize the importance of long-term follow-up, and the need for more efficacious and less toxic therapeutic approaches for advanced-stage HL patients. This trial was registered at www.clinicaltrials.gov as #NCT00822120.
Introduction
For patients with newly diagnosed Ann Arbor stage III/IV (advanced-stage) Hodgkin lymphoma (HL), ∼70% can expect to be cured after treatment with doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD), which has been the preferred standard of care in the United States for many years.1,2 An alternate treatment regimen for advanced-stage HL, escalated bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, and prednisone (eBEACOPP), has demonstrated a higher cure rate (>80%) with a tradeoff of increased short- and long-term toxicities including second cancers and infertility.3,-5 The desire to maximize efficacy and minimize toxicity has prompted concerted efforts to better identify patients at highest risk for shorter survival.
A key prognostic factor is interim metabolic response based on positron emission tomography (PET) scan as assessed by Deauville score following cycle 2 of ABVD (PET2).6 Failing to achieve complete response (CR) at this time point has been associated with substantially shorter progression-free survival (PFS).7,-9 As such, many groups have developed response-adapted therapy where response on the PET2 scan determines whether to reduce, maintain, or increase the intensity of therapy on an individual basis. Notable PET2-adapted therapy regimens for advanced-stage HL patients include SWOG S0816, the Risk-Adapted Therapy of Hodgkin Lymphoma (RATHL) study, and the Gruppo Italiano Terapie Innovative nei Linfomi (GITIL) 0607 study.10,-12
The first published response-adapted therapy was S0816, which is a collaborative effort between the US SWOG, Eastern Cooperative Oncology Group (ECOG), Cancer and Leukemia Group B (CALGB)/Alliance for Clinical Trials in Oncology (Alliance), and AIDS Malignancy Consortium.10 In this study, patients with newly diagnosed advanced-stage HL received 2 cycles of ABVD. Patients achieving CR on PET2 (Deauville score ≤3) continued 4 additional cycles of ABVD. Those patients who were unable to achieve CR on PET2 (Deauville score >3) were switched to eBEACOPP therapy for 6 cycles. The primary analysis of S0816, published after a median of 3.3 years of follow-up, achieved the primary end point by demonstrating a 2-year PFS of 79% for all patients treated on the study.10 In patients receiving eBEACOPP, the survival advantage came with a tradeoff of increased short-term toxicity.10 Herein, we report the long-term outcomes in patients who received response-adapted therapy on S0816, now with over 5 years of follow-up.
Methods
Patients/treatment
Eligible patients aged 18 to 60 years with advanced-stage HL were enrolled in S0816 as previously described.10 Investigators at each institution obtained informed consent from each participant. Patients were treated with PET-adapted therapy as described in the Introduction and as previously published.10 No radiotherapy was planned. Extended follow-up evaluations were completed at days 276 and 365, every 6 months for years 2 to 5, and then annually until year 7. Follow-up evaluations recorded events of death, relapse, new primary cancer, new nonprotocol cancer therapy, and new severe (grade ≥3) toxicity related to treatment. The study was approved by local institutional review boards.
Imaging
PET scans were performed at baseline, after 2 cycles of ABVD (PET2), and 6 to 8 weeks after the end of protocol treatment. Each PET scan was electronically transmitted to the CALGB Imaging Core Laboratory for centralized review and assignment of a Deauville score as described previously.10 PET scans with a score ≤3 were deemed PET−. PET scans with a score of >3 were deemed PET+. PET2 scans were reviewed in real time, and patients underwent a second registration after receipt of results. Disease response was performed according to Cheson criteria.13
Statistical analysis
The original design of S0816 had 2 primary end points: to estimate the 2-year PFS rate in the patients treated with PET response-adapted therapy and to estimate the 2-year PFS rate in the PET2+ patients subsequently treated with eBEACOPP. As such, 278 eligible HIV− patients and 60 patients with a positive PET2 scan were required to estimate the 2-year PFS rates of these groups to within 6% and 13%, respectively. The original publication reported that the study met its primary end points by demonstrating excellent 2-year PFS in all patients with notable improvement in the PET2+ subset of patients when compared with historical controls.10 The primary analysis of the current long-term follow-up was to estimate the 5-year PFS rates of patients with a negative and positive PET2. PFS was measured from the date of registration to the first observation of progressive disease, relapse, or death. Patients last known to be alive and progression-free were censored at the date of last contact. Overall survival (OS) was measured from the date of registration to the date of death. Patients last known to be alive were censored at the date of last contact. PFS and OS estimates were calculated using the Kaplan-Meier method. The 2-sided Fisher's exact test was used to compare: (1) the baseline patient characteristics of patients who had a negative PET2 scan between those patients who relapsed and those who did not relapse; (2) the rate of second cancer between patients switched to treatment eBEACOPP and those treated with continued ABVD after 2 initial cycles of ABVD; and (3) the baseline characteristics of patients who received eBEACOPP between those patients who developed a second cancer and those who did not develop a second cancer. The Wilcoxon score test was used to compare age and time to second cancer between groups. Data as of 20 June 2018 were included in this analysis.
Results
Patient characteristics
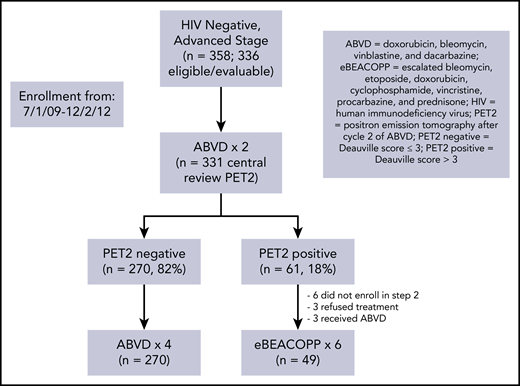
From July 2009 to December 2012, 371 patients with advanced-stage HL were enrolled in S0816. Of this group, 336 eligible, evaluable, and HIV− patients are analyzed herein. Central review of the PET2 scan was performed in 331 patients of this subset (Figure 1). The patients had a median age of 31 years (range, 18-60 years). Eighteen percent of the patients had bulky disease (>10 cm) and 51% had an international prognostic score (IPS) of ≥3 (Table 1).
Baseline patient characteristics
| Characteristic . | All patients, n = 336 . |
|---|---|
| Age, median (range), y | 31 (18-60) |
| Male sex, % | 56 |
| White, % | 82 |
| Stage, % | |
| III | 52 |
| IV | 48 |
| B symptoms, % | 62 |
| Bulk > 10 cm, % | 18 |
| IPS, % | |
| 0-2 | 49 |
| 3-7 | 51 |
| Characteristic . | All patients, n = 336 . |
|---|---|
| Age, median (range), y | 31 (18-60) |
| Male sex, % | 56 |
| White, % | 82 |
| Stage, % | |
| III | 52 |
| IV | 48 |
| B symptoms, % | 62 |
| Bulk > 10 cm, % | 18 |
| IPS, % | |
| 0-2 | 49 |
| 3-7 | 51 |
Imaging and treatment
Of the 331 patients with central review of PET2, 270 (82%) and 61 (18%) had negative and positive scans, respectively (Figure 1; supplemental Appendix, available on the Blood Web site). All 270 PET2− patients continued therapy with 4 additional cycles of ABVD. Of the 61 patients with a positive PET2, 49 patients (80%) switched to therapy with eBEACOPP. Six patients did not register for part 2 of the study, 3 patients chose to continue ABVD therapy, and 3 patients refused any further protocol-related therapy.
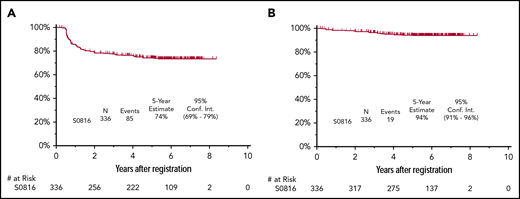
PFS
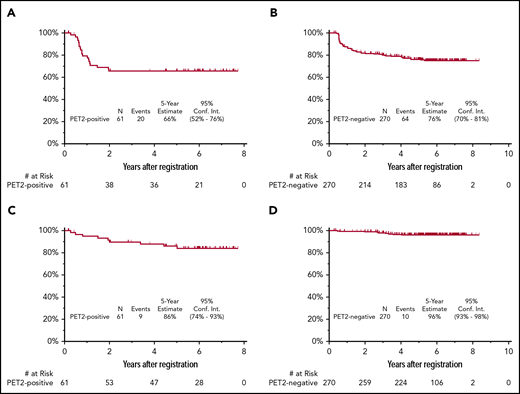
The median follow-up for patients treated on S0816 is now 5.9 years (range, 0.2-8.3 years). Among all patients, 85 patients (26%) have either progressed or died, for an estimated 5-year PFS of 74% (95% confidence interval [CI], 69%-79%; Figure 2A). Thirty-three percent of patients who had a positive PET2 (20 of 61) experienced a progression event for a 5-year PFS of 66% (95% CI, 52%-76%; Figure 3A). The estimated 5-year PFS for patients with a positive PET2 who received eBEACOPP per protocol (n = 49) was similar to the total group of 61 patients who had a positive PET2 (63% vs 66%; see supplemental Figure 1A). Twenty-four percent of patients who had a negative PET2 (64 of 270) experienced a progression event for a 5-year PFS of 76% (95% CI, 70%-81%; Figure 3B). One event occurred in a patient without central review of PET2. The baseline characteristics of patients with a negative PET2 categorized by progression status are detailed in Table 2. When the baseline characteristics seen in patients with negative PET2 who relapsed vs those who did not relapse were compared, there was no statistical difference in the characteristics of age, sex, stage, presence of B symptoms, bulk of disease, IPS, or PET2 Deauville score 1 to 2 vs 3. Accordingly, the estimated rates of 5-year PFS were similar in patients with a PET2 Deauville score of 1 to 2 vs 3 (see supplemental Figure 2). Patients who registered their race as nonwhite and had a negative PET2 scan were more likely to have progression of HL (17 of 47 = 36%) than those patients who registered their race as white and had a negative PET2 scan (47 of 223 = 21%; 2-sided Fisher's exact P = .04). In the patients with a negative PET2, there were no differences in baseline characteristics of the white vs nonwhite patients. Among the 85 patients with a progression event, 15 patients (18%) experienced the event ≥2 years after therapy. When the baseline characteristics of patients with late (≥2 years) vs early (<2 years) progression events were compared, there was no statistical difference in the characteristics of age, sex, stage, presence of B symptoms, bulk of disease, and IPS. All patients with late progression and 71% of patients with early progression had a negative PET2 (2-sided Fisher's exact P = .017). A more detailed description of the timing of progression events by PET2 status can be found in Table 3.
Survival of 336 HIV−patients on SWOG S0816. PFS (A) and OS (B). Conf. Int., confidence interval.
Survival of 336 HIV−patients on SWOG S0816. PFS (A) and OS (B). Conf. Int., confidence interval.
PFS and OS of 331 HIV−patients treated on SWOG S0816. (A) PFS for patients with PET2+; (B) PFS for patients with PET2−; (C) OS for patients with PET2+; and (D) OS for patients with PET2−.
PFS and OS of 331 HIV−patients treated on SWOG S0816. (A) PFS for patients with PET2+; (B) PFS for patients with PET2−; (C) OS for patients with PET2+; and (D) OS for patients with PET2−.
Baseline patient characteristics of patients with a negative PET2 scan by progression status
| . | Nonprogressor, n = 206 . | Progressor, n = 64 . | 2-sided Fisher's exact P . |
|---|---|---|---|
| Age, median (range), y | 31.8 (18.1-60.6) | 32.25 (19.1-60.8) | .52* |
| Sex, n (%) | .32 | ||
| Male | 118 (78.7) | 32 (21.3) | |
| Female | 88 (73.3) | 32 (26.7) | |
| Race, n (%) | .04 | ||
| White | 176 (78.9) | 47 (21.1) | |
| Other | 30 (63.8) | 17 (36.2) | |
| Stage, n (%) | .06 | ||
| III | 116 (81.1) | 27 (18.9) | |
| IV | 90 (70.8) | 37 (29.1) | |
| B symptoms, n (%) | 123 (77.4) | 36 (22.6) | .66 |
| Bulk >10 cm, n (%) | 36 (76.6) | 11 (23.4) | 1.00 |
| IPS, n (%) | .15 | ||
| 0-2 | 119 (79.9) | 30 (20.1) | |
| 3-7 | 87 (71.9) | 34 (28.1) | |
| PET2 Deauville score, n (%) | .19 | ||
| 1-2 | 127 (79.4) | 33 (20.6) | |
| 3 | 79 (71.8) | 31 (28.2) |
| . | Nonprogressor, n = 206 . | Progressor, n = 64 . | 2-sided Fisher's exact P . |
|---|---|---|---|
| Age, median (range), y | 31.8 (18.1-60.6) | 32.25 (19.1-60.8) | .52* |
| Sex, n (%) | .32 | ||
| Male | 118 (78.7) | 32 (21.3) | |
| Female | 88 (73.3) | 32 (26.7) | |
| Race, n (%) | .04 | ||
| White | 176 (78.9) | 47 (21.1) | |
| Other | 30 (63.8) | 17 (36.2) | |
| Stage, n (%) | .06 | ||
| III | 116 (81.1) | 27 (18.9) | |
| IV | 90 (70.8) | 37 (29.1) | |
| B symptoms, n (%) | 123 (77.4) | 36 (22.6) | .66 |
| Bulk >10 cm, n (%) | 36 (76.6) | 11 (23.4) | 1.00 |
| IPS, n (%) | .15 | ||
| 0-2 | 119 (79.9) | 30 (20.1) | |
| 3-7 | 87 (71.9) | 34 (28.1) | |
| PET2 Deauville score, n (%) | .19 | ||
| 1-2 | 127 (79.4) | 33 (20.6) | |
| 3 | 79 (71.8) | 31 (28.2) |
Two-sided Wilcoxon score test.
OS
Nineteen patients have died for an estimated 5-year OS of 94% (95% CI, 91%-96%; Figure 2B). Fifteen percent of patients who had a positive PET2 (9 of 61) died for a 5-year OS of 86% (95% CI, 74%-93%; Figure 3C). The estimated 5-year OS for patients with a positive PET2 who received eBEACOPP per protocol (n = 49) was similar to the total group of 61 patients who had a positive PET2 (85% vs 86%; see supplemental Figure 1B). Four percent of patients who had a negative PET2 (10 of 270) died for a 5-year PFS of 96% (95% CI, 93%-98%; Figure 3D). HL was the most common cause of death with 3% of patients (11 of 331) dying of their disease during follow-up. Of these 11 patients, 6 (6 of 270 = 2%) had a negative PET2 and 5 (5 of 61 = 8%) had a positive PET2. Other causes of death included: treatment-related toxicity (sepsis [eBEACOPP, n = 1], bleomycin lung injury [eBEACOPP, n = 1; ABVD, n = 1], and graft-versus-host disease [ABVD n = 1]); second primary cancers (eBEACOPP, n = 2; cervical cancer and non-Hodgkin lymphoma [NHL]); and unknown causes (ABVD, n = 2).
Posttherapy adverse events
Posttherapy grade 3 adverse events were uncommon but included 1 case each of heart failure, peripheral neuropathy, prolonged neutropenia, diarrhea, deep venous thrombosis (catheter-related) in patients who received continued ABVD, and osteonecrosis of hips/shoulders in patients who received eBEACOPP.
Second malignancies
There were 13 cases of reported second cancers, including 7 (7 of 49 = 14%) in patients treated with eBEACOPP (1 each of myelodysplastic syndrome [MDS], kidney, melanoma, NHL, cervical, medullary thyroid, and basal cell carcinoma) and 6 (6 of 270 = 2%) in patients treated with ABVD (1 each of kidney, melanoma, NHL, bladder, prostate, and squamous cell carcinoma; 2-sided Fisher's exact P = .001). There were no statistical differences in the baseline characteristics of the patients who received eBEACOPP and developed a second cancer and those who did not develop a second cancer, including the variables of age, sex, race, stage, B symptoms, bulky disease, and IPS. The patient who developed MDS had a relapse of HL following eBEACOPP therapy and was subsequently treated with 1 cycle of ifosfamide, carboplatin, and etoposide therapy and 2 cycles of gemcitabine. The patient did not receive stem cell transplantation (SCT) and developed MDS 4 years after the completion of all HL treatments. None of the other patients who developed second cancers had additional therapy (including SCT) for HL prior to development of second cancer. Only 2 patients received radiation therapy off-protocol and neither patient developed a second cancer. The median time to the development of second cancer is 2.9 years (range, 0.8-5.2 years) in patients treated with eBEACOPP and 4.2 years (range, 0.6-5.2 years) in patients treated with ABVD (2-sided Wilcoxon score test P = .72). Additional details regarding second cancers can be found in Table 4.
Second primary cancers reported by patients treated on S0816
| S0816 therapy received . | Type of second cancer . | Salvage therapy for HL prior to second cancer . | SCT prior to second cancer . | Time from S0816 treatment to second cancer, y . |
|---|---|---|---|---|
| eBEACOPP | Myelodysplastic syndrome | Y | N | 5.1 |
| eBEACOPP | Kidney | N | N | 2.9 |
| eBEACOPP | Melanoma | N | N | 5.0 |
| eBEACOPP | Non-HL | N | N | 1.1 |
| eBEACOPP | Cervical | N | N | 0.8 |
| eBEACOPP | Medullary thyroid | N | N | 5.2 |
| eBEACOPP | Basal cell carcinoma | N | N | 2.9 |
| ABVD | Kidney | N | N | 0.6 |
| ABVD | Melanoma | N | N | 3.9 |
| ABVD | Non-HL | N | N | 0.6 |
| ABVD | Bladder | N | N | 4.8 |
| ABVD | Prostate | N | N | 4.4 |
| ABVD | Squamous cell carcinoma | N | N | 5.2 |
| S0816 therapy received . | Type of second cancer . | Salvage therapy for HL prior to second cancer . | SCT prior to second cancer . | Time from S0816 treatment to second cancer, y . |
|---|---|---|---|---|
| eBEACOPP | Myelodysplastic syndrome | Y | N | 5.1 |
| eBEACOPP | Kidney | N | N | 2.9 |
| eBEACOPP | Melanoma | N | N | 5.0 |
| eBEACOPP | Non-HL | N | N | 1.1 |
| eBEACOPP | Cervical | N | N | 0.8 |
| eBEACOPP | Medullary thyroid | N | N | 5.2 |
| eBEACOPP | Basal cell carcinoma | N | N | 2.9 |
| ABVD | Kidney | N | N | 0.6 |
| ABVD | Melanoma | N | N | 3.9 |
| ABVD | Non-HL | N | N | 0.6 |
| ABVD | Bladder | N | N | 4.8 |
| ABVD | Prostate | N | N | 4.4 |
| ABVD | Squamous cell carcinoma | N | N | 5.2 |
N, no; Y, yes.
Discussion
In S0816, PET response-adapted therapy led to an impressive 5-year OS rate of 94%. Although the OS rate remains high, the 5-year PFS rate for all patients has dropped to 74% despite an interim PET-adapted strategy. Of note, nearly one-quarter of the patients with a negative PET2 experienced progression events during this longer-term follow-up period with a drop in estimated PFS from 82% after 2 years to 76% after 5 years.10 In this subset, a negative PET2 was ineffective at fully identifying those patients who would remain free of progression. This demonstrates a low negative predictive value of PET2, limitation in ABVD induction therapy, and the importance of long-term follow-up for this patient group to detect late relapses.
In our analysis, baseline clinical characteristics could not accurately predict which patients would experience relapse events following a negative PET2 scan. The only baseline characteristic associated with increased risk of relapse events after a negative PET2 was nonwhite race. Although this factor may be falsely inflated secondary to the small number patients of a nonwhite race vs the number of patients of white race treated on the study (48 patients vs 223 patients), disparities based on race are an area of interest in HL. Several groups have noted that racial minorities have worse outcomes.14,-16 These studies have postulated that access to medical care, lifestyle choices, or underlying biologic factors may contribute to these disparities. Our study was not designed to further investigate these factors, although access to care was presumably similar for all patients on the study. Other than nonwhite status, we found no difference in clinical pretreatment characteristics, including IPS, age, and sex, between PET2− patients who experienced relapse events vs those who did not experience relapse events.
Predicting relapse at the time of diagnosis remains a very difficult task, and there are currently no validated biologic or clinical tools to apply at an individual level. Attempts to identify novel molecular biomarkers at diagnosis have been made. A 26-gene expression-based model was developed but was not validated when used on patient samples from this trial.17,18 Alternative methods including evaluation of circulating tumor DNA or quantification of HL-infiltrating macrophages or serum soluble chemokines/cytokines produced by HL cells or the tumor microenvironment are promising but have not yet been validated for routine practice.19,-21
As standard assessment of PET2− status as determined by Deauville criteria missed 24% of patients who experienced progression events on S0816, other types of metabolic imaging and analysis may be more prognostic and should be evaluated. The current approach uses standardized uptake values to make a determination whether the PET2 scan is considered positive or negative. Promising novel approaches to analyze PET scan data include calculation of metabolic tumor volume (MTV; additive volume of all metabolic lesions) and total lesion glycolysis index (MTV multiplied by the mean standardized uptake value within the lesion).22,23 Retrospective and prospective analyses have shown that baseline MTV and total lesion glycolysis are independent prognostic factors for patients with HL.24,,,-28 Additional studies are under way to validate these promising findings.
Because patients who had a negative PET scan after 2 cycles of ABVD continued to experience relapse events during the 5-year follow-up of S0816, alternate induction regimens may be required to maximize efficacy in this population. Frontline therapy with eBEACOPP is advocated by some groups. In the recently published German HL Study Group (GHSG) HD18 study, 1964 patients with advanced-stage HL were initially treated with eBEACOPP. After central review, patients with a negative PET scan after 2 cycles of eBEACOPP (n = 940; 48%) demonstrated an estimated 5-year PFS of 91%, which was higher than the 76% reported on the S0816 study.5 However, the estimated 5-year OS rates were very similar between the 2 studies at ∼95% for all patients.5 Another key finding of the HD18 study is that the patients with a negative PET2 who were randomized to only 2 additional cycles of eBEACOPP (n = 501) had similar 5-year PFS to those patients with a negative PET2 who were randomized to receive 4 to 6 additional cycles of eBEACOPP (92% vs 91%). This group also had less severe infections and organ toxicity than those who received 6 to 8 cycles of eBEACOPP. Therefore, a substantial number of patients with negative PET2 after eBEACOPP could limit toxicity and derive similar efficacy when given 4 total cycles of eBEACOPP compared with >4 cycles. Of note, only 48% of patients on the HD18 study had a negative PET2 scan compared with 82% on the S0816 study. This difference can be explained by the fact that definitions of what is considered a negative PET2 scan are variable and the HD18 study only considered those patients with a Deauville score of 1 to 2 to have a negative PET2 scan as opposed to a Deauville score of 1 to 3 defining a negative PET2 on the S0816 study. The percentage of patients with a Deauville score of 1 to 2 (n = 160) in the S0816 study was similar at 48%. Conversely, 76% of patients in the HD18 study had a PET2 with a Deauville score of 1 to 3, which was similar to the 82% seen in the S0816 study.29 In addition to the HD18 study data, a randomized study comparing eBEACOPP to ABVD in the frontline setting demonstrated an improved PFS for advanced-stage HL patients who received eBEACOPP, which was confirmed in a meta-analysis.30,31 Despite these data, utilization of eBEACOPP for all advanced-stage HL patients has not gained wide acceptance given concerns over short- and long-term toxicity, as well as the ability to salvage patients with relapsed disease.
Another alternate strategy to enhance efficacy in this patient population is to use novel, targeted, and potentially less toxic combination therapies. The ECHELON-1 study randomized patients with newly diagnosed advanced-stage HL to ABVD to adriamycin, vinblastine, doxorubicin (AVD) plus brentuximab vedotin (brentuximab, adriamycin, vinblastine, doxorubicin [AAVD]).32 In this study, patients who received AAVD had a lower combined risk of progression, death, non-CR, and use of subsequent anticancer therapy at 2 years when compared with patients who received ABVD.32,33 A recent update demonstrated continued benefit of AAVD at 3 years.34 Longer follow-up will reveal whether the late progressions that we observed with ABVD are prevented in the brentuximab-containing arm.
Another novel and potentially more efficacious regimen under evaluation for frontline therapy for advanced-stage HL is AVD plus nivolumab (ANVD; a checkpoint inhibitor approved for relapsed HL). Although length of follow-up is short compared with studies using ABVD, early results of the phase 2 Checkmate 205 study, in which 51 patients with newly diagnosed advanced-stage HL (cohort D) were treated with ANVD, demonstrate reasonable tolerability, an overall response rate of 84%, and a 9-month PFS of 94%.35 As such, the next North American intergroup study planned as a collaboration of the US SWOG, ECOG, Alliance, Children’s Oncology Group, and the Canadian Clinical Trials Group will randomize patients to receive AAVD vs ANVD (S1826). Notably, these targeted combinations do not rely on PET-based response and S1826 will not alter therapy based on PET2 response given these findings in S0816. Additionally, as opposed to PET-adapted approaches that intensify therapy to eBEACOPP, AAVD and ANVD can be safely administered to older patients with HL. Therefore, unlike the S0816 study, the upcoming S1826 study will not exclude patients over the age of 60 years.
In contrast to the PET2− patients treated on S0816, the use of PET as a biomarker to escalate therapy significantly improved outcomes for the patients who had a positive PET2. The historically expected 2-year PFS rate of 12% to 30% was improved to 64% in this subgroup.7,8,10 This PFS rate advantage remained stable after nearly 6 years of follow-up at 65% in this long-term analysis.
Although escalation of therapy to eBEACOPP after a positive PET2 improved PFS for these patients, this long-term follow-up detected an alarmingly high rate of second malignancies (14%) in this group. There were no differences in the baseline characteristics of the patients who received eBEACOPP who did and did not develop a second cancer. With the exception of the patient who developed MDS, no patient who received eBEACOPP and developed a second cancer had received subsequent chemotherapy or radiotherapy for HL. In this limited retrospective analysis, the only statistically significant distinguishing feature of patients who did and did not develop a second cancer was the type of HL therapy received (ABVD vs eBEACOPP). However, there is no clear pathobiological mechanism to implicate eBEACOPP therapy as a cause for second malignancy. The rate of second malignancies reported on the S0816 study is higher than what has been reported in patients receiving eBEACOPP on other response-adapted studies, potentially due to the small number of patients and our longer follow-up. In the RATHL study, patients with newly diagnosed stage II-IV HL received 2 cycles of ABVD. If the PET2 was positive (Deauville >3), therapy was switched to either full-dose BEACOPP (every 14 days; BEACOPP-14) for 6 cycles or eBEACOPP for 4 cycles.11 Among the 172 patients who received BEACOPP therapy, only 3 second cancers were detected (2%) after 3.4 years of follow-up.11 In the GITIL 0607 study, patients with newly diagnosed stage IIB-IV HL received 2 cycles of ABVD. If the PET2 scan was positive (Deauville >3), therapy was switched to eBEACOPP for 4 cycles followed by 4 cycles of standard BEACOPP with or without rituximab. After 3.6 years of follow-up, no second cancers were reported.12 The rate of second malignancies reported on the S0816 study is also higher than what has been reported in patients receiving eBEACOPP as primary HL therapy in other prospective trials, most notably the GHSG HD9, HD15, and HD18 studies.4,5,36,37 Direct comparison of these studies is limited by differences in chemotherapy regimens, use of radiotherapy, and length of follow-up. Regardless of cause, the high rate of second cancers (reported up to 5 years from therapy) emphasizes the importance of long-term follow-up for these patients and careful screening for second cancers.
In summary, the long-term OS of patients treated on S0816 remains high (94%) at 5 years. Despite historical data suggesting favorable clinical outcomes in patients with a negative PET2, nearly one-quarter of these patients ultimately experienced relapse events, demonstrating limitations of the current PET-adapted approach and of standard frontline therapy with ABVD. In patients who were PET2+ and received eBEACOPP, PFS was favorable relative to historical series, but was associated with a high rate of secondary malignancies. Our results emphasize the importance of long-term follow-up of clinical trials in this disease, and the need for better biomarkers at diagnosis of HL and less toxic, more active therapies for advanced-stage presentations of HL.
Presented at the 60th annual meeting of the American Society of Hematology, 1-4 December 2018, San Diego, CA.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by National Institutes of Health, National Cancer Institute National Clinical Trials Network grants CA180888, CA180819, CA180821, CA180820, CA180833, CA180818, CA11083, and CA04919.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
The long-term follow-up results of this study are presented in memory of Oliver W. Press, principal investigator of this trial, who died in 2017.
Authorship
Contribution: D.M.S., H.L., S.M.S., and J.W.F. collected and analyzed data, drafted the original manuscript, revised the manuscript, and approved the final draft; and H.S., D.J.S., C.H.M., M.L., L.M.R., N.L.B., A.M.E., A.S.L., P.M.B., M.V.K., E.D.H., J.P.L., and B.S.K. collected and analyzed data, revised the manuscript, and approved the final draft.
Conflict-of-interest disclosure: D.M.S. has received research funding from Acerta, Gilead, Karyopharm and honoraria from Genentech. H.S. has served as a consultant for Allero Therapeutics. D.J.S. has received research funding from and serves as a consultant for Seattle Genetics. L.M.R. holds a patent through NanoString. N.L.B. has received research funding from Gilead, Kite, Seattle Genetics, Affimed, Bristol-Myers Squibb, Celgene, Forty Seven, Genentech, Immune Design, Janssen, Merck, Millennium, and Pharmacyclics, and serves as a consultant for Pfizer and Acerta. A.M.E. serves as a consultant for and has received honoraria from Seattle Genetics, Bayer, Verastem, and Pharmacyclics. A.S.L. serves as a consultant for Seattle Genetics and Bristol-Myers Squibb. P.M.B. serves as a consultant for AbbVie. E.D.H. serves as a consultant for Celgene, Seattle Genetics, and Jazz and has received research funding from AbbVie and Eli Lilly. J.P.L. serves as a consultant for Celgene, Juno, Bristol-Myers Squibb, Sutro, Gilead, Genentech, Pfizer, Bayer, Biotest, United Therapeutics, Karyopharm, ADC Therapeutics, MEI Pharma, AstraZeneca, and Novartis. B.S.K. serves as a consultant for Genentech, Celgene, Acerta, AstraZeneca, Juno, CTI, ADC Therapeutics, AbbVie, Gilead, and Seattle Genetics. S.M.S. serves as a consultant for Bristol-Myers Squibb, Humanigen, and Seattle Genetics and has received research funding from Forty Seven and Sanofi. J.W.F. has received honoraria from Bayer. The remaining authors declare no competing financial interests.
Correspondence: Deborah M. Stephens, University of Utah, 2000 Circle of Hope, Research South 5509, Salt Lake City, UT 84112; e-mail: deborah.stephens@hci.utah.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal