TO THE EDITOR:
The introduction of plasmapheresis with plasma exchange has increased survival in patients with thrombotic thrombocytopenic purpura (TTP) from <10% to 80%.1 More than 30% of patients with TTP have at least 1 relapse within 2 years.2 Treatment with rituximab normalizes ADAMTS13 and induces durable remissions in >90% of patients with refractory or relapsing TTP.3 Rituximab is increasingly used in the upfront setting with evidence of improved rates of remission and disease-free interval.4,5
The standard dosing regimen for rituximab is 375 mg/m2 weekly for 4 weeks, which is the dose adopted from regimens developed to treat of non-Hodgkin lymphoma.6 In autoimmune conditions with a lower total lymphocyte mass, an efficacious dose of rituximab is likely less than what is administered for the treatment of lymphoma. Lower doses of rituximab are attractive for potential cost savings, decreased infusion time, and less toxicity. A fixed rituximab dose of 100 mg weekly has been used successfully to treat other autoimmune hematologic conditions.7,-9 A retrospective cohort study recently analyzed outcomes for patients with TTP treated with doses ranging between 200 mg/m2 and 500 mg/m2 and did not identify differences in treatment outcomes,10 and adaptive rituximab 375 mg/m2 administration based on lymphocyte depletion appeared promising in a phase 2 study.11 A prospective clinical trial evaluating the efficacy and safety of low-dose rituximab in TTP has not been conducted.
A prospective, multicenter, single-arm, phase 2 trial was conducted at 4 university hospitals. The primary endpoint of the study was the composite outcome of exacerbation or refractory TTP. Exacerbation was defined as recurrent TTP ≤30 days after treatment response (ie, 2 consecutive days with a platelet count ≥150 × 109/L). A durable treatment response required a platelet count to remain >150 × 109/L ≥30 days after discontinuation of plasma exchange. Criterium for refractory TTP was the failure at day 28 to achieve a treatment response or a failure to achieve a durable treatment response by day 60.
The protocol was approved by the institutional review boards of participating hospitals. Eligible patients were ≥18 years of age, with evidence of TTP: (1) platelet count <80 × 109/L for new diagnosis or <120 × 109/L for relapse; (2) red cell fragmentation; (3) an elevated lactate dehydrogenase; and (4) ADAMTS13 activity <10%.12 Study subjects were excluded if they received treatment of TTP within the previous 2 months, had active malignancy, or received organ or stem cell transplant, congenital TTP, rituximab within the prior year, or evidence of HIV, hepatitis B, or hepatitis C exposure.
Rituximab 100 mg was administered every 5 to 9 days for 4 weeks. Study subjects were required to undergo plasma exchange, not to exceed the fourth plasma exchange prior to the first rituximab administration. Rituximab was to be administered immediately after plasma exchange, allowing maximal exposure prior to next exchange (with a goal of at least a 20-hour time interval between exchanges). Prednisone was administered at 1 mg/kg daily until response and slowly tapered.
A total of 19 patients were enrolled in the study with 1 patient later diagnosed with congenital TTP and not included in the analyses. There were 12 women, and the median age was 49 years. The median body mass index was 33.5 kg/m2. Seven patients were enrolled during initial diagnosis of TTP, and 11 were enrolled at time of relapse. Baseline ADAMTS13 activity was undetectable in all but 1 subject, who had an activity of 1.7% with 13.1 inhibitor units.
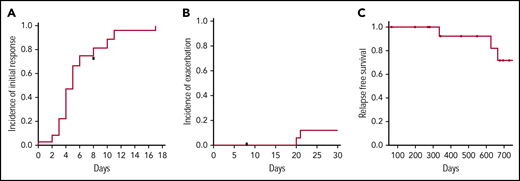
The median time to treatment response was 5 days (Figure 1A). There were no cases of primary refractory TTP, but there were 2 episodes of exacerbations that required reinitiation of plasma exchange with a cumulative incidence of exacerbation or refractory TTP at 30 days of 12.5% (Figure 1B). Prior to meeting the definition of refractory TTP, 1 patient was taken off study at day 8 with a platelet count of 9 × 109/L after receiving only 1 dose of rituximab and was switched to an alternative regimen (full-dose rituximab, vincristine, and twice-daily plasma exchange). There were 3 relapses that occurred within 2 years of follow-up (Figure 1C) and only 1 recurrence within 12 months of enrollment.
Outcomes following low-dose rituximab and plasma exchange for the treatment of acquired TTP. (A) Time to initial treatment response (ie, platelet count >150 000/mL on 2 consecutive days). (B) The incidence of exacerbation prior to day 30 was 12%. (C) The relapse-free survival at 2 years was 72%. The cumulative incidence of response and relapse was estimated by the method of Kaplan-Meier.
Outcomes following low-dose rituximab and plasma exchange for the treatment of acquired TTP. (A) Time to initial treatment response (ie, platelet count >150 000/mL on 2 consecutive days). (B) The incidence of exacerbation prior to day 30 was 12%. (C) The relapse-free survival at 2 years was 72%. The cumulative incidence of response and relapse was estimated by the method of Kaplan-Meier.
The observed response rates compare favorably with published experience. Plasma exchange and in the absence of rituximab is associated with exacerbation rates of between 30% and 40% and relapse rates between 30% to 40% among those with ADAMTS13 levels <10%.2,13,14 Rituximab administered at 375 mg/m2 weekly dosing in conjunction with plasma exchange and steroids is associated with high rates of response and low rates of relapse, similar to those observed in the current trial. For instance, in a phase 2 study of 40 patients treated with rituximab and plasma exchange for acute TTP, the median time to normalization of platelet count was 12 days with 10.8% relapse at a median time of 27 months.4
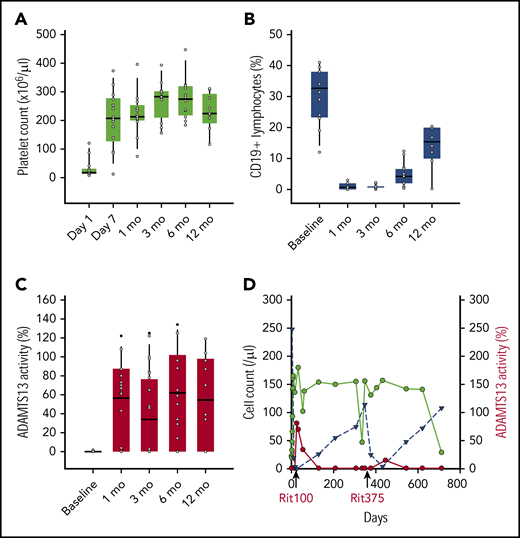
CD19 lymphocyte recovery typically occurs between 12 and 18 months following a cycle of standard-dose rituximab.4,15 As shown in Figure 2, the median CD19 lymphocyte percentage remained significantly below baseline levels at 12 months post–rituximab infusion (Wilcoxon signed-rank, P = .003). There were no differences in 6-month CD19+ lymphocyte counts in those who remained free of relapse compared with those who relapsed (median 4.0% and 4.6%, respectively, Mann-Whitney rank sum, P = .6).
Changes in platelets, CD19 lymphocytes, ADAMTS13 activity following initial treatment with weekly rituximab 100 mg and plasma exchange. Box plots of platelet count (A) and absolute CD19+ lymphocyte % (B), and ADAMTS13% activity (C) are shown at time intervals. An Illustrative case of relapse (D) following 4 weekly doses of rituxan 100 mg (labeled rit100) and 4 weekly doses of rituxan 375 mg/m2 (labeled rit375) administered at time of relapse. Serial measurements of platelet count (green) and absolute CD19 lymphocyte count (blue) shown over time with relapse occurring around years 1 and 2. ADAMTS13 activity shown in red. A similar pattern of CD19 lymphocyte count rise and platelet decline observed following both dosing regimens of rituximab.
Changes in platelets, CD19 lymphocytes, ADAMTS13 activity following initial treatment with weekly rituximab 100 mg and plasma exchange. Box plots of platelet count (A) and absolute CD19+ lymphocyte % (B), and ADAMTS13% activity (C) are shown at time intervals. An Illustrative case of relapse (D) following 4 weekly doses of rituxan 100 mg (labeled rit100) and 4 weekly doses of rituxan 375 mg/m2 (labeled rit375) administered at time of relapse. Serial measurements of platelet count (green) and absolute CD19 lymphocyte count (blue) shown over time with relapse occurring around years 1 and 2. ADAMTS13 activity shown in red. A similar pattern of CD19 lymphocyte count rise and platelet decline observed following both dosing regimens of rituximab.
Normalization of ADAMTS13 (defined as levels ≥60%) occurred in 72% (13 of 18) of patients in whom follow-up ADAMTS13 values were available (median response 36 days). Of the remaining 5 patients, 3 achieved a partial response (levels ≥30%). There were 2 patients whose ADAMTS13 activity levels remained <5% at all measurements, and neither developed relapsed TTP during the period of observation. In all instances of relapse, the ADAMTS13 activity was undetectable. The overall response rate for recovery of ADAMTS13 plasma concentrations was 89% (complete and partial response), which is similar to recent cohorts reporting 92% with standard rituximab dosing.10
A case of recurrent relapse that occurred at 1 and 2 years following enrollment serves as an illustrative comparison of patterns of CD19 lymphocyte, platelet, and ADAMTS13 recovery following reduced-dose vs standard-dose rituximab (Figure 2D). Following initial therapy with plasma exchange and rituximab 100 mg × 4 doses, there was a rapid recovery of platelets and ADAMTS13 activity along with complete depletion of CD19 lymphocytes. Following relapse, prednisone and rituximab 375 mg/m2 × 4 doses were administered. Over the ensuing year, there was a gradual recovery in lymphocyte count with another relapse 1 year later, suggesting that rituximab dosing did not alter the kinetics of disease in this patient.
With shorter infusion times, a theoretical benefit of lower rituximab dosing is less toxicity. However, there were several severe adverse events that were considered possibly related to rituximab (see supplemental Table 1, available on the Blood Web site). This included an acute episode of grade 4 respiratory failure during infusion of the third dose of rituximab (occurring 8 days after last plasma exchange). There was 1 death on study in the setting of advanced cancer occurring at 1.5 years following enrollment, which was considered unrelated to rituximab administration.
Consistent with findings of other trials evaluating the efficacy of low-dose rituximab in the treatment of autoimmune cytopenias, the results of this phase 2 study of low-dose rituximab when administered in conjunction with plasma exchange and steroids demonstrated encouraging activity for the treatment of acquired TTP. Low-dose rituximab has clear advantages, including shortened infusion time and lower cost, but whether there is a toxicity benefit is less clear. Low-dose rituximab in combination with plasma exchange achieved high rates of initial response coupled with sustained reduction in CD19 lymphocyte populations, but comparative studies are needed to establish whether rituximab dosing influences long-term durability of response.
The online version of this article contains a data supplement.
Acknowledgments
This work was supported by funding from the National Institutes of Health, National Heart, Lung, and Blood Institute grant U54HL112303 (J.E.S.) and by a Scientist Development Grant from the American Heart Association 16SDG30740011 (J.M.).
The authors dedicate this article to J. Evan Sadler, who led this trial until his untimely passing. J. Evan Sadler was an outstanding scholar, mentor, and colleague, whose passion for science was accented by a gentle humility; he will be missed dearly.
Authorship
Contribution: The manuscript was principally authored by J.I.Z. and J.E.S. (introduction); the final version was reviewed by all authors except J.E.S.; the study was designed by J.E.S., J.M., and L.D.; and data collection was performed by J.E.S., L.A.W., J.M., L.D., P.N., A.R., A.G.A., A.M., A.H., and J.I.Z., with analysis performed by J.E.S., J.M., L.A.W., P.N., and J.I.Z.
Conflict-of-interest disclosure: J.I.Z. discloses prior research funding from Quercegen Pharma and Incyte and has served on scientific advisory boards for Portola Pharmaceuticals, Bayer, Pfizer, and Seattle Genetics. J.M. and J.E.S. have a patent, fluorogenic substrate for ADAMTS‐13, US 8663912, issued to Washington University. The remaining authors declare no competing financial interests.
J. Evan Sadler died on 13 December 2018.
Additional members of the ART Investigators appear in the supplemental appendix.
Correspondence: Jeffrey I. Zwicker, Beth Israel Deaconess Medical Center, Harvard Medical School, 330 Brookline Ave, Boston, MA 02215; e-mail: jzwicker@bidmc.harvard.edu.