In this issue of Blood, Holthenrich et al used a proximity labeling approach to pull, from out of the crowded intracellular milieu, proteins that specifically interact with Weibel-Palade bodies (WPBs). From the resulting catalog of proteins, the authors identified Munc13-2 as a novel WPB-associated SNARE-interacting protein that positively regulates hormone-evoked WPB exocytosis.1
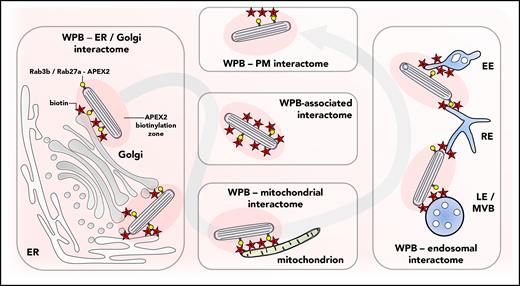
Proximity ligation identifies the WPB interactome. Newly formed WPBs acquire the Rab3b-APEX2 or Rab27a-APEX2 fusion proteins which, upon addition of biotin, generate an active zone of biotinylation around the organelle, randomly labeling proteins that are associated with the granule or that are in its immediate vicinity. As the WPB makes its way through the cell it encounters (and interacts with) various subcellular organelles and intracellular membranes, a path that is illuminated through the identification of sets of proteins that are found on the endoplasmic reticulum (ER), Golgi, mitochondria, and endosomal compartments such as the late endosomes or multivesicular bodies (LE/MVB), recycling endosomes (RE), and early endosomes (EE). PM, plasma membrane.
Proximity ligation identifies the WPB interactome. Newly formed WPBs acquire the Rab3b-APEX2 or Rab27a-APEX2 fusion proteins which, upon addition of biotin, generate an active zone of biotinylation around the organelle, randomly labeling proteins that are associated with the granule or that are in its immediate vicinity. As the WPB makes its way through the cell it encounters (and interacts with) various subcellular organelles and intracellular membranes, a path that is illuminated through the identification of sets of proteins that are found on the endoplasmic reticulum (ER), Golgi, mitochondria, and endosomal compartments such as the late endosomes or multivesicular bodies (LE/MVB), recycling endosomes (RE), and early endosomes (EE). PM, plasma membrane.
WPBs are specialized secretory granules containing the adhesive glycoprotein von Willebrand factor, the leukocyte adhesion molecule P-selectin, and a host of angiogenic and inflammatory mediators.2 These unusual organelles have been likened to a vascular emergency kit that functions to minimize blood loss, fight infections, and repair damaged blood vessels at sites of injury. Correct formation, trafficking, and exocytosis of WPBs involves a huge workforce comprising a great many proteins, most of which we know very little about. In their study, Holthenrich et al have attempted to identify these proteins and so narrow this major gap in our knowledge.
To do this, Holthenrich et al applied a fast and highly sensitive proximity labeling methodology based on fusions of WPB-specific Rab GTPases Rab27a or Rab3b and a modified ascorbate peroxidase, APEX2.3 In the presence of its substrate, biotin-phenol, and hydrogen peroxide, APEX2 catalyzes the biotinylation of proteins located in its immediate vicinity (10-20 nm).3 The speed (in seconds) and efficiency of APEX2 makes it particularly useful in catching weak or transient protein interactions, and the biotin-tagged proteins can then be extracted, purified, and identified by using mass spectrometry to yield a protein interactome for the Rab-APEX2-labeled WPBs (see figure).
The choice of Rab27a and Rab3b was important for several reasons. First, these Rab proteins are recruited in a highly specific fashion to WPBs soon after they bud from the trans-Golgi network and remain with the organelle until its destruction upon exocytosis. This allowed the identification of interactors at all stages in the life cycle of the WPB. Second, both Rab proteins are fairly promiscuous in their interactions with cytosolic effectors, so they provided an ideal base from which to sample and catch direct interactions with potential effectors. Third, because both Rabs use a C-terminal lipid anchor to attach to the outer leaflet of the WPB membrane, they are highly mobile,4 rapidly (subseconds) exploring the surface of the WPB to provide a dynamic active zone for APEX2 to mark proteins on or close to the organelle. Finally, Rab27a and Rab3b are likely to mediate very distinct functions on the WPB that may potentially be revealed through the specific protein interactomes.
The approach was successful. First, Holthenrich et al were able to identify most of the known WPB regulators, including integral membrane proteins incorporated into the organelle during biogenesis and maturation (VAMP3, syntaxin-3, SLEP, ATP6V02) and effectors recruited to the organelle from the cytoplasm (eg, Rab27a, Rab3a/b/c, Slp4-a, MyRIP) or through interactions with endomembrane compartments or the plasma membrane (eg, CD63, PLD1, STXBP1).
More important was that this elegant study revealed that the known components are just the tip of the iceberg that comprises the WPB interactome. In total, 183 proteins were identified of which 158 were common to both Rab27a and Rab3b. Among these was a new component of the WPB secretory machinery, Munc13-2, a SNARE-interacting protein that was shown to promote WPB exocytosis. Newly formed WPBs remain within the crowded environment of the trans-Golgi network while they acquire Rab27a or Rab3b, a process that takes up to 30 minutes.5 It is perhaps not surprising that a number of the 183 proteins identified by both Rabs comprised ER or Golgi components, including the Golgi-specific brefeldin A-resistance guanine nucleotide exchange factor 1 (GBF1), recently identified as a master regulator of environmental cue–driven WPB formation.6 Whether these ER/Golgi proteins reflect innocent bystanders labeled within the dense confines of the cellular biosynthetic factory or in fact play a direct role in the itinerary of the WPB remains to be established. Certainly, en route to the plasma membrane, the WPB must navigate a cytoplasm crowded with endomembrane compartments and cytoskeletal components, most graphically illustrated in recent cryo-electron microscopy studies of WPBs in intact vitrified endothelial cells.7,8 Incredibly close physical interactions are observed between WPBs and a variety of endomembrane components that may account for APEX2 detection of Rab7, Rab11, SNAP29, Rab14, and the Rab5 effector Rab11FIP5. Many of these close interactions are likely to be of functional importance, for example, in the delivery of CD63 and possibly other components to the WPB.8,-10 Indeed, the pattern of detected proteins is almost akin to a ghost of the path taken by the organelle as it moves through the cell.
Of interest was the apparent absence of molecules implicated in the final stages of WPB fusion at the plasma membrane (eg, S100A10/AnnexinA2, syntaxin-2, syntaxin-4, STXBP3, STXBP5, RalGDS, RalA). The reason for this may lie in the requirement for active translocation of such molecules to the plasma membrane during cellular activation, or because WPBs, unlike secretory organelles of many neuroendocrine cells, do not seem to be docked in close proximity to the plasma membrane prior to activation. The Holthenrich et al study was carried out in resting cells, and one can envisage that future studies in cells stimulated with a range of agonists operating through different signaling pathways may yet reveal these and novel components of the machinery that regulates WPB trafficking and exocytosis.
In conclusion, the study by Holthenrich et al has provided a wealth of new information about the itinerary and interaction of WPBs as they traverse the intracellular milieu. The catalog of new interactors identified will provide fertile ground for new studies, and a detailed analysis of the molecules captured by Rab27a or Rab3b may provide clues to functions not previously recognized.
Conflict-of-interest disclosure: The authors declare no competing financial interests.