Key Points
In vivo proteomics with Weibel-Palade body-targeted APEX2 constructs reveals the organelle-associated proteome.
Munc13-2 is a novel positive regulator of von Willebrand factor secretion from endothelial cells.
Abstract
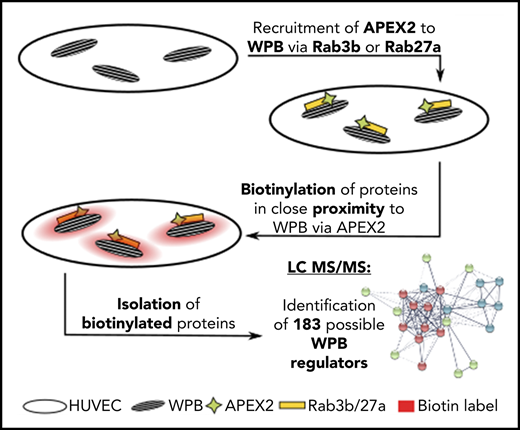
Weibel-Palade bodies (WPB) are unique secretory organelles of endothelial cells that store factors regulating vascular hemostasis and local inflammation. Endothelial activation triggers rapid exocytosis of WPB, leading to the surface presentation of adhesion molecules relevant for leukocyte rolling (P-selectin) and platelet capture (von Willebrand factor [VWF]). Despite its role as an important secretory organelle, a comprehensive compilation of factors associated with WPB has not been carried out. We addressed this via a proximity proteomics approach employing the peroxidase APEX2 coupled with 2 known WPB-associated proteins: the Rab GTPases Rab3b and Rab27a. We show that APEX2-Rab3b/27a fusion constructs are correctly targeted to WPB of primary endothelial cells, and that proteins in their close proximity can be biotinylated through the WPB-recruited APEX2. Mass spectrometry analysis of the biotinylated proteins identified 183 WPB-associated proteins. Whereas these include factors reported before to localize to WPB, the majority comprises proteins not previously associated with WPB biology. Among them, the SNARE-interacting protein Munc13-2 was shown here to specifically localize to WPB and to serve as a novel factor promoting histamine-evoked WPB exocytosis and VWF secretion. Thus, APEX2-based proximity proteomics can be used to specifically identify novel organelle-associated factors in primary endothelial cells.
Introduction
Vascular endothelial cells react to local perturbations such as tissue inflammation or vascular injury by the acute secretion of factors that control coagulation and leukocyte, as well as platelet adhesion. Many of these factors are stored in specialized secretory granules, Weibel-Palade bodies (WPB), that undergo exocytosis after endothelial activation. WPB are elongated structures1 that form at the trans-Golgi network and further mature during their microtubule-dependent transport to the cell periphery.2 Major WPB constituents are the coagulation-promoting von Willebrand factor (VWF) that forms condensely packed tubules within WPB, the leukocyte receptor P-selectin, and the tetraspanin CD63 (Lamp3) that is acquired from the endosomal system during WPB maturation.3,4
Maturation, motility, and secretagogue-evoked exocytosis of WPB are mediated by a number of associated factors, in particular Rab GTPase family members. Rab27a is the most prominent among them. It is recruited to more mature WPB2,5 and most likely serves multiple functions in cortical actin anchorage and exocytosis of WPB, depending on the engagement of different effectors.6,-8 Other Rab proteins associated with WPB are Rab3 isoforms that have been implicated in the biogenesis and final exocytotic events.8,9
However, the molecular complexity of WPB as an important secretory organelle is far from being understood. Up to now, only a single proteomic study addressed this issue by isolating WPB through density gradient fractionation and identifying the proteins present by mass spectrometry (MS).10 This analysis focused on the content of WPB; that is, proteins residing inside WPB and not associated cytosolic factors that could be involved in regulating WPB dynamics and exocytosis. Therefore, we employed here a different strategy to identify novel proteins associated with WPB. We used proximity proteomics involving the peroxidase APEX211 to biotinylate proteins residing at or in the immediate vicinity of WPB in live cells, which were subsequently enriched by streptavidin pulldown and identified by tandem MS. This revealed a total of 183 WPB-associated proteins, most of which had not been described in conjunction with WPB before. One of the novel, WPB-associated proteins is the tethering/priming factor Munc13-2 (UNC13B), which acts as a positive regulator of histamine-evoked WPB exocytosis, and thus VWF secretion.
Methods
HUVEC (human umbilical vein endothelial cells) culture, transfection, and the VWF secretion assay were carried out according to Disse et al.12 APEX2-mediated biotinylation and streptavidin pulldown were performed as described by Hung et al,11 with minor changes listed in the supplemental Materials and Methods, available on the Blood Web site. Plasmids, siRNAs, antibodies, fluorescence microscopy techniques, statistical analyses, and MS are described in the supplemental Materials and Methods.
Results and discussion
Targeting of APEX2 to WPB in primary endothelial cells
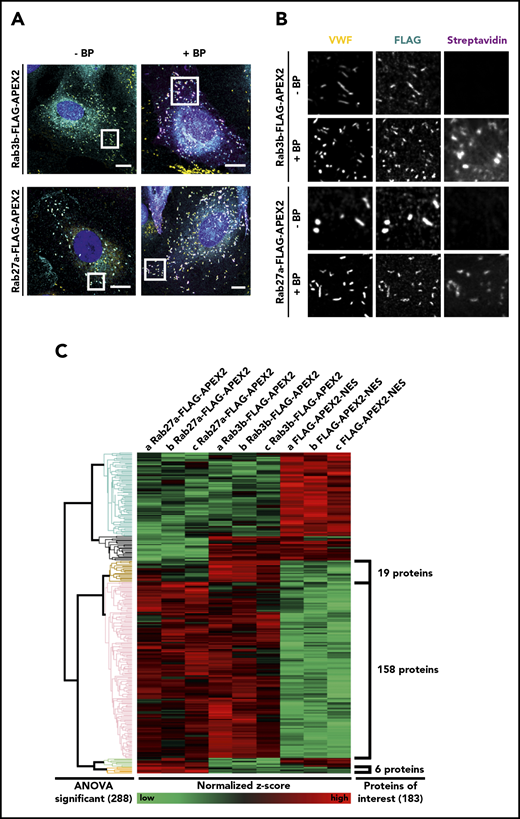
To identify novel WPB-associated proteins by proximity proteomics in HUVEC, we directed APEX2 to WPB by fusing the enzyme to 2 Rab GTPases known to specifically associate with WPB, Rab3b, and Rab27a.5,8 A fusion construct of APEX2 and a nuclear export signal (NES) served as control for cytosolic proteins unspecifically biotinylated by APEX2 proximity (supplemental Figure 1). Figure 1 shows that the Rab3b- and Rab27a-APEX2 constructs are specifically targeted to WPB, and that almost all WPB of the transfected HUVEC are positive for the respective Rab-APEX construct. Importantly, the addition of biotin-phenol (BP) and H2O2 for 60 seconds resulted in a peroxidase-catalyzed biotinylation of proteins residing at or in close proximity to WPB, as revealed by streptavidin staining of the accordingly treated HUVEC (Figure 1). In comparison with the anti-VWF and anti-FLAG antibody signals that delineate the WPB, the streptavidin signal appears slightly more diffuse around the WPB, most likely because of the proximity labeling of WPB-associated proteins.
Rab3b- and Rab27a-APEX2 fusion proteins localize to WPB and biotinylate proteins in close proximity. (A-B) HUVEC were transfected with either Rab3b-FLAG-APEX2 or Rab27a-FLAG-APEX2 constructs. APEX2 fusion proteins were stained by anti-FLAG antibodies (cyan), VWF used as a WPB marker was visualized by anti-VWF antibodies (yellow), and biotinylated proteins were labeled by AF647-streptavidin (magenta). Images on the left side show a merge of the whole cell (A), whereas images on the right show the boxed areas at higher magnification (B). Both Rab3b- and Rab27a-FLAG-APEX2 localize to WPB, and no streptavidin signal is visible in cells before BP/hydrogen peroxide treatment (−BP). After treatment with BP and hydrogen peroxide (+BP), cells show a notably enhanced streptavidin signal that is concentrated in the proximity of WPB. Nuclei are shown in dark blue. Scale bars, 10 µm. (C) Streptavidin pulldowns from lysates of BP/H2O2-treated HUVEC expressing Rab27a-FLAG-APEX2, Rab3b-FLAG-APEX2, or FLAG-APEX2-NES, respectively, were carried out in triplicates, and proteins enriched were identified by tandem MS. Proteins that were present in all 3 replicates (a, b, c) of at least 1 sample group (Rab27a-FLAG-APEX2, Rab3b-FLAG-APEX2, or FLAG-APEX2-NES) were included in the calculation of relative protein enrichment by ANOVA. Because of the very low yield of biotinylated proteins from the nontransfected cells, this sample group was not included. Proteins with P < .02 were considered significantly enriched, leading to the identification of 288 proteins that were clustered into different groups according to their similar enrichment pattern in the different samples. This pattern is visualized by showing high relative enrichment in red and reduced amounts of protein in green. A total of 183 proteins were identified as highly enriched in the WPB-associated samples compared with cytosolic proteins biotinylated by FLAG-APEX2-NES: 19 proteins enriched only in pulldowns from Rab3b-FLAG-APEX2 expressing cells, 6 proteins enriched only in pulldowns from Rab27a-FLAG-APEX2 expressing cells, and a large cluster of 158 proteins enriched in pulldowns from both Rab27a- and Rab3b-FLAG-APEX2 expressing cells (supplemental Tables 1-3).
Rab3b- and Rab27a-APEX2 fusion proteins localize to WPB and biotinylate proteins in close proximity. (A-B) HUVEC were transfected with either Rab3b-FLAG-APEX2 or Rab27a-FLAG-APEX2 constructs. APEX2 fusion proteins were stained by anti-FLAG antibodies (cyan), VWF used as a WPB marker was visualized by anti-VWF antibodies (yellow), and biotinylated proteins were labeled by AF647-streptavidin (magenta). Images on the left side show a merge of the whole cell (A), whereas images on the right show the boxed areas at higher magnification (B). Both Rab3b- and Rab27a-FLAG-APEX2 localize to WPB, and no streptavidin signal is visible in cells before BP/hydrogen peroxide treatment (−BP). After treatment with BP and hydrogen peroxide (+BP), cells show a notably enhanced streptavidin signal that is concentrated in the proximity of WPB. Nuclei are shown in dark blue. Scale bars, 10 µm. (C) Streptavidin pulldowns from lysates of BP/H2O2-treated HUVEC expressing Rab27a-FLAG-APEX2, Rab3b-FLAG-APEX2, or FLAG-APEX2-NES, respectively, were carried out in triplicates, and proteins enriched were identified by tandem MS. Proteins that were present in all 3 replicates (a, b, c) of at least 1 sample group (Rab27a-FLAG-APEX2, Rab3b-FLAG-APEX2, or FLAG-APEX2-NES) were included in the calculation of relative protein enrichment by ANOVA. Because of the very low yield of biotinylated proteins from the nontransfected cells, this sample group was not included. Proteins with P < .02 were considered significantly enriched, leading to the identification of 288 proteins that were clustered into different groups according to their similar enrichment pattern in the different samples. This pattern is visualized by showing high relative enrichment in red and reduced amounts of protein in green. A total of 183 proteins were identified as highly enriched in the WPB-associated samples compared with cytosolic proteins biotinylated by FLAG-APEX2-NES: 19 proteins enriched only in pulldowns from Rab3b-FLAG-APEX2 expressing cells, 6 proteins enriched only in pulldowns from Rab27a-FLAG-APEX2 expressing cells, and a large cluster of 158 proteins enriched in pulldowns from both Rab27a- and Rab3b-FLAG-APEX2 expressing cells (supplemental Tables 1-3).
WPB-associated proteins identified by proximity biotinylation
Given the specific biotinylation of the WPB proximity, we next isolated the biotinylated proteins by streptavidin pulldown (supplemental Figure 2). Pulldowns were prepared in triplicates from 3 batches of independently transfected HUVEC and subjected to tandem MS. The MS analysis identified a total of 183 proteins that were considered significantly enriched in one of the Rab samples with a P < .02. These hits clustered into different groups according to their relative enrichment patterns (ie, 19 proteins were enriched in the Rab3b-APEX2, 6 in the Rab27a-APEX2, and 158 in both the Rab3b-APEX2 and Rab27a-APEX2 samples; Figure 1).
Proteins enriched in the Rab3b- and/or Rab27a-APEX2 groups are listed in the supplemental Tables 1-3. Although many of these proteins had not been implicated in WPB biology before, gene ontology term analysis revealed that most of them are involved in membrane/protein transport and organelle organization (supplemental Figures 3 and 4). Moreover, some known WPB-associated factors were identified, verifying the specificity of our approach. These include well-described WPB markers such as P-selectin, CD63, VAMP3, MyRIP, and Slp4a, but also proteins that had been identified before as functioning in WPB biology (eg, syntaxin binding protein 1,13 phosphatidylinositol 4-kinase,14 phospholipase D1,12 Rab 46,15 and GBF116 and proteins enriched in isolated WPB,10,13 highlighted in supplemental Tables 1-3). Further supporting the validity of our approach, we identified multiple subunits of known protein complexes as hits in the proteomic analysis (eg, of the vacuolar ATPase ATP6V previously also identified in the proteome of isolated WPB).10 A number of the proteins identified in our APEX2 screen had previously been implicated in endosomal trafficking (eg, Rab7A), suggesting a possible function in endosome-to-WPB transport. Moreover, factors in Golgi-associated processes were identified (eg, Golgin family members17 and the secretory pathway Ca2+ ATPase type 1 [SPAC1]).18 Although Rab3b-APEX2 and Rab27a-APEX2 are specifically localized to WPB (Figure 1), we cannot exclude that some of these Golgi-associated proteins were biotinylated by Rab3b/27a-APEX2 residing on WPB in close vicinity to the Golgi.
Munc13-2 is a novel WPB-associated factor involved in VWF secretion
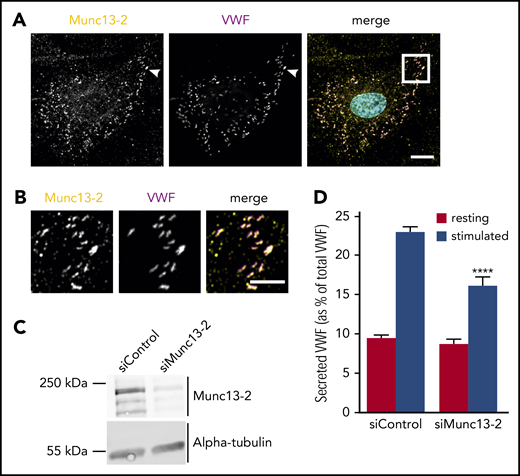
Munc13-2, a member of the Munc13 family of vesicle tethering/priming factors, is highly enriched in the group of proteins biotinylated in the Rab3b-APEX2 and Rab27a-APEX2 samples (supplemental Table 1). It had previously been implicated in the secretion of the WPB cargo angiopoietin 2 from resting brain endothelial cells19 and, based on its membrane fusion-promoting functions, was chosen for further analysis. Antibody staining revealed that Munc13-2 is specifically associated with VWF-positive WPB, thus verifying the validity of our proximity proteome approach (Figure 2). To test a functional involvement of Munc13-2 in WPB exocytosis, we analyzed the effect of Munc13-2 knockdown on histamine-evoked secretion of VWF. Figure 2C-D shows that the siRNA-mediated depletion of Munc13-2 results in a marked inhibition of evoked VWF secretion identifying Munc13-2 as a positive regulator of Ca2+-dependent WPB exocytosis. In several processes including mast cell granule exocytosis,20 Munc13-2 cooperates with another family member, Munc13-4, to promote vesicle priming, and thus regulated exocytosis. To assess whether this situation could be similar in HUVEC, in which Munc13-4 is involved in WPB-plasma membrane tethering21 and evoked WPB exocytosis,21,22 we carried out double-knockdown experiments. As compared with single knockdowns, no additional inhibitory effect of a depletion of both Munc13-2 and Munc13-4 on histamine-evoked VWF secretion was observed (supplemental Figure 5). This suggests that the 2 Munc13 isoforms act at separate steps of WPB maturation/exocytosis: Munc13-4 as a plasma membrane tethering factor21 and Munc13-2 possibly during WPB biogenesis in a way similar to its role in phagolysosome maturation.23
Munc13-2 localizes to WPB and positively regulates histamine-induced secretion of VWF. (A) HUVEC were fixed and stained for Munc13-2 (yellow) and VWF (magenta) using specific antibodies. Nuclei are shown in cyan. Scale bar, 10 µm. (B) Higher magnification of the boxed region in panel A (merge). Note that almost all VWF-containing WPB are also positive for Munc13-2. As Rab3b and Rab27a are markers for more mature WPB, this could suggest that Munc13-2 might be recruited to WPB in a Rab3b/27a-independent manner. Scale bar, 5 µm. (C-D) Munc13-2 was depleted by transfection with specific siRNAs (siMunc13-2), nontargeting siRNA served as control (siControl). Knockdown efficiency was confirmed by western blot analysis using anti-Munc13-2 antibodies and anti-α-tubulin antibodies as loading control (C). The effect of Munc13-2 depletion on histamine-induced secretion of VWF was measured by an enzyme-linked immunosorbent assay quantifying the amount of VWF released into the cell culture supernatant (D). Note the significant reduction of histamine-evoked VWF secretion after Munc13-2 knockdown. n = 3. Error bars + standard error of the mean. ****P ≤ .0001.
Munc13-2 localizes to WPB and positively regulates histamine-induced secretion of VWF. (A) HUVEC were fixed and stained for Munc13-2 (yellow) and VWF (magenta) using specific antibodies. Nuclei are shown in cyan. Scale bar, 10 µm. (B) Higher magnification of the boxed region in panel A (merge). Note that almost all VWF-containing WPB are also positive for Munc13-2. As Rab3b and Rab27a are markers for more mature WPB, this could suggest that Munc13-2 might be recruited to WPB in a Rab3b/27a-independent manner. Scale bar, 5 µm. (C-D) Munc13-2 was depleted by transfection with specific siRNAs (siMunc13-2), nontargeting siRNA served as control (siControl). Knockdown efficiency was confirmed by western blot analysis using anti-Munc13-2 antibodies and anti-α-tubulin antibodies as loading control (C). The effect of Munc13-2 depletion on histamine-induced secretion of VWF was measured by an enzyme-linked immunosorbent assay quantifying the amount of VWF released into the cell culture supernatant (D). Note the significant reduction of histamine-evoked VWF secretion after Munc13-2 knockdown. n = 3. Error bars + standard error of the mean. ****P ≤ .0001.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This study was supported by the German Research Foundation (SFB1009/A06, SFB1348/A04, and Ge514/6).
Authorship
Contribution: A.H., H.C.A.D., T.C., and J.N. designed and performed experiments, analyzed data, and wrote the manuscript; and V.G. designed the project, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Volker Gerke, Institute of Medical Biochemistry, Center for Molecular Biology of Inflammation, University of Münster, Von-Esmarch-Str 56, D-48149 Münster, Germany; e-mail: gerke@uni-muenster.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal