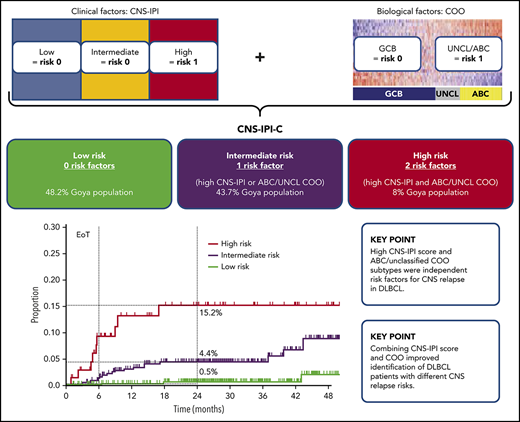
Key Points
High CNS-IPI score and ABC/unclassified COO subtypes were independent risk factors for CNS relapse in DLBCL.
Combining CNS-IPI score and COO improved identification of DLBCL patients with different CNS relapse risks.
Abstract
Central nervous system (CNS) relapse carries a poor prognosis in diffuse large B-cell lymphoma (DLBCL). Integrating biomarkers into the CNS–International Prognostic Index (CNS-IPI) risk model may improve identification of patients at high risk for developing secondary CNS disease. CNS relapse was analyzed in 1418 DLBCL patients treated with obinutuzumab or rituximab plus cyclophosphamide, doxorubicin, vincristine, prednisone chemotherapy in the phase 3 GOYA study. Cell of origin (COO) was assessed using gene-expression profiling. BCL2 and MYC protein expression was analyzed by immunohistochemistry. The impact of CNS-IPI, COO, and BCL2/MYC dual-expression status on CNS relapse was assessed using a multivariate Cox regression model (data available in n = 1418, n = 933, and n = 688, respectively). High CNS-IPI score (hazard ratio [HR], 4.0; 95% confidence interval [CI], 1.3-12.3; P = .02) and activated B-cell‒like (ABC) (HR, 5.2; 95% CI, 2.1-12.9; P = .0004) or unclassified COO subtypes (HR, 4.2; 95% CI, 1.5-11.7; P = .006) were independently associated with CNS relapse. BCL2/MYC dual-expression status did not impact CNS relapse risk. Three risk subgroups were identified based on the presence of high CNS-IPI score and/or ABC/unclassified COO (CNS-IPI-C model): low risk (no risk factors, n = 450 [48.2%]), intermediate risk (1 factor, n = 408 [43.7%]), and high risk (both factors, n = 75 [8.0%]). Two-year CNS relapse rates were 0.5%, 4.4%, and 15.2% in the respective risk subgroups. Combining high CNS-IPI and ABC/unclassified COO improved CNS relapse prediction and identified a patient subgroup at high risk for developing CNS relapse. The study was registered at www.clinicaltrials.gov as #NCT01287741.
Introduction
Central nervous system (CNS) relapse is a rare, usually fatal, event in diffuse large B-cell lymphoma (DLBCL); median overall survival (OS) after its occurrence is 3.5 to 7 months.1,2 Addition of rituximab (R) to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves outcomes in DLBCL patients3,4 ; however, its impact on the incidence of secondary CNS disease remains unclear, with some studies demonstrating reduced CNS relapse risk in DLBCL patients treated with R-CHOP vs CHOP5,6 and others showing similar CNS relapse rates.7
Reliable identification of patients at higher risk of developing secondary CNS disease is needed. Several clinical prognostic models have been proposed.1,2,8 The CNS International Prognostic Index (CNS-IPI) model is the most recently developed1 and was built using a large dataset of patients with aggressive B-cell lymphomas (80% DLBCL), who were enrolled in studies from the German High-Grade Non-Hodgkin Lymphoma Study Group and MabThera International Trial, and it was successfully validated in population-based DLBCL cohorts.9,10 The model includes the International Prognostic Index (IPI) risk factors plus involvement of the kidneys and/or adrenal glands. Implementation of biomarkers into the CNS-IPI model may improve identification of patients with a high risk for CNS relapse.9
DLBCL represents a biologically heterogeneous disease with germinal center B-cell–like (GCB) and activated B-cell–like (ABC) subtypes, each arising from different nonmalignant lymphoid counterparts.11 DLBCL cell-of-origin (COO) subtypes harbor specific genetic abnormalities12-14 ; for example, GCB DLBCL is characterized by frequent translocations of the BCL2 gene and loss of PTEN, whereas ABC DLBCL is characterized by biallelic loss of the CDKN2A gene, which encodes proteins implicated in regulation of the cell cycle (p16INK4A) and p53 (ARF) and chronically active B-cell receptor and NF-κB signaling.12,15-18 The impact of COO subtype on prognosis has been confirmed in several studies, with the ABC subtype predicting worse outcomes.19,20 ABC DLBCL was also shown to be the most common COO subtype in primary CNS lymphomas.21 Data are limited on the association of COO subtype with the risk of secondary CNS disease in DLBCL, with only 1 retrospective study published to date. Savage et al showed that ABC (or non-GCB) DLBCL is associated with higher CNS relapse risk.9 In a multivariate analysis including COO subtype, dual-expression status of BCL2 and MYC proteins, and CNS-IPI, only high CNS-IPI score and BCL2/MYC dual expression were significantly associated with CNS relapse risk.9
GOYA is a multicenter randomized phase 3 trial (NCT01287741) investigating the efficacy and safety of obinutuzumab (G) or R plus CHOP in patients with previously untreated DLBCL. After a median observation time of 29.0 months, there were no significant differences between G-CHOP and R-CHOP with regard to progression-free survival (PFS) and OS22 ; 3-year investigator-assessed PFS rates were 70% and 67%, respectively. Patients with GCB DLBCL demonstrated better outcomes than those with ABC or unclassified DLBCL, with 3-year PFS rates of 75%, 59%, and 63%, respectively. Using data from GOYA, we aimed to evaluate the impact of distinct COO subtypes and dual expression of BCL2 and MYC proteins on CNS relapse risk.
Methods
Patients, treatment, and clinical assessments
The GOYA study design is described in full elsewhere.22 Patients had previously untreated histologically documented CD20+ DLBCL and an IPI score ≥ 2, an IPI score of 1 (if age ≤ 60 years, with or without bulky disease), or an IPI score of 0 (with bulky disease [1 lesion ≥ 7.5 cm]). Patients with CNS involvement at diagnosis were excluded.
Patients received 8 21-day cycles of G or R plus 6 to 8 cycles of CHOP chemotherapy. CNS prophylaxis with intrathecal chemotherapy was recommended to be given according to institutional practice. No systemic CNS-directed prophylaxis was administered.
Staging investigations included computed tomography scan and bone marrow biopsy. Baseline lumbar puncture was recommended in patients with high-risk disease or with ≥1 of the following sites of involvement: paranasal sinuses, testicular, parameningeal, periorbital, paravertebral, or bone marrow. CNS relapse was diagnosed according to institutional practice via imaging (magnetic resonance imaging or computed tomography scan) and/or the presence of malignant cells in cerebrospinal fluid or affected tissue. The protocol was approved by the ethics committees of participating centers. All patients provided written informed consent.
COO, immunohistochemical, and FISH analyses
COO classification was performed by a central laboratory based on gene-expression profiling using the NanoString Lymphoma Subtyping Research-Use-Only assay (NanoString Technologies, Seattle, WA). Immunohistochemical analysis using BCL2 (clone 124) and MYC (clone Y69) assays (Ventana Medical Systems, Tucson, AZ) was conducted on slides cut from diagnostic formalin-fixed paraffin-embedded (FFPE) blocks. Cut slide stability was not considered for selection of tissue sections for analysis. BCL2 protein expression was assessed according to the percentage of tumor cells with BCL2 expression and staining intensity; positivity was defined as moderate or strong staining in ≥50% of tumor cells. MYC positivity was defined as expression in ≥40% of tumor cells. Immunohistochemical analyses were conducted in a central laboratory (Hematogenix Laboratory Services, Chicago, IL). Fluorescence in situ hybridization (FISH) was performed in a central laboratory (HistoGeneX, Antwerp, Belgium) on the diagnostic FFPE tissue sections using Vysis LSI Dual Color, Break Apart probes for BCL2 and MYC rearrangement detection, as previously described.23
Targeted next-generation sequencing
Genomic DNA was extracted from diagnostic FFPE tissue sections containing ≥ 20% tumor cells. Samples were submitted to a central laboratory (Foundation Medicine, Cambridge, MA) for next-generation sequencing–based genomic profiling. Adaptor-ligated DNA underwent hybrid capture for all coding exons of 465 cancer-related genes (FoundationOne Heme platform). Captured libraries were sequenced to a median exon coverage depth > 500× (DNA) using Illumina sequencing, and resultant sequences were analyzed for base substitutions, small insertions and deletions, copy number alterations (focal amplifications and homozygous deletions), and gene fusions/rearrangements, as previously described.24 Frequent germline variants from the 1000 Genomes Project (dbSNP142) were removed. To maximize mutation-detection accuracy (sensitivity and specificity) in impure clinical specimens, the test was previously optimized and validated to detect base substitutions at ≥5% mutant allele frequency, insertions and deletions with ≥10% mutant allele frequency with ≥99% accuracy, and fusions occurring within baited introns/exons with >99% sensitivity.24 Known confirmed somatic alterations deposited in the Catalogue Of Somatic Mutations In Cancer (COSMIC v62) are called at allele frequencies ≥ 1%.25 Next-generation sequencing–based genomic profiling was performed in a subset of patients (617 of 1418) who provided an optional written informed consent; data that passed the quality check criteria were evaluable in 499 of 617 patients.
Statistical analysis
The event-specific cumulative incidence of CNS relapse and time to CNS relapse were estimated with Kaplan-Meier statistics. The impact of variables of interest (CNS-IPI, COO, BCL2/MYC dual-expression status, CDKN2A alteration, and the GOYA study randomization stratification factors [number of planned chemotherapy cycles, geographical region]) on CNS relapse was assessed using univariate and multivariate Cox regression models. In these models, the end point of interest was time to CNS relapse, defined through the manual review of patients with disease progression or a death event at the time of the primary analysis cutoff (29 April 2016). The significance level of 5% was used consistently; all tests are 2-sided. No multiplicity adjustment was performed in order to avoid loss of power due to the low number of events, which is a structural limitation of such rare phenomena. R statistical software package version 3.4.0,26 together with RStudio version 1.0.153,27 was used for all analyses.
Results
Overall, 1418 DLBCL patients, randomized and treated with G-CHOP (n = 706) or R-CHOP (n = 712) in GOYA, were analyzed for CNS relapse occurrence. Baseline characteristics are shown in Table 1. According to CNS-IPI score, 279 (19.7%) patients were categorized as being at low risk (0-1), 894 (63.0%) patients were categorized as being at intermediate risk (2-3), and 245 (17.3%) patients were categorized as being at high risk (4-6) for developing CNS relapse. COO was available for 933 patients, of whom 540 (57.9%), 243 (26.0%), and 150 (16.1%) were classified as having GCB, ABC, and unclassified DLBCL, respectively. Both COO and BCL2/MYC protein expression were available in 688 patients; 295 (42.9%) were BCL2/MYC dual expressers. More patients with ABC DLBCL were BCL2/MYC dual expressers compared with GCB or unclassified DLBCL (136 [70.5%] vs 117 [30.7%] vs 42 [36.8%], respectively; Table 2).
Key baseline clinical characteristics (CNS-IPI risk factors, CNS-IPI score) of patients who developed CNS relapse compared with patients with no CNS relapse and the overall GOYA study population
| Characteristic . | CNS relapse (n = 38) . | No CNS relapse (n = 1380) . | All patients (N = 1418) . |
|---|---|---|---|
| Age, median (range), y | 66.5 (21-81) | 61.0 (18-86) | 62.0 (18-86) |
| <60 | 13 (34.2) | 591 (42.8) | 604 (42.6) |
| ≥60 | 25 (65.8) | 789 (57.2) | 814 (57.4) |
| ECOG PS | |||
| 0-1 | 31 (81.6) | 1200 (87.0) | 1231 (86.9) |
| 2-3 | 7 (18.4) | 179 (13.0) | 186 (13.1) |
| Ann Arbor stage | |||
| I or II | 4 (10.5) | 337 (24.4) | 341 (24.1) |
| III or IV | 34 (89.5) | 1042 (75.6) | 1076 (75.9) |
| Elevated LDH | 26 (68.4) | 790 (57.5) | 816 (57.7) |
| Extranodal sites, n | |||
| 0-1 | 15 (39.5) | 900 (65.2) | 915 (64.5) |
| >1 | 23 (60.5) | 480 (34.8) | 503 (35.5) |
| Involvement of kidneys and/or adrenal glands | 11 (28.9) | 80 (5.8) | 91 (6.4) |
| CNS-IPI | |||
| Low (0-1) | 4 (10.5) | 275 (20.0) | 279 (19.7) |
| Intermediate (2-3) | 16 (42.1) | 878 (63.6) | 894 (63.0) |
| High (4-6) | 18 (47.4) | 227 (16.5) | 245 (17.3) |
| Characteristic . | CNS relapse (n = 38) . | No CNS relapse (n = 1380) . | All patients (N = 1418) . |
|---|---|---|---|
| Age, median (range), y | 66.5 (21-81) | 61.0 (18-86) | 62.0 (18-86) |
| <60 | 13 (34.2) | 591 (42.8) | 604 (42.6) |
| ≥60 | 25 (65.8) | 789 (57.2) | 814 (57.4) |
| ECOG PS | |||
| 0-1 | 31 (81.6) | 1200 (87.0) | 1231 (86.9) |
| 2-3 | 7 (18.4) | 179 (13.0) | 186 (13.1) |
| Ann Arbor stage | |||
| I or II | 4 (10.5) | 337 (24.4) | 341 (24.1) |
| III or IV | 34 (89.5) | 1042 (75.6) | 1076 (75.9) |
| Elevated LDH | 26 (68.4) | 790 (57.5) | 816 (57.7) |
| Extranodal sites, n | |||
| 0-1 | 15 (39.5) | 900 (65.2) | 915 (64.5) |
| >1 | 23 (60.5) | 480 (34.8) | 503 (35.5) |
| Involvement of kidneys and/or adrenal glands | 11 (28.9) | 80 (5.8) | 91 (6.4) |
| CNS-IPI | |||
| Low (0-1) | 4 (10.5) | 275 (20.0) | 279 (19.7) |
| Intermediate (2-3) | 16 (42.1) | 878 (63.6) | 894 (63.0) |
| High (4-6) | 18 (47.4) | 227 (16.5) | 245 (17.3) |
Data are presented as n (%), unless otherwise noted. Data for Eastern Cooperative Oncology Group performance status (ECOG PS) and Ann Arbor Stage were not available in 1 case, and data on lactate dehydrogenase (LDH) were not available in 5 cases. Differences ≥ 10% between CNS relapse/no relapse groups are highlighted in bold type.
Key clinical and biomarker characteristics of patients with distinct COO subtypes: GCB, unclassified, and ABC DLBCL
| Characteristic . | GCB (n = 540) . | Unclassified (n = 150) . | ABC (n = 243) . |
|---|---|---|---|
| Age, median (range), y | 62.5 (18-83) | 62.0 (21-83) | 64.0 (29-86) |
| <60 | 228 (42.2) | 59 (39.3) | 70 (28.8) |
| ≥60 | 312 (57.8) | 91 (60.7) | 173 (71.2) |
| ECOG PS | |||
| 0-1 | 475 (88.1) | 126 (84.0) | 209 (86.0) |
| 2-3 | 64 (11.9) | 24 (16.0) | 34 (14.0) |
| Ann Arbor stage | |||
| I or II | 146 (27.0) | 34 (22.7) | 52 (21.4) |
| III or IV | 394 (73.0) | 116 (77.3) | 191 (78.6) |
| Elevated LDH | 308 (57.1) | 76 (50.7) | 169 (70.4) |
| Extranodal sites, n | |||
| 0-1 | 355 (65.7) | 95 (63.3) | 158 (65.0) |
| >1 | 185 (34.3) | 55 (36.7) | 85 (35.0) |
| Involvement of kidneys and/or adrenal glands | 36 (6.7) | 9 (6.0) | 13 (5.3) |
| CNS-IPI | |||
| Low (0-1) | 115 (21.3) | 29 (19.3) | 28 (11.5) |
| Intermediate (2-3) | 335 (62.0) | 97 (64.7) | 164 (67.5) |
| High (4-6) | 90 (16.7) | 24 (16.0) | 51 (21.0) |
| BCL2/MYC dual expression | n = 381 | n = 114 | n = 193 |
| Nodual expressers | 264 (69.3) | 72 (63.2) | 57 (29.5) |
| Dual expressers | 117 (30.7) | 42 (36.8) | 136 (70.5) |
| Characteristic . | GCB (n = 540) . | Unclassified (n = 150) . | ABC (n = 243) . |
|---|---|---|---|
| Age, median (range), y | 62.5 (18-83) | 62.0 (21-83) | 64.0 (29-86) |
| <60 | 228 (42.2) | 59 (39.3) | 70 (28.8) |
| ≥60 | 312 (57.8) | 91 (60.7) | 173 (71.2) |
| ECOG PS | |||
| 0-1 | 475 (88.1) | 126 (84.0) | 209 (86.0) |
| 2-3 | 64 (11.9) | 24 (16.0) | 34 (14.0) |
| Ann Arbor stage | |||
| I or II | 146 (27.0) | 34 (22.7) | 52 (21.4) |
| III or IV | 394 (73.0) | 116 (77.3) | 191 (78.6) |
| Elevated LDH | 308 (57.1) | 76 (50.7) | 169 (70.4) |
| Extranodal sites, n | |||
| 0-1 | 355 (65.7) | 95 (63.3) | 158 (65.0) |
| >1 | 185 (34.3) | 55 (36.7) | 85 (35.0) |
| Involvement of kidneys and/or adrenal glands | 36 (6.7) | 9 (6.0) | 13 (5.3) |
| CNS-IPI | |||
| Low (0-1) | 115 (21.3) | 29 (19.3) | 28 (11.5) |
| Intermediate (2-3) | 335 (62.0) | 97 (64.7) | 164 (67.5) |
| High (4-6) | 90 (16.7) | 24 (16.0) | 51 (21.0) |
| BCL2/MYC dual expression | n = 381 | n = 114 | n = 193 |
| Nodual expressers | 264 (69.3) | 72 (63.2) | 57 (29.5) |
| Dual expressers | 117 (30.7) | 42 (36.8) | 136 (70.5) |
Data are presented as n (%), unless otherwise noted. Data for ECOG PS were not available in 1 case, and data on LDH were not available in 3 cases.
Incidence and outcome of CNS relapse
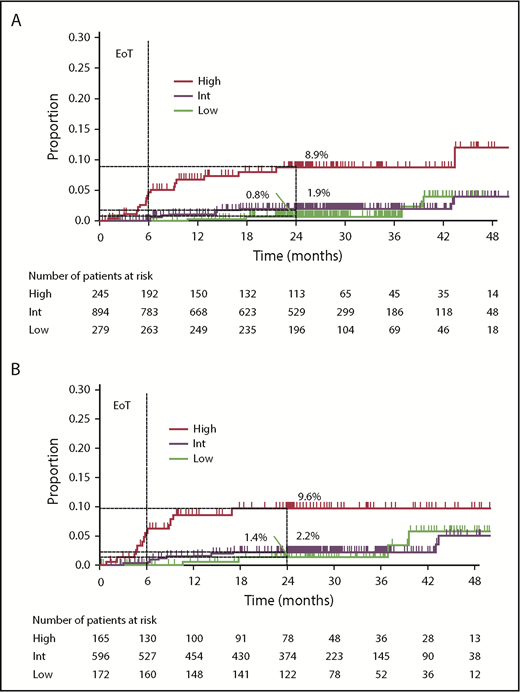
After a median observation of 29.0 months (interquartile range, 24.5-37.4), 38 (2.7%) of the 1418 patients developed CNS relapse (17 patients treated with chemotherapy only, 6 treated with chemotherapy and radiotherapy, 4 treated with radiotherapy only, 6 received no treatment; data not available in 5 patients); 37 of these had radiological signs of CNS relapse and/or infiltrated CSF. In 1 patient, CNS relapse (intraocular) was diagnosed via cytological evaluation of corpus vitreum. Most CNS relapses were localized in the brain parenchyma (parenchymal only, n = 27 [71.1%]; leptomeningeal only, n = 6 [15.8%]; parenchymal and leptomeningeal, n = 3 [7.9%]; intraocular, n = 1 [2.6%], and data not available, n = 1 [2.6%]). Median time to CNS relapse was 8.5 months (range, 0.9-43.5). The majority (34 [89.5%]) of CNS relapses occurred within 2 years of randomization. The 2-year CNS relapse rate for the entire cohort was 2.8%. Twenty-four (63%) of 38 patients with CNS relapse were dead at the time of the analysis; median survival after CNS relapse was 5.9 months. According to CNS-IPI, 10.5% of patients with CNS relapse were categorized as low risk, 42.1% were categorized as intermediate risk, and 47.4% were categorized as high risk. Two-year CNS relapse rates were 0.8% (95% confidence interval [CI], 0.0-1.9), 1.9% (95% CI, 0.9-2.9), and 8.9% (95% CI, 4.7-12.9) for the low-, intermediate-, and high-risk CNS-IPI subgroups, respectively (Figure 1A).
Risk for CNS relapse by CNS-IPI categories. (A) Overall GOYA study population (N = 1418); (B) population with COO data available (n = 933). EoT, end of treatment.
Risk for CNS relapse by CNS-IPI categories. (A) Overall GOYA study population (N = 1418); (B) population with COO data available (n = 933). EoT, end of treatment.
Treatment arm and prophylaxis with intrathecal chemotherapy and CNS relapse risk
The number of CNS relapses was similar in the G-CHOP and R-CHOP arms (20 vs 18, respectively), with no impact of treatment arm on the incidence of CNS relapse (hazard ratio [HR], 1.13; 95% CI, 0.60-2.15; P = .70). Overall, 140 (9.9%) of 1418 patients received intrathecal methotrexate or cytarabine or a combination of both as CNS relapse prophylaxis. Within the low-, intermediate-, and high-risk CNS-IPI groups, 16 (5.7%) of 279, 94 (10.5%) of 894, and 30 (12.2%) of 245 patients received intrathecal CNS relapse prophylaxis, respectively (supplemental Table 1, available on the Blood Web site). Two-year CNS relapse rates were not different between patients who did or did not receive CNS relapse prophylaxis (2.8% vs 2.6%). Similarly, the number of CNS relapses was not different in patients treated or not with prophylaxis in any of the CNS-IPI categories (0.0% vs 0.9%, 1.3% vs 2.0%, and 8.5% vs 9.0% for the low-, intermediate-, and high-risk CNS-IPI subgroups, respectively; supplemental Table 1).
COO and BCL2/MYC dual-expression status and CNS relapse risk
In patients with COO available (n = 933, 30 CNS-relapse events; supplemental Table 2), 2-year CNS relapse rates were 1.4% (95% CI, 0.0-3.2), 2.2% (95% CI, 0.9-3.5), and 9.6% (95% CI, 4.5-14.5) for the low-, intermediate-, and high-risk CNS-IPI subgroups, respectively (Figure 1B). On univariate analysis, patients with ABC and unclassified DLBCL had significantly higher CNS relapse risk than those with GCB DLBCL (HR, 5.2; 95% CI, 2.1-12.7; P = .0003; and HR, 4.2; 95% CI, 1.5-11.7; P = .005; respectively). Two-year CNS relapse rates were 6.9%, 4.8%, and 1.3% for patients with ABC, unclassified, and GCB DLBCL, respectively. There was no significant association between BCL2/MYC dual expression and the risk for CNS relapse on univariate analysis (HR, 1.5; 95% CI, 0.7-3.5; P = .3196; 2-year CNS relapse rate: dual-expressers 4.0% vs nondual expressers 2.2%; n = 688). In a multivariate analysis of the COO-available population (n = 933), ABC subtype (HR, 5.2; 95% CI, 2.1-12.9; P = .0004), unclassified COO subtype (HR, 4.2; 95% CI, 1.5-11.7; P = .006), and high CNS-IPI (HR, 4.0; 95% CI, 1.3-12.3; P = .02) were associated with greater CNS relapse risk (Table 3). In a multivariate analysis of the population with COO and BCL2/MYC dual-expression status available (n = 688, 22 CNS-relapse events; supplemental Table 2), there was no impact of BCL2/MYC dual expression (HR, 0.8; 95% CI, 0.3-2.1; P = .69) on CNS relapse risk, whereas the ABC and unclassified COO subtypes remained significantly associated with higher CNS relapse risk (Table 4). In this population, high CNS-IPI score was not significantly associated with CNS relapse risk, although a trend for greater risk was observed (HR, 2.8; 95% CI, 0.8-9.4; P = .10).
Results of multivariate Cox regression analysis on factors associated with CNS relapse in the COO-available population (n = 933)
| Factor . | HR* . | 95% CI . | P . |
|---|---|---|---|
| CNS-IPI intermediate (vs low) | 0.88 | 0.29-2.74 | .8312 |
| CNS-IPI high (vs low) | 3.97 | 1.28-12.33 | .0172 |
| ABC COO (vs GCB) | 5.18 | 2.09-12.87 | .0004 |
| Unclassified COO (vs GCB) | 4.18 | 1.50-11.66 | .0062 |
| Factor . | HR* . | 95% CI . | P . |
|---|---|---|---|
| CNS-IPI intermediate (vs low) | 0.88 | 0.29-2.74 | .8312 |
| CNS-IPI high (vs low) | 3.97 | 1.28-12.33 | .0172 |
| ABC COO (vs GCB) | 5.18 | 2.09-12.87 | .0004 |
| Unclassified COO (vs GCB) | 4.18 | 1.50-11.66 | .0062 |
CNS relapses, n = 30. Factors that were significantly associated with greater CNS relapse risk are highlighted in bold.
Adjusted for study randomization stratification factors (number of planned chemotherapy cycles, geographic region).
Results of multivariate Cox regression analysis on factors associated with CNS relapse in the COO and BCL2/MYC dual-expression status–available population (n = 688)
| Factor . | HR* . | 95% CI . | P . |
|---|---|---|---|
| CNS-IPI intermediate (vs low) | 0.75 | 0.23-2.45 | .6378 |
| CNS-IPI high (vs low) | 2.76 | 0.81-9.42 | .1042 |
| ABC COO (vs GCB) | 4.78 | 1.49-15.29 | .0084 |
| Unclassified COO (vs GCB) | 4.24 | 1.32-13.61 | .0151 |
| BCL2/MYC dual expresser (vs nondual expresser) | 0.83 | 0.34-2.06 | .6931 |
| Factor . | HR* . | 95% CI . | P . |
|---|---|---|---|
| CNS-IPI intermediate (vs low) | 0.75 | 0.23-2.45 | .6378 |
| CNS-IPI high (vs low) | 2.76 | 0.81-9.42 | .1042 |
| ABC COO (vs GCB) | 4.78 | 1.49-15.29 | .0084 |
| Unclassified COO (vs GCB) | 4.24 | 1.32-13.61 | .0151 |
| BCL2/MYC dual expresser (vs nondual expresser) | 0.83 | 0.34-2.06 | .6931 |
CNS relapses, n = 22. Factors that were significantly associated with greater CNS relapse risk are highlighted in bold.
Adjusted for study randomization stratification factors (number of planned chemotherapy cycles, geographic region).
Overall, 560 (39.5%) of 1418 patients had FISH results available. Twenty patients (3.6%) harbored both BCL2 and MYC translocations, of whom only 1 patient developed CNS relapse (FISH data were not included in the statistical analysis because of the low number of CNS relapses within the double-hit DLBCL).
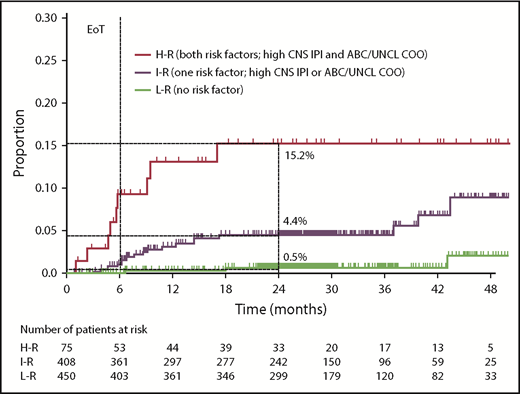
CNS-IPI and COO were combined (1 point for high CNS-IPI, 1 point for ABC or unclassified COO) to create a modified risk-stratification model, CNS-IPI-C. Three CNS-IPI-C subgroups were identified as having low (no risk factor, n = 450 [48.2%]), intermediate (1 risk factor, n = 408 [43.7%]), and high (2 risk factors, n = 75 [8.0%]) CNS relapse risk. The 2-year CNS relapse rates were 0.5% (95% CI, 0.0-1.3), 4.4% (95% CI, 2.2-6.6), and 15.2% (95% CI, 5.4-24.0), respectively, resulting in a 22-fold higher risk for CNS relapse in the high- vs low-risk groups (Figure 2; supplemental Table 3).
Risk for CNS relapse by CNS-IPI and COO (CNS-IPI-C) in the COO available population (n = 933). H-R, high risk; I-R, intermediate risk; L-R, low risk; UNCL, unclassified.
Risk for CNS relapse by CNS-IPI and COO (CNS-IPI-C) in the COO available population (n = 933). H-R, high risk; I-R, intermediate risk; L-R, low risk; UNCL, unclassified.
Mutational profile
Mutational profiles were available in 499 of 1418 patients (12 of 38 patients with CNS relapse; 487 of 1380 without CNS relapse; supplemental Table 2). A detailed description of all gene alterations for the patients with CNS relapse is listed in supplemental Table 4. CDKN2A was the most frequently (8 of 12; 66.6%) altered gene in patients who developed CNS relapse, with 7 cases having homozygous deletion of CDKN2A and 1 case harboring nonsynonymous CDKN2A mutation; in the population of patients without CNS relapse, the prevalence of CDKN2A gene alterations was 21.6% (105 of 487). On multivariate analysis, CDKN2A gene alterations were associated with higher risk for CNS relapse (HR, 7.2; 95% CI, 2.1-25.0; P = .002), independent of clinical factors. The impact of CDKN2A gene alterations on CNS relapse risk was weakened after inclusion of COO in the model (HR, 3.6; 95% CI, 0.93-14.0; P = .064). Alterations of genes known to deregulate NF-κB signaling were also observed, such as mutations of MYD88, which were found in 5 (42%) of 12 cases compared with 78 (16.0%) of 487 cases in the cohort with no CNS relapse. Three of the 5 patients with MYD88 mutation had simultaneous CD79B mutation.
Discussion
The current analysis of GOYA evaluated risk factors associated with CNS relapse in newly diagnosed DLBCL patients treated with anti-CD20–based immunochemotherapy (R-CHOP or G-CHOP). We found no difference in CNS relapse risk between R and G, with the incidence of CNS relapse similar in both treatment arms and consistent with the literature.1
With these data, we have provided an independent validation of the CNS-IPI prognostic model.1 Patients with high CNS-IPI scores had significantly higher risk for CNS relapse than did those with intermediate or low CNS-IPI scores. High CNS-IPI score was also an independent risk factor for CNS relapse on multivariate analysis. The 2-year CNS relapse rate for the high-risk CNS-IPI subgroup in GOYA (8.9%) was consistent with previous data from Schmitz et al (10.2%).1 No significant difference in the incidence of CNS relapse was observed between the intermediate- and low-risk CNS-IPI groups. This may be due to differences in baseline patient characteristics in the German High-Grade Non-Hodgkin Lymphoma Study Group and MabThera International Trial (testing cohort for CNS-IPI building) and GOYA study cohorts.1 In the current study, we confirmed that CNS-IPI is a valuable clinical tool for identification of DLBCL patients with high CNS relapse risk.
Most primary DLBCLs of the CNS resemble the ABC subtype, suggesting that this biological subtype may be prone to CNS infiltration.21 In the current study, patients with ABC and unclassified DLBCL had significantly higher CNS relapse risk compared with those with GCB DLBCL, and, in the multivariate analysis, COO and a high CNS-IPI score were shown to be independent risk factors for CNS relapse. Previous data from Savage et al showed that BCL2/MYC dual expression is associated with a higher probability of CNS relapse.9 Given the association of the ABC subtype with dual expression of BCL2 and MYC proteins, we analyzed whether the higher risk of CNS relapse, at least in patients with ABC DLBCL, is related to the high prevalence of BCL2/MYC dual expression. Surprisingly, we did not observe a higher incidence of CNS relapse in BCL2/MYC dual expressers compared with nondual expressers in univariate or multivariate analyses, which may be due to the higher prevalence of BCL2/MYC dual expression (driven by a high rate of MYC positivity) in the GOYA study compared with the population examined by Savage et al (42.1% vs 29.7%, respectively).9,23 The reason for the high rate of MYC positivity detected in the GOYA study is not entirely clear. One possible explanation is that the proportion of patients enrolled with low IPI scores (or low CNS-IPI) was relatively low, and, therefore, there was a high proportion of high-risk patients who are more likely to be BCL2/MYC dual expressers. Larger studies may provide further insight.
Primary CNS lymphomas frequently, if not uniformly, exhibit biallelic loss of CDKN2A, resulting in cell cycle and p53 pathway deregulation, or mutations in MYD88 and CD79B, thereby deregulating NF-κB and B-cell receptor signaling.21,28-31 Although data on the mutational profile were only available for a limited number of patients, CDKN2A loss and mutation of MYD88 were the most commonly observed alterations in patients with CNS relapse. In the multivariate analysis, CDKN2A loss was associated with a higher risk for CNS relapse, independent of clinical factors. However, the impact of CDKN2A loss on the risk of CNS relapse was weaker in a model that included COO, probably due to the association of CDKN2A alterations with the ABC subtype, which has been demonstrated in GOYA, as well as in other studies.32,33 Because of the limited number of patients with CNS relapse and mutational profile data available in the GOYA study, further studies are needed to confirm our hypothesis and to explore the impact of specific gene alterations on the risk of CNS relapse, especially in the context of particular COO subtypes.
Because ABC/unclassified COO subtypes and high CNS-IPI score were independent risk factors for CNS relapse, we combined both factors to improve the risk-stratification ability of CNS-IPI, resulting in a modified CNS-IPI-C model. CNS-IPI-C allowed the identification of 3 subgroups with different 2-year CNS relapse risks. This incorporation of biomarkers into the CNS-IPI-C model improved the discrimination of subgroups with a very low and high 2-year CNS relapse risk compared with the CNS-IPI model (2-year relapse rate in low- and high-risk subgroups 0.5% vs 1.4% and 15.2% vs 9.6%, respectively). This could help to identify patients who should undergo a more comprehensive examination of the CNS to exclude asymptomatic CNS lymphoma involvement. It may also identify patients who could potentially benefit from treatment with effective prophylaxis to reduce CNS relapse risk.34,35 Last, but not least, CNS-IPI-C identifies a large subgroup of patients with a very low risk for CNS relapse who could be spared invasive diagnostic and prophylactic interventions. However, it must be noted that CNS-IPI-C needs to be validated in an independent cohort of DLBCL patients before its potential clinical use.
There is growing evidence that CNS prophylaxis with intrathecal methotrexate is not sufficient to prevent CNS relapse.5,36 Some trials indicate that high-dose IV methotrexate (3 g/m2) can prevent CNS relapse37 ; however, treatment can be associated with significant toxicity, and an acceptable risk-benefit ratio should be carefully considered. Overall, 9.9% of patients were treated with prophylactic intrathecal chemotherapy in GOYA. We did not observe a significant difference in the incidence of CNS relapse in patients who did or did not receive intrathecal chemotherapy, either in the entire cohort or in the different risk groups according to CNS-IPI. However, it must be noted that GOYA was not designed to assess the impact of CNS prophylaxis on CNS relapse risk. CNS prophylaxis was indicated and administered upon investigator decision, based on institutional practice, resulting in heterogeneous schedules and doses. Randomized clinical trials would be required to define appropriate CNS prophylaxis in DLBCL.
In conclusion, using the largest prospective dataset of previously untreated DLBCL with relevant biomarker data to date, we validated the CNS-IPI clinical prognostic model and demonstrated that ABC and unclassified DLBCL are associated with higher CNS relapse risk compared with GCB DLBCL. Combining CNS-IPI and COO helped to improve stratification of DLBCL patients with different CNS relapse risks.
Presented in part at the 14th International Conference for Malignant Lymphoma, Lugano, Switzerland, 14 June 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the GOYA study team, investigators, nurses, and patients for their contributions and participation.
GOYA was sponsored by F. Hoffmann-La Roche Ltd. with scientific support from the Fondazione Italiana Linfomi. Third-party editorial assistance, under the direction of M.K., was provided by Lynda McEvoy of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche Ltd.
The funder was involved in trial design, administration or conduct of study procedures, coordination of data collection, and data analysis and interpretation. The corresponding authors (M.K. and M.T.) and Roche authors (E.A.P., E.S.-G., C.R.B., M.Z.O., G.R.F.-R., and T.N.) had access to all data in the study; all other authors had access to the final study report.
Authorship
Contribution: M.K., M.Z.O., G.R.F.-R., T.N., and M.T. conceived and designed the analysis, and L.H.S., I.B.-B., F.C., J.J., M.M., D.S., U.V., F.Z., Q.Z., and M.T. provided study materials or patients; all authors contributed to analyzing and interpreting the data and writing of the manuscript and provided final approval of the manuscript; and M.K. had final responsibility for the decision to submit the manuscript for publication.
Conflict-of-interest disclosure: M.K. was employed by F. Hoffmann-La Roche Ltd. during the time of the analysis. L.H.S. has acted as a consultant for and received honoraria from Roche/Genentech, Amgen, Janssen, Celgene, AbbVie, and Seattle Genetics. I.B.-B. has served as a member of the advisory board for F. Hoffmann-La Roche Ltd. M.M. has acted as a consultant for F. Hoffmann-La Roche Ltd., Janssen, Sandoz, Takeda, Celgene, and Mundipharma; has served as a member of the speaker’s bureau for F. Hoffman-La Roche Ltd. and Takeda; and has served on the advisory board for F. Hoffmann-La Roche Ltd., Janssen, and Sandoz. D.S. has served on the advisory board for, and received research funding from, as part of a clinical trial, F. Hoffmann-La Roche Ltd. U.V. has received research funding from F. Hoffmann-La Roche Ltd.; has received honoraria from F. Hoffmann-La Roche Ltd., Takeda, and Gilead; and has served as a member of the advisory board for F. Hoffmann-La Roche Ltd., Celgene, and Janssen. F.Z. has acted as a consult or advisor for F. Hoffmann-La Roche Ltd., Janssen, Novartis, and Celgene; has served as a member of the speaker’s bureau for F. Hoffmann-La Roche Ltd., Celgene, Novartis, Gilead, and Takeda; and has received research funding from Celgene and Novartis. F.M., G.S., M.Z.O., G.R.F.-R., and T.N. are employees of F. Hoffmann-La Roche Ltd., and G.R.F.-R. owns stock in F. Hoffmann-La Roche Ltd. E.A.P., E.S.-G., and C.R.B. are employees of Genentech Inc. M.T. has received honoraria, research funding, travel, accommodations and/or expenses from, and has acted as a consultant or advisor to, F. Hoffmann-La Roche Ltd. The remaining authors declare no competing financial interests.
Correspondence: Magdalena Klanova, 1st Department of Medicine, Charles University General Hospital, U Nemocnice 2, Prague 2, 12808, Czech Republic; e-mail: magdalena.klanova@gmail.com; and Marek Trneny, 1st Department of Medicine, Charles University General Hospital, U Nemocnice 2, Prague 2, 12808, Czech Republic; e-mail: trneny@cesnet.cz.