Key Points
Seven of 10 patients with cold agglutinin disease responded to the anti-C1s antibody with a median 4-g/dL increase in hemoglobin levels.
Upstream inhibition of the classical complement cascade is an effective treatment for patients with cold agglutinin disease.
Abstract
Cold agglutinin disease is a difficult-to-treat autoimmune hemolytic anemia in which immunoglobulin M antibodies bind to erythrocytes and fix complement, resulting in predominantly extravascular hemolysis. This trial tested the hypothesis that the anti-C1s antibody sutimlimab would ameliorate hemolytic anemia. Ten patients with cold agglutinin disease participated in the phase 1b component of a first-in-human trial. Patients received a test dose of 10-mg/kg sutimlimab followed by a full dose of 60 mg/kg 1 to 4 days later and 3 additional weekly doses of 60 mg/kg. All infusions were well tolerated without premedication. No drug-related serious adverse events were observed. Seven of 10 patients with cold agglutinin disease responded with a hemoglobin increase >2 g/dL. Sutimlimab rapidly increased hemoglobin levels by a median of 1.6 g/dL within the first week, and by a median of 3.9 g/dL (interquartile range, 1.3-4.5 g/dL; 95% confidence interval, 2.1-4.5) within 6 weeks (P = .005). Sutimlimab rapidly abrogated extravascular hemolysis, normalizing bilirubin levels within 24 hours in most patients and normalizing haptoglobin levels in 4 patients within 1 week. Hemolytic anemia recurred when drug levels were cleared from the circulation 3 to 4 weeks after the last dose of sutimlimab. Reexposure to sutimlimab in a named patient program recapitulated the control of hemolytic anemia. All 6 previously transfused patients became transfusion-free during treatment. Sutimlimab was safe, well tolerated, and rapidly stopped C1s complement–mediated hemolysis in patients with cold agglutinin disease, significantly increasing hemoglobin levels and precluding the need for transfusions. This trial was registered at www.clinicaltrials.gov as #NCT02502903.
Introduction
Cold agglutinin disease is a subtype of autoimmune hemolytic anemia (AIHA), usually caused by high concentrations of circulating immunoglobulin M autoantibodies (cold agglutinins), which bind to the “I” antigen on erythrocytes.1-3 Cold agglutinins preferentially bind to erythrocytes at lower-than-core body temperature and can cause erythrocyte agglutination due to their multivalent structure.4 The ensuing activation of the classical pathway of complement leads C1 esterase to activate C2 and C4, generating the C3 convertase, which cleaves C3 to C3a and C3b that opsonizes erythrocytes.5 These are subsequently phagocytosed by the liver6,7 (Figure 1). This extravascular hemolysis is considered to be the predominant mechanism of erythrocyte destruction in patients with cold agglutinin disease.8,9 Intravascular hemolysis can occur by the cleavage of complement component 5 (C5) and formation of the membrane attack complex in some patients10 but is largely curtailed by the presence of complement regulatory proteins (CD55 and CD59) on the erythrocyte surface. However, the limited hemoglobin increase (<1 g/dL) after treatment with the C5 inhibitor eculizumab emphasizes the need to target upstream in the classical pathway to prevent complement opsonization in patients with cold agglutinin disease.11 Blocking C1, the most upstream component of the classical pathway, seems more promising: a mouse monoclonal antibody (mAb) that inhibits the classical complement pathway–specific protease C1s prevented samples from patients with cold agglutinin disease from inducing complement deposition on human erythrocytes, thereby rescuing them from subsequent phagocytosis by macrophages in vitro.12
Extravascular hemolysis caused by cold agglutinin–induced complement-mediated opsonization. Cold agglutinins (mostly pentameric immunoglobulin M [IgM]) agglutinate erythrocytes and fix C1, triggering the classical complement cascade and leading to C3 split product opsonization of the red blood cell. Complement-opsonized erythrocytes then travel to the liver where they are phagocytosed, a process known as extravascular hemolysis. Although complement-mediated intravascular hemolysis can occur, which requires C5 cleavage and formation of the membrane attack complex, it is generally prevented by complement regulatory proteins on the erythrocyte surface (ie, CD55 and CD59). Regardless of the hemolytic mechanism, upstream C1s blockade prevents both extravascular and intravascular hemolysis. Figure adapted and modified from Berentsen and Sundic4 and from Shi et al.12
Extravascular hemolysis caused by cold agglutinin–induced complement-mediated opsonization. Cold agglutinins (mostly pentameric immunoglobulin M [IgM]) agglutinate erythrocytes and fix C1, triggering the classical complement cascade and leading to C3 split product opsonization of the red blood cell. Complement-opsonized erythrocytes then travel to the liver where they are phagocytosed, a process known as extravascular hemolysis. Although complement-mediated intravascular hemolysis can occur, which requires C5 cleavage and formation of the membrane attack complex, it is generally prevented by complement regulatory proteins on the erythrocyte surface (ie, CD55 and CD59). Regardless of the hemolytic mechanism, upstream C1s blockade prevents both extravascular and intravascular hemolysis. Figure adapted and modified from Berentsen and Sundic4 and from Shi et al.12
Primary cold agglutinin disease is associated with a low-grade clonal B-cell lymphoproliferative disorder.13,14 Secondary forms, referred to as secondary cold agglutinin syndrome, result from an underlying condition such as aggressive lymphoma in adults9 or Mycoplasma pneumoniae or Epstein-Barr virus infections.15 At first presentation, hemoglobin levels vary substantially between patients: average hemoglobin levels ranged from 8.2 to 10.2 g/dL,15-17 and 45% of patients had severe anemia (<8 g/dL) in another study.18
Anemia can be life-threatening18 and complicated by thromboembolic events.19 No drugs have been approved for the treatment of cold agglutinin disease. Corticosteroids are generally ineffective and require unacceptably high doses to maintain clinical benefit in those who do respond.9,15,19 The anti-CD20 antibody rituximab depletes B cells9 and induces mainly partial responses in approximately one-half of patients after an average delay of 1.5 months.9,14 Relapses frequently occur within 1 year.20,21 The combination of rituximab with cytostatic agents increases the response rates and duration of responses, but they are often accompanied by pronounced toxicity.22,23 Secondary cases of cold agglutinin disease may respond to antilymphoma therapy.1,24,25 However, patients may remain transfusion-dependent despite previous therapies.17 Although fatalities have been reported,26 transfusions can be safely administered if indicated; however, clinical benefit may be fleeting owing to cold agglutinin–mediated complement attack on the donor erythrocytes in the circulation of the patient. Furthermore, chronic transfusions may be complicated by alloimmunization and iron overload.27 Thus, there remains an unmet medical need for alternative nontoxic treatments to rapidly and permanently control anemia.
We therefore hypothesized that the humanized anti-C1s antibody sutimlimab (previously BIVV009 or TNT009) would be able to correct anemia in patients with cold agglutinin disease. Because of the rarity of cold agglutinin disease, we leveraged the rapid on/off effects observed in the phase 1 trial to show causality of the treatment effects in a series of N-of-1 trials. N-of-1 trials are multiple crossover trials in individual patients; our specific case involved multiple crossover periods between on-treatment and off-treatment (a withdrawal design utilizing the rapid on/off effects) using different doses to facilitate dose finding for a biweekly regimen.
Materials and methods
Trial design
This study was a first-in-human trial using an integrated protocol design that studied single- and multiple-ascending doses of sutimlimab in a randomized, placebo-controlled setting in healthy volunteers (phase 1a), as well as a prospective open-label trial design in 4 different diseases that share a common underlying pathophysiology (ie, antibody-mediated complement activation) (phase 1b). The rationale for this trial design has been explained in more detail elsewhere.28 The present article primarily focuses on the observed efficacy data of sutimlimab in the patient group affected by cold agglutinin disease (treated from January 2016 until March 2017) and is augmented by a series of N-of-1 trials to confirm the cause-and-effect relationship upon re-exposure to the drug under a named patient program. To exclude spontaneous regression to the mean, we studied the reversal of effects (ie, recurrence of anemia and hemolysis) when the drug washed out and its recapitulation upon re-challenge. This concept was adapted from the guideline of Clinical Trials in Small Populations (CHMP/EWP/83561/2005) and represents a series of nonrandomized N-of-1 trials with several crossover periods from “on-treatment” to “off-treatment.” The duration of the off-treatment periods was mainly driven by the recurrence of hemolysis and severe anemia after discontinuation of treatment, with simultaneous avoidance of unnecessary transfusions between periods.
The trial protocol and its amendments were approved by the National Competent Authority and the Ethics Committee of the Medical University of Vienna, which is the tertiary care center where the trial was performed. This trial was registered at www.clinicaltrials.gov as #NCT02502903 and at the European Clinical Trials Database as #2014-003881-26. To request data, the Medical Information department at Bioverativ, a Sanofi company (medinfo@bioverativ.com), can be contacted.
Patients
Inclusion criteria comprised patients ≥18 years of age who were previously vaccinated against encapsulated bacterial pathogens (Neisseria meningitidis, Haemophilus influenzae, and Streptococcus pneumoniae) or willing to undergo vaccination and were able to give informed consent. The diagnosis of cold agglutinin disease was established by active hemolytic anemia, a cold agglutinin titer ≥64 at 4°C, and a direct antiglobulin test (DAT) result that was negative or only weakly positive for immunoglobulin G (IgG) but strongly positive for C3d. Initially, patients with a hemoglobin level <12 g/dL were allowed in the trial; this criterion was later amended to a hemoglobin level <11 g/dL. Exclusion criteria were active infection within the preceding month, an autoimmune disorder other than cold agglutinin disease, other known complement-mediated disorders, known malignancy (other than lymphoproliferative disorders causally related to the complement-mediated diseases under study, or locally limited and previously removed basal cell carcinoma of the skin), clinically significant hepatobiliary disorder, history of infusion hypersensitivity, allergic or anaphylactic reactions to other therapeutic proteins, substance abuse, inability to understand or follow the trial procedure, women of childbearing age not using contraception, concurrent treatment with other experimental drugs or participation in another trial with any investigational drug within 30 days before treatment start or rituximab treatment within the last 3 months, and body weight >98 kg.
Treatment
Sutimlimab is a humanized anti-C1s IgG4 mAb that has attained orphan drug designation in the United States and the European Union. Patients underwent a screening examination and could start drug infusions a minimum of 14 days after vaccinations against N meningitidis, H influenzae, and S pneumoniae. Upon request by the Austrian National Competent Authority, an initial 10-mg/kg IV test dose of sutimlimab was infused to allow a rapid washout of drug in case of unforeseen adverse effects upon first infusion. One to 4 days later, patients received a full 60-mg/kg dose, followed by 3 additional 60-mg/kg infusions at weekly intervals. Patients were observed for a total of 49 to 53 days in accordance with the protocol. Treatment in the series of N-of-1 trials is described in the supplemental Appendix, available on the Blood Web site.
Transfusion policy
In general, the transfusion policy of the Medical University of Vienna’s Division of Hematology is restrictive and foresees transfusions for patients with autoimmune hemolysis when hemoglobin levels are <6 to 8 g/dL if patients are stable and asymptomatic. However, symptomatic patients and/or those with heart disease may receive transfusions at higher hemoglobin levels.
Laboratory analysis
All laboratory parameters were measured in a fully automated manner at the central laboratory of the Medical University of Vienna. Technicians performed microscopic differential blood counts. All samples were collected by fresh venipunctures into 37°C prewarmed evacuated blood tubes and transported in a prewarmed steel block. In some instances, strong agglutination and ex vivo hemolysis occurred after blood withdrawal, which prevented accurate measurements of secondary outcome parameters. The DAT specimen was analyzed with LISS/Coombs Gel Cards (monospecific IgM, immunoglobulin A, IgG, C3c, and C3d; Bio-Rad GmbH, Vienna, Austria). The supplemental Appendix includes additional information on flow cytometric analysis of erythrocytes.
Serum sutimlimab levels were determined by using a direct binding enzyme–linked immunosorbent assay in which C1s-coated plates were used to capture free sutimlimab, detected by using a goat anti-human horseradish peroxidase–conjugated secondary antibody, and developed with the colorimetric substrate 3,3′,5,5′-tetramethylbenzidine. Pharmacokinetic analysis was performed by Certara Inc. (Princeton, NJ). Pharmacokinetic parameters of serum sutimlimab were calculated by using a validated version of WinNonlin Enterprise version 5.2 (Certara Inc.). Pharmacodynamic parameters of classical complement pathway were calculated by using a validated version of Phoenix WinNonlin version 6.3 (Certara Inc.). Modeling of serum sutimlimab and complement pathway activity was performed by using Phoenix NLME version 7.0 (Certara Inc.) via the first-order conditional estimation–extended least squares minimization algorithm.
Statistical analysis
A sample size calculation was not performed for this trial in patients with cold agglutinin disease because no data were available to estimate the effect size and variability thereof. The primary outcome variable of interest in patients with cold agglutinin disease is the hemoglobin level because it determines symptoms and circulatory instability and is the main trigger for transfusions. Hemoglobin changes are expressed as 95% confidence intervals. Strong ex vivo agglutination of erythrocytes occasionally resulted in unmeasurable values for reticulocyte counts, lactate dehydrogenase, and DAT despite using sampling tubes preheated to 37°C. However, no missing data were imputed. A clinically meaningful response was defined as a ≥2-g/dL increase in hemoglobin. No inferential statistical testing was planned, but a Friedman analysis of variance was used for time courses, and a Wilcoxon test was used for differences between baseline and maximum individual effects. The other biomarkers are nonindependent secondary outcome parameters; hence, no correction for multiplicity was performed. Data are summarized descriptively by using median and the interquartile range (IQR). In cases in which values were below the detection limit of the assays, values of the detection limit minus 1 were assigned for statistical comparisons (eg, haptoglobin = 11 mg/dL instead of <12 mg/dL).
Results
Study population
Patient characteristics are shown in Table 1, illustrating multiple lines of previous treatments in most patients, including 2 who recently failed treatment with eculizumab. Thirteen patients with a history of cold agglutinin disease were screened; 3 female patients were excluded because of iron-deficiency anemia and inactive cold agglutinin disease, negative cold agglutinin titer, or hemoglobin levels >11 g/dL. Ten patients (1 Asian, 1 Hispanic, and 8 white patients) were eventually included, with a median disease duration of 5 years (range, 1-20 years). Three patients were referred to the trial while receiving moderate doses of prednisolone (10-25 mg/d), which were reduced to <10 mg on the first trial day and could be tapered and discontinued within the first weeks of sutimlimab treatment.
Baseline characteristics of patients with cold agglutinin disease
| Patient . | Additional diagnosis . | Disease duration, y . | Age, y . | Sex . | Previous treatment . | Thromboembolic events . | Recent transfusion history . |
|---|---|---|---|---|---|---|---|
| C1001 | After LPL therapy | 4 | 70 | F | Steroids, bendamustine/rituximab | Venous thrombosis | 2 U 2014, 4 U 2015 |
| C1002 | 10 | 76 | F | Steroids, rituximab, IV immunoglobulins | None | 4 U 2014, 2 U before inclusion | |
| C1003 | LPL MYD88+ | 10 | 68 | F | Steroids | None | Never |
| C1004 | 1 | 74 | F | Steroids, erythropoietin previously and on study | Stroke | 4 U before inclusion | |
| C1006 | 4 | 70 | F | Steroids, azathioprine | None | 2-4 U monthly | |
| C1008 | 1 | 76 | F | Steroids, IV immunoglobulins | Pulmonary embolism | 4 U 2016 | |
| C1009 | LPL MYD88+ | 20 | 68 | M | None | None | Never |
| C1010 | After LPL therapy MYD88+ | 5 | 56 | F | R-CVP, rituximab, rituximab/fludarabine, eculizumab, R-ESHAP, erythropoietin (on study) | None | 4 U monthly |
| C1011 | Mixed AIHA (IgG+2) after LPL therapy | 12 | 76 | M | Cyclophosphamide, azathioprine, mycophenolate, rituximab, bendamustine/rituximab, eculizumab | None | 20 U 2015, 8 U 2016 |
| C1013 | Indolent* lymphoma | 3 | 59 | F | Steroids, rituximab | None | 7 U 2015 |
| Patient . | Additional diagnosis . | Disease duration, y . | Age, y . | Sex . | Previous treatment . | Thromboembolic events . | Recent transfusion history . |
|---|---|---|---|---|---|---|---|
| C1001 | After LPL therapy | 4 | 70 | F | Steroids, bendamustine/rituximab | Venous thrombosis | 2 U 2014, 4 U 2015 |
| C1002 | 10 | 76 | F | Steroids, rituximab, IV immunoglobulins | None | 4 U 2014, 2 U before inclusion | |
| C1003 | LPL MYD88+ | 10 | 68 | F | Steroids | None | Never |
| C1004 | 1 | 74 | F | Steroids, erythropoietin previously and on study | Stroke | 4 U before inclusion | |
| C1006 | 4 | 70 | F | Steroids, azathioprine | None | 2-4 U monthly | |
| C1008 | 1 | 76 | F | Steroids, IV immunoglobulins | Pulmonary embolism | 4 U 2016 | |
| C1009 | LPL MYD88+ | 20 | 68 | M | None | None | Never |
| C1010 | After LPL therapy MYD88+ | 5 | 56 | F | R-CVP, rituximab, rituximab/fludarabine, eculizumab, R-ESHAP, erythropoietin (on study) | None | 4 U monthly |
| C1011 | Mixed AIHA (IgG+2) after LPL therapy | 12 | 76 | M | Cyclophosphamide, azathioprine, mycophenolate, rituximab, bendamustine/rituximab, eculizumab | None | 20 U 2015, 8 U 2016 |
| C1013 | Indolent* lymphoma | 3 | 59 | F | Steroids, rituximab | None | 7 U 2015 |
Transfusions within 6 months of inclusion are presented in boldface.
F, female; LPL, lymphoplasmacytic lymphoma; M, male; R-CVP, rituximab with cyclophosphamide, vincristine, and prednisone; R-ESHAP, rituximab plus etoposide, cytarabine, cisplatin, and methylprednisolone; U, units of packed red blood cells.
CD19++, CD5–, CD10–, CD20+, CD22+, CD79b+, CD81+, FMC7+, partially CD38+, immunoglobulin M (IgM)+.
Pharmacokinetic and pharmacodynamic variables
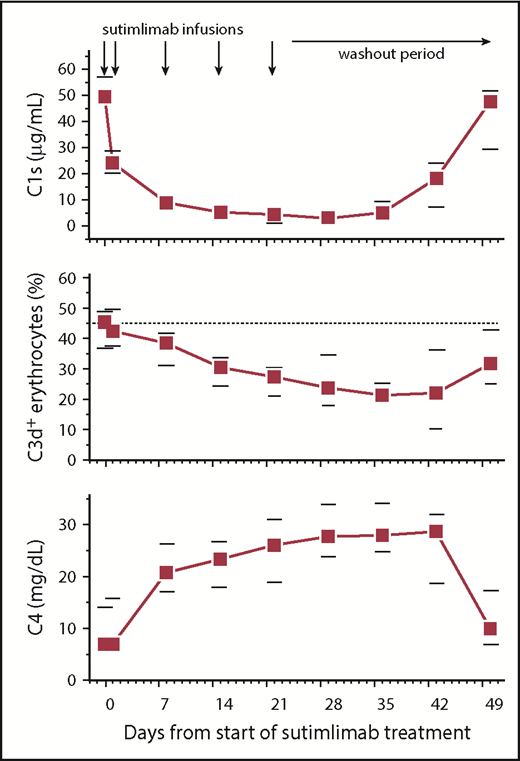
Analysis of sutimlimab pharmacokinetic variables revealed median maximal concentrations of 1885 µg/mL (supplemental Figure 1), and sutimlimab levels remained well above the level of 15.5 µg/mL required to inhibit classical complement pathway activation by >90%29 for 22 days (n = 9) or 28 days (n = 1) after the last dose, maintaining complement inhibition for at least 6 weeks. Only 1 patient developed low levels (22 ng/mL) of antidrug antibodies, which had no apparent impact on the sutimlimab concentrations (which were >1000-fold higher) or safety. In addition to blockade of C1s activity,12 the mechanism of action of sutimlimab also involves removal of C1s from the circulation (Figure 2). Sutimlimab significantly reduced total serum complement activity measured by CH50 from a median of 52% (IQR, 21%-104%) at baseline to 22% (IQR, 18%-29%) within 24 hours (P = .02). This effect was less apparent in the 3 patients with a concurrent lymphoma whose baseline CH50 levels were the lowest (<8% to 21%). Plasma levels of complement component C4, the first substrate cleaved by C1s, were initially low at baseline (as has been previously shown in patients with cold agglutinin disease) but steadily increased by 3.8-fold over the course of the study (P = .008). Circulating C3d-positive erythrocytes decreased from 40% (IQR, 27%-49%) to a nadir of 21% (IQR, 14%-27%) 5 weeks after the first dose (P = .02). These in vivo measures of pharmacodynamic activity are consistent with the mechanism of action of sutimlimab.
Sutimlimab infusion reduces plasma levels of complement C1s, gradually reduces the percentage of C3d-positive (C3d+) erythrocytes, and increases plasma C4 concentrations. Data are presented as medians with 25th to 75th percentiles for 10 patients.
Sutimlimab infusion reduces plasma levels of complement C1s, gradually reduces the percentage of C3d-positive (C3d+) erythrocytes, and increases plasma C4 concentrations. Data are presented as medians with 25th to 75th percentiles for 10 patients.
Sutimlimab increases hemoglobin levels and rapidly inhibits hemolysis
Ten patients with cold agglutinin disease were treated with sutimlimab. Median hemoglobin levels increased by 1.6 g/dL within the first week (P = .007) and by a median best response of 3.9 g/dL (IQR, 1.3-4.5 g/dL; 95% confidence interval, 2.1-4.5; P = .005) after 6 weeks (Figure 3). In 7 of 10 patients, the hemoglobin level increased by >2 g/dL, including those recently failing to respond to or relapsing after treatment with rituximab, rituximab plus bendamustine, or eculizumab. Furthermore, hemoglobin levels increased by ≥4 g/dL in 5 patients and completely normalized (≥12 g/dL) in 4 patients. Reticulocyte counts increased by a median of 41% within the first 24 hours (P = .0381) and then gradually declined as hemoglobin levels rose.
Sutimlimab rapidly normalized bilirubin levels and increased hemoglobin levels. The horizontal dotted lines indicate normal limits. Data are presented as medians with 25th to 75th percentiles for 10 patients (solid squares). The open triangles represent the median levels in the subgroup of patients with no concurrent lymphoma or mixed AIHA (n = 6). Reciprocal changes in bilirubin and hemoglobin levels occurred after effective drug levels washed out.
Sutimlimab rapidly normalized bilirubin levels and increased hemoglobin levels. The horizontal dotted lines indicate normal limits. Data are presented as medians with 25th to 75th percentiles for 10 patients (solid squares). The open triangles represent the median levels in the subgroup of patients with no concurrent lymphoma or mixed AIHA (n = 6). Reciprocal changes in bilirubin and hemoglobin levels occurred after effective drug levels washed out.
Haptoglobin levels normalized in 4 patients within 1 to 2 weeks (Table 2) and were accompanied by a normalization of lactate dehydrogenase levels in 5 patients. Eight of 10 patients had abnormal bilirubin levels at baseline, reflecting an increased erythrocyte turnover by the liver. Sutimlimab infusions decreased median bilirubin levels by 61%, normalizing in most patients within 24 hours of the first infusion (P = .007) (Figure 3). Immediately upon sutimlimab washout, bilirubin levels increased again, demonstrating the recurrence of hemolysis. The rapidity of the response provided a disease-related biomarker to examine the relationship between sutimlimab concentration and extravascular hemolysis. At sutimlimab concentrations >40 µg/mL, bilirubin levels were within the normal range, with few borderline exceptions (Figure 4).
Clinical laboratory parameters before treatment and maximal absolute changes during treatment
| Variable . | Hemoglobin . | Reticulocytes . | Bilirubin . | Haptoglobin . | Lactate dehydrogenase . | Complement C4 . | CA titer . |
|---|---|---|---|---|---|---|---|
| Normal range | 12-16 g/dL | 32-110 × 109/L | 0.1-1.2 mg/dL | 30-200 mg/dL | <250 U/L | 0-40 mg/mL | 1-32 titer |
| Patient | |||||||
| C1001 | 8.3/+3.8 | 131/−100 | 1.6/−1.4 | <12/+55.4 | 212/−40 | 10.9/+20.8 | 512/−384 |
| C1002 | 7.5/+4.8 | 140/−114 | 2.5/−2.1 | <12/+30.5 | 375/−185 | 14.5/+23.1 | 32/+32* |
| C1003 | 7.9/+0.5 | 133/−62 | 0.9/−0.2 | <12/0 | 537/−96 | 7.0/0† | 1024/−512 |
| C1004 | 6.8/+4.0 | 101/−65 | 1.7/−1.4 | <12/+121 | 501/−334 | 7.0/+21.4 | 1024/0 |
| C1006 | 7.7/+4.5 | 171/NA | 3.8/−3.0 | <12/0 | 371/−133 | 7.0/+18.2 | 1024/0 |
| C1008 | 8.2/+5.0 | 115/−68 | 4.8/−4.3 | <12/+96 | 357/−194 | 7.0/+19.6 | 256/+768 |
| C1009 | 6.1/+3.7 | NA/NA | 6.7/−1.4‡ | <12/0 | 342/−57 | 7.0/+9.3 | 1024/0 |
| C1010 | 7.6/+4.0 | 136/−60 | 2.2/−1.6 | <12/0 | 506/−190 | 20.4/+21.6 | 1024/0 |
| C1011 | 9.3/+1.3 | 183/−70 | 1.7/−0.7 | <12/5.5 | 309/−34 | 14.1/+20.4 | 512/+512 |
| C1013 | 10.4/+0.9 | 125/−96 | 1.0/−0.7 | <12/0 | 352/−38 | 7.0/+14.3 | 1024/0 |
| Median/change | 7.8/3.9 | 133/−97 | 2.0/−1.5 | <12/3 | 364/−174 | 7.0/+19.6 | 1024/16 |
| Variable . | Hemoglobin . | Reticulocytes . | Bilirubin . | Haptoglobin . | Lactate dehydrogenase . | Complement C4 . | CA titer . |
|---|---|---|---|---|---|---|---|
| Normal range | 12-16 g/dL | 32-110 × 109/L | 0.1-1.2 mg/dL | 30-200 mg/dL | <250 U/L | 0-40 mg/mL | 1-32 titer |
| Patient | |||||||
| C1001 | 8.3/+3.8 | 131/−100 | 1.6/−1.4 | <12/+55.4 | 212/−40 | 10.9/+20.8 | 512/−384 |
| C1002 | 7.5/+4.8 | 140/−114 | 2.5/−2.1 | <12/+30.5 | 375/−185 | 14.5/+23.1 | 32/+32* |
| C1003 | 7.9/+0.5 | 133/−62 | 0.9/−0.2 | <12/0 | 537/−96 | 7.0/0† | 1024/−512 |
| C1004 | 6.8/+4.0 | 101/−65 | 1.7/−1.4 | <12/+121 | 501/−334 | 7.0/+21.4 | 1024/0 |
| C1006 | 7.7/+4.5 | 171/NA | 3.8/−3.0 | <12/0 | 371/−133 | 7.0/+18.2 | 1024/0 |
| C1008 | 8.2/+5.0 | 115/−68 | 4.8/−4.3 | <12/+96 | 357/−194 | 7.0/+19.6 | 256/+768 |
| C1009 | 6.1/+3.7 | NA/NA | 6.7/−1.4‡ | <12/0 | 342/−57 | 7.0/+9.3 | 1024/0 |
| C1010 | 7.6/+4.0 | 136/−60 | 2.2/−1.6 | <12/0 | 506/−190 | 20.4/+21.6 | 1024/0 |
| C1011 | 9.3/+1.3 | 183/−70 | 1.7/−0.7 | <12/5.5 | 309/−34 | 14.1/+20.4 | 512/+512 |
| C1013 | 10.4/+0.9 | 125/−96 | 1.0/−0.7 | <12/0 | 352/−38 | 7.0/+14.3 | 1024/0 |
| Median/change | 7.8/3.9 | 133/−97 | 2.0/−1.5 | <12/3 | 364/−174 | 7.0/+19.6 | 1024/16 |
For haptoglobin, 11 mg/dL was used for calculations when <12 mg/dL. Cold agglutinin (CA) titers indicate fold-dilutions (1024 maximum tested).
C4, complement component 4 (7 was used for calculations when <8 mg/dL); NA, not assessable due to agglutination.
The cold agglutinin titer was 1:64 during screening.
Patient with complement deficiency and 70% bone marrow infiltration whose hemoglobin values were censored before start of ibrutinib for conservative statistical comparison.
Gilbert disease.
Relationship between serum concentrations of sutimlimab and bilirubin. The horizontal dotted line represents sutimlimab 20 µg/mL, and the vertical dotted line represents 1.2 mg/dL of bilirubin (upper limit of normal).
Relationship between serum concentrations of sutimlimab and bilirubin. The horizontal dotted line represents sutimlimab 20 µg/mL, and the vertical dotted line represents 1.2 mg/dL of bilirubin (upper limit of normal).
In contrast to hemolysis, sutimlimab did not change agglutination of erythrocytes on blood smears or symptoms of acrocyanosis (data not shown).
Three patients did not respond sufficiently to treatment with sutimlimab (0.5-1.3 g/dL increase in hemoglobin). One of these nonresponders turned out to be repeatedly positive for both C3d and IgG (>1+) in the Coombs test (mixed AIHA). The 2 other nonresponders had active lymphoma with lymphocytic bone marrow infiltrates of 15% (CD5–, MYD88– clonal B-cell disorder) and 70% (MYD88+). However, 1 patient with 60% bone marrow infiltration (MYD88+) responded with a >2-g/dL rise in hemoglobin. Of the patients who had neither a history of lymphoma nor mixed disease (n = 6), all had adequate responses with a median maximum hemoglobin increase of 4.3 g/dL and resolution of hemolysis as shown by normalization of bilirubin levels.
Patients with histologic evidence of lymphoma in the bone marrow had extremely low C1q levels (range, 16-49 mg/L; 3 standard deviations lower than normal) compared with the other patients (range, 73-110 mg/L). Nevertheless, C4 levels increased in 2 of the 3 patients with concurrent lymphoma and in the patient with mixed AIHA upon treatment, still indicating pharmacodynamic activity.
None of the patients required packed red blood cell transfusions while receiving sutimlimab treatment, including 6 patients who had recently undergone transfusion (≥2 units of packed red blood cells within the last 6 months).
Recapitulation of response after washout and re-challenge with sutimlimab
Complement deposition on erythrocytes, hemolysis, and anemia recurred in all responders ∼3 to 4 weeks after the last dose of sutimlimab.28 All 6 responders without concurrent lymphoma or mixed AIHA were enrolled in a named patient program to prove causality by serial treatments in a series of N-of-1 trials. Re-exposure to sutimlimab recapitulated the immediate onset of effect (supplemental Table 1; supplemental Figure 2) and the rapid and complete inhibition of hemolysis in all patients. The fall in hemoglobin level upon each drug withdrawal indicated that seasonal variations played no role in the improvement of the anemia. All 6 patients remained transfusion-free while undergoing treatment (up to 18 months). Two patients discontinued the named patient program, one because she was diagnosed as having a massive gynecologic tumor requiring radiation therapy of the pelvis, and the other patient because she had to care for her husband with Alzheimer disease. Both have again become transfusion-dependent, requiring transfusion support approximately every other week.
Alternative doses and dose regimens were explored in the named patient program because pharmacokinetic analyses showed that weekly 60-mg/kg doses resulted in increasing trough levels of sutimlimab, suggesting accumulation of the drug. Lower doses administered at 14-day intervals led to breakthrough hemolysis due to inadequate trough concentrations <20 µg/mL, which was accompanied by restoration of CH50 levels. An increase of the dose to 65 mg/kg or a fixed dose of 5.5 g every other week prevented further breakthrough events. All 6 patients achieved normal hemoglobin levels (>12 g/dL) after sutimlimab treatment. Longer observation periods suggested that some of these patients will continue to have a hemoglobin level >12 g/dL, whereas others may have hemoglobin values ∼11 g/dL.
Safety
All infusions were given over 1 hour and were well tolerated without premedication and without relevant drug-related adverse effects. There were few adverse events during the trial; all were mild or moderate and considered unrelated or unlikely related to the study drug (supplemental Table 2). One unrelated serious adverse event occurred in a patient who was hospitalized for elective pain treatment of a new vertebral fracture (in addition to preexisting ones), which was attributed to long-term steroid therapy. Pruritus and exanthema occurred in another patient but was transient despite continuing sutimlimab re-exposure.
Discussion
In this first-in-human trial, blockade of C1s by the mAb sutimlimab rapidly halted hemolysis, corrected anemia, and precluded the need for transfusions in patients with cold agglutinin disease. Based on these data, sutimlimab was granted breakthrough therapy designation by the US Food and Drug Administration for the treatment of this condition.
Sutimlimab exposure and maximal concentrations in the patients with cold agglutinin disease were only 10% to 15% lower than in young healthy volunteers,29 indicating that the higher systemic turnover of complement in cold agglutinin disease does not have a major impact on pharmacokinetic variables. Pharmacokinetic modeling in normal volunteers showed a steep concentration–effect relationship for the inhibition of classical pathway activity, reaching >90% inhibition at sutimlimab concentrations of ∼15.5 µg/mL. Accordingly, bilirubin levels were, in general, normal when plasma concentrations exceeded that threshold in patients with cold agglutinin disease (Figure 4).
As expected, sutimlimab reduced CH50 levels, a pharmacodynamic marker of its activity, which reflected the presence of active drug concentrations, particularly in those patients who had high baseline levels. In contrast, baseline CH50 levels and C1q were already low in patients with cold agglutinin disease with concurrent lymphoma, consistent with low complement activity in patients with lymphatic malignancies.30 A larger study is needed to determine whether low C1q may be a useful marker to predict limited response. Furthermore, baseline levels of C4, the first substrate cleaved following activation of C1s, were low in patients with cold agglutinin disease, owing to cold agglutinin–mediated classical pathway consumption.6,12 Blockade of C1s by sutimlimab increased circulating levels of C4 several-fold in patients, providing an in vivo readout of sutimlimab activity (Figure 2). Finally, sutimlimab treatment decreased the percentage of C3d-positive erythrocytes in the circulation of patients, which corresponded to the rapid and substantial rise in hemoglobin levels.
Extravascular hemolysis was inhibited by sutimlimab as reflected by a normalization of bilirubin levels within 24 hours of treatment and recurred upon drug washout. The nearly instantaneous response of bilirubin to sutimlimab allowed us to assess the relationship between plasma concentrations of sutimlimab and bilirubin, a disease-relevant biomarker. Plasma concentrations of sutimlimab 15.5 µg/mL caused near-complete inhibition (>90%) of serum classical pathway activity in healthy volunteers.29 Consistently, bilirubin levels were largely normal when sutimlimab concentrations exceeded that threshold in patients (Figure 4). Taken together, these results strongly suggest that the inhibition of the classical complement pathway by sutimlimab stops extravascular-mediated hemolysis in patients with cold agglutinin disease.
Haptoglobin, a very sensitive and specific marker of hemolysis, normalized in 4 patients, indicating that sutimlimab can fully inhibit hemolysis in some patients. However, sutimlimab would not be expected to affect cold agglutinin titers or prevent their binding to erythrocytes. Therefore, the remaining agglutination and associated microvascular erythrocyte disruption may have prevented a normalization of haptoglobin and lactate dehydrogenase in the other responders, despite them experiencing a substantial increase in hemoglobin levels.
In contrast to the median 3.9-g/dL rise in hemoglobin level within weeks after the start of C1s blockade by sutimlimab, the C5 inhibitor eculizumab increased hemoglobin levels only modestly, from a median of 9.3 to 10.2 g/dL after 6 months, even in a carefully chosen subset of patients with cold agglutinin disease exhibiting increased intravascular hemolysis (ie, patients with lactate dehydrogenase levels >2 times the upper limit of normal).31 Collectively, these results strongly support the concept that anemia in cold agglutinin disease is driven predominantly by the classical complement pathway and upstream C3 opsonin–mediated phagocytosis of erythrocytes (Figure 1).9 It further highlights the critical need to block complement activation upstream of C5 to correct the anemia experienced by these patients.
Of the 4 patients who experienced the smallest increases in hemoglobin, 3 had a concurrent documented lymphoma and the fourth had mixed AIHA (Tables 1 and 2). Figure 3 indicates that bilirubin response was similar in those patients; thus, patients with bone marrow infiltration may at least in part have a decreased ability to produce erythrocytes. Interestingly, C4 levels nevertheless increased in 2 of the 3 patients with concurrent lymphoma and in the patient with mixed AIHA upon treatment, confirming the pharmacodynamic effect of sutimlimab in these patients and suggesting that cold agglutinin–mediated classical pathway activity was not driving their anemia. Hence, the beneficial effects seen in cold agglutinin disease cannot be extrapolated to patients experiencing IgG-mediated removal of erythrocytes via the spleen. A limitation of this study is the lack of information on recent bone marrow histology in some patients and often missing mutation analysis for MYD88.13 Neither of these factors were considered as inclusion criteria, partly to facilitate participation of as many patients as possible. The sample size was limited because of the rarity of this disease, and further testing is warranted in a larger, multicenter, multinational trial.
Although we chose an uncontrolled design to demonstrate proof-of-concept, several lines of evidence support the attribution of the hematologic response to sutimlimab. First, no spontaneous improvements in hemolysis or anemia had been observed in the history of the enrolled patients to indicate a placebo effect in the trial. An illustrative example is provided in supplemental Figure 3. In addition, the duration of effect seemed to be dose dependent in the named patient program; that is, higher maintenance doses were required to avoid episodes of hemolytic breakthrough if an interval of 14 days was chosen between subsequent doses. Finally, the repeat crossover between on-drug and off-drug periods in a series of N-of-1 trials unequivocally showed that cessation of hemolysis and the concurrent rise in hemoglobin level occurred during periods of complement inhibition as reflected by the inhibition of CH50 activity.
The good safety and tolerability profile of the IgG4 antibody sutimlimab in the population with cold agglutinin disease is consistent with that in healthy volunteers29 and patients with antibody-mediated rejection after kidney transplantation.32 The IgG4 subclass of antibodies is known for its low or no binding affinity for FcyRIIIa and lack of complement activation. This excellent tolerability of sutimlimab is in striking contrast to cytotoxic antibodies such as rituximab, where even a fraction of <1/3750 of an authorized dose induces B-cell killing with ensuing—possibly complement-mediated—adverse infusion reactions.33 Although the safety data of sutimlimab are encouraging, they must be interpreted cautiously given the limited duration of the trial.
In conclusion, blockade of C1s by sutimlimab rapidly and effectively prevented further hemolysis in patients with cold agglutinin disease. Moreover, sutimlimab was well tolerated and induced clinically meaningful increases in hemoglobin levels, even in patients with multiple previous lines of therapy, precluding the need for transfusions.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Editorial support (formatting of the manuscript according to journal style) was requested by the sponsor and provided by Fishawack Communications (Conshohocken, PA).
This research was funded by True North Therapeutics, Inc. (now part of Bioverativ, a Sanofi company).
Authorship
Contribution: The manuscript was written by B.J. and B.J. in conjunction with S.D., C. Schöergenhofer, J.B., U.D., C. Sillaber, P.J.-S., M.F., T.S., G.P., S.P., G.C.P., and J.C.G. without any writing assistance provided by a third party; and all authors contributed to the data collection, interpretation of data, and drafting and revising of the manuscript.
Conflict-of-interest disclosure: U.J. reports personal fees from True North Therapeutics during the conduct of the study, grants and personal fees from Roche, grants and personal fees from Celgene, grants and personal fees from Gilead, personal fees from Amgen, grants and personal fees from Novartis, personal fees from Takeda, personal fees from AbbVie, and personal fees from Infinity; outside the submitted work, grants and personal fees from True North Therapeutics. G.P. is a consultant to Bioverativ and was an employee and stockholder of the study sponsor, True North Therapeutics, at the time the study was designed and conducted. G.C.P. is an employee of Bioverativ. S.P. was an employee and stockholder of the study sponsor, True North Therapeutics, at the time the study was designed and conducted, and was subsequently an employee of Bioverativ. J.C.G. was an employee and stockholder of the study sponsor, True North Therapeutics, at the time the study was designed and conducted. B.J. received reimbursement from True North Therapeutics related to travel costs for scientific presentations and scientific advice. The remaining authors declare no competing financial interests.
Correspondence: Bernd Jilma, Department of Clinical Pharmacology, Medical University of Vienna, Währinger Gürtel 18-20, 1090 Vienna, Austria; e-mail: bernd.jilma@meduniwien.ac.at.


![Figure 1. Extravascular hemolysis caused by cold agglutinin–induced complement-mediated opsonization. Cold agglutinins (mostly pentameric immunoglobulin M [IgM]) agglutinate erythrocytes and fix C1, triggering the classical complement cascade and leading to C3 split product opsonization of the red blood cell. Complement-opsonized erythrocytes then travel to the liver where they are phagocytosed, a process known as extravascular hemolysis. Although complement-mediated intravascular hemolysis can occur, which requires C5 cleavage and formation of the membrane attack complex, it is generally prevented by complement regulatory proteins on the erythrocyte surface (ie, CD55 and CD59). Regardless of the hemolytic mechanism, upstream C1s blockade prevents both extravascular and intravascular hemolysis. Figure adapted and modified from Berentsen and Sundic4 and from Shi et al.12](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/133/9/10.1182_blood-2018-06-856930/4/m_blood856930f1.png?Expires=1767751012&Signature=1uW2pyagv8crleybDTZLQAavkkPTUVD~9pGloC9jM-6IcGbiGQOx1~P7BR08dDZIMqJGWl-GGB6cjy11TIodr4a5w-JyYt7LuBA~lAz16HvMd2MGv85mU~kulUqO~1K9Xx5~LtgXRjS0wgPZjUL8S1Y1mWeFv0kFDztjHvBhGUx2OiZ0YJMvI1qZfDn0vxI3fPZG6BYOZxt0XMbk6U7sH8x4Hj5C42spBnKeLQgvNm0mtrzjIkwCh-lUyO9xqB1oXAwm0aavGUYVJ85IZYleVKQ6JyoMhMIHsguNN2RDrhOcFgWGrZENDxnX4JRF3fAzr3hA-BYFQDpVrGk7Hw7gQg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)