In this issue of Blood, Yu et al have identified a new role for the spliceosome factor breast carcinoma amplified sequence 2 (BCAS2) in developmental hematopoiesis.1
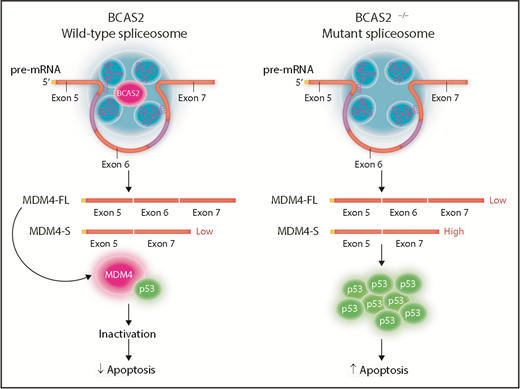
BCAS2 participates in splicing reactions of MDM4 pre-mRNA in HSCs and influences splice site selection.8 In the presence of wild-type levels of BCAS2, the spliceosome preferentially produces MDM4-FL mRNA, which includes exon 6. This results in production of normal MDM4 protein that binds to and inactivates p53. In contrast, in the absence of BCAS2, MDM4 preferentially undergoes alternative spicing, resulting in a shortened version (MDM4-S) that skips exon 6. In BCAS2-null zebrafish, expression of MDM4-S mRNA is increased but MDM4-FL is decreased. The MDM4-S has a premature stop codon, encoding a truncated MDM4-S protein. Together with decreased MDM4-FL mRNA, this results in decreased functional MDM4 protein. Without MDM4 to inactivate it, p53 expression increases leading to increased apoptosis of HSCs.
BCAS2 participates in splicing reactions of MDM4 pre-mRNA in HSCs and influences splice site selection.8 In the presence of wild-type levels of BCAS2, the spliceosome preferentially produces MDM4-FL mRNA, which includes exon 6. This results in production of normal MDM4 protein that binds to and inactivates p53. In contrast, in the absence of BCAS2, MDM4 preferentially undergoes alternative spicing, resulting in a shortened version (MDM4-S) that skips exon 6. In BCAS2-null zebrafish, expression of MDM4-S mRNA is increased but MDM4-FL is decreased. The MDM4-S has a premature stop codon, encoding a truncated MDM4-S protein. Together with decreased MDM4-FL mRNA, this results in decreased functional MDM4 protein. Without MDM4 to inactivate it, p53 expression increases leading to increased apoptosis of HSCs.
The highly coordinated process of RNA splicing is carried out by major and minor spliceosomes to remove noncoding regions (introns) of the pre–messenger RNA (mRNA) before protein translation. Alternative RNA splicing, a previously underappreciated mechanism for regulating gene expression in normal tissues and cancer, has recently reemerged.2 First discovered in 2011, mutations in spliceosomal proteins and regulatory splicing factors are now recognized as being common in patients with myelodysplastic syndrome and are also found in leukemia and clonal disorders of hematopoiesis.3,4 However, published data are limited regarding the role of these same splicing components in regulating normal hematopoiesis during development or homeostasis.5,6
Yu et al capitalize on the zebrafish animal model to examine hematopoietic development in bcas2−/− mutants. Hematopoiesis is highly conserved across vertebrates, occurring in multiple waves during embryogenesis.7 The first (primitive) wave of hematopoietic specification consists of erythroid and myeloid cells, which provide short-term support for the embryo. Subsequently, the definitive wave of hematopoiesis generates the first multipotent hematopoietic stem and progenitor cells (HSPCs), which emerge from the hemogenic endothelium in the ventral wall of the dorsal aorta of all vertebrates, producing all of the blood cells required throughout the animal’s lifetime. These first HSPCs travel from the dorsal aorta to seed the fetal liver, placenta, thymus, and bone marrow in mammals. In zebrafish, the first HSPCs seed the caudal hematopoietic tissue (CHT), which is the fetal liver equivalent, and later seed the thymus and kidney marrow.
To study the role of BCAS2 in hematopoietic development, the authors generated bcas2 zebrafish mutants using targeted mutagenesis with transcription activator-like effector nucleases and then examined the various waves of hematopoiesis during early development. Previous studies had revealed that BCAS2 is expressed in numerous cell types throughout embryonic development, and knocking out Bcas2 in drosophila8 and mice9 revealed profound effects on development through regulation of Δ-Notch signaling and the DNA damage response, respectively. However, before the study by Yu et al, there was no known role for BCAS2 in hematopoiesis. The authors showed that the development of definitive HSPCs and all definitive blood lineages were curtailed in bcas2−/− embryos starting around 3 days postfertilization (dpf).
In contrast, primitive hematopoiesis (both erythropoiesis and myelopoiesis) in early development was not altered in bcas2−/− embryos. Likewise, the specification of HSPCs in the ventral wall of the aorta was normal in bcas2−/− mutants compared with their wild-type siblings at 36 hours post fertilization and 2 dpf. After migrating from the dorsal aorta to the CHT, the HSPCs in bcas2−/− embryos failed to undergo normal expansion at 3 to 4 dpf. Additional experiments revealed that HSPCs in the CHT of bcas2−/− embryos had normal proliferation but increased apoptosis and increased expression of p53 and its target genes.
Because BCAS2 was known to play a role in the response to DNA damage,9 the authors asked whether this could explain the increased expression of p53 in the bcas2−/− mutant embryos. They discovered DNA damage response was not different from that in wild-type embryos; they realized instead that mdm4, an inhibitor of p53, had decreased expression in bcas2−/− embryos. This led to the hypothesis that the increase in p53 could be the result of alternative splicing of mdm4. The authors then showed that in the absence of bcas2, the zebrafish mdm4 pre-mRNA was preferentially spliced to remove exon 6. The ratio of full-length MDM4 (MDM4-FL) to this shortened form (MDM4-S) was decreased in bcas2−/− embryos, leading to lower levels of mdm4 protein (see figure). Because MDM4 binds to the N-terminal transactivation domain of p53 to inactivate it, a lack of MDM4 results in increased p53.10
These data indicate that BCAS2 is vital for HSPC survival by regulating p53 signaling to inhibit apoptosis. Although many transcription factors and epigenetic modifiers have been identified to regulate embryonic and adult hematopoiesis,7 BCAS2 is among the first spliceosomal components found to regulate HSPCs. Future experiments will no doubt reveal more alternatively spiced mRNAs that regulate the development and malignant transformation of HSPCs. Discovering how to manipulate this mechanism with small molecules or other therapeutics and thereby direct HSPC differentiation or target hematopoietic cancer cells remains a challenge for the future.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal