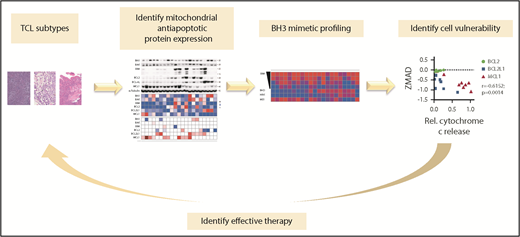
In this issue of Blood,Koch et al1 demonstrate the utility of an integrated functional biomarker-driven approach for the selection of candidate drugs and combinations that may be effective in the treatment of T-cell lymphomas (TCLs) (see figure). Specifically, they combine a functional screening strategy assessing susceptibility to BH3 mimetics and BCL2 family inhibitors and conventional chemotherapy to predict sensitivity of TCLs to a combination regimen.
Functional precision medicine for patients with TCL using BH3 mimetic profiling. Functional profiling of T-cell lymphoma–derived cell lines was utilized to screen for cell susceptibilities to BH3 mimetics and BCL2 family inhibitors which can be used in combination with conventional chemotherapeutic agents. The figure has been adapted from the article by Koch et al that begins on page 566.
Functional precision medicine for patients with TCL using BH3 mimetic profiling. Functional profiling of T-cell lymphoma–derived cell lines was utilized to screen for cell susceptibilities to BH3 mimetics and BCL2 family inhibitors which can be used in combination with conventional chemotherapeutic agents. The figure has been adapted from the article by Koch et al that begins on page 566.
A critical goal of precision oncology is to tailor therapies to malignancies that exhibit maximal susceptibility to the selected agent. Traditional approaches for assessing context-specific sensitivity of cancers to specific drugs have relied upon detection strategies targeting structural biomolecules, such as nucleic acids or proteins expressed in the tumor sample. Although biomarker strategies that are exclusively based on genomic or protein expression are predictive in many instances, they may not offer a functional indication of the efficacy of the biomarker-directed therapy in a clinically relevant context. Thus, the development and implementation of functional strategies to evaluate the vulnerability of tumors to specific drugs and drug combinations are critically needed.
Accordingly, functional strategies to explore therapeutic vulnerabilities in cancers are being increasingly explored to directly interrogate which drugs may yield the most optimal killing efficiency for treating specific malignancies.
Peripheral TCLs represent a diverse category of poorly understood malignant lymphomas. The diversity of entities encompassed within the TCL category is reflected in the World Health Organization classification, which recognizes 29 subtypes, including cutaneous and peripheral TCLs,2 with aggressive subtypes exhibiting especially poor clinical outcomes. In this regard, most patients treated with anthracycline-based induction chemotherapy, such as CHOP (cyclophosphamide, adriamycin, vincristine and prednisone), do not achieve remission or suffer disease relapse within 2 years of completing front-line therapy.3 Further, the overall survival of relapsed/refractory disease is quite poor, and treatment with newly approved drugs has not resulted in improved outcomes.4 The dismal outcomes, as well as the desirable goal of administering more effective and less toxic personalized therapeutic strategies, necessitate the development of novel efficacious therapies and better ways to identify to which drugs patients’ tumors are most sensitive.
Apoptosis is an important mechanism of cancer cell death and is induced by antiapoptotic peptide inhibitors and cytotoxic chemotherapeutic agents.5 For apoptosis to occur, the proapoptotic molecules BAX and BAK oligomerize to form pores that facilitate permeabilization of mitochondrial outer membranes. BH3 profiling offers a strategy to interrogate whether intact cells exposed to BH3 peptides, alone or in combination with other therapeutic agents, are primed or undergoing apoptotic cell death.6 BH3 profiling involves utilization of a library of synthetic peptides largely derived from the BH3 domains of the different proapoptotic BCL2 family members to assess the ability for cells to undergo mitochondrial outer membrane permeabilization (MOMP), an irreversible stage in the induction of apoptosis.7 Following treatment with a specific peptide or drug, the ensuing release of mitochondrial cytochrome C is used as an indicator of MOMP. The approach requires preparation of single-cell suspensions of the tumor cells and ex vivo treatment with individual drugs or drug combinations that are being considered for therapy. Cell death within 16 hours of exposure to a drug or drug combination in cell line, murine, and human clinical experiments suggests that this approach could predict in vivo response to therapy.7 This provides a valuable tool for selection of drugs to which the tumors are susceptible in a clinically relevant timeline and context.
CHOP-based chemotherapy achieves <40% curative success, and this underscores the need for more effective and optimally personalized therapeutic strategies in TCL. Thus, Koch et al leveraged a BH3-profiling approach to investigate the utility of BH3 peptides in the combinatorial therapy of patients with TCL.
The concept of targeting the BCL2 and apoptosis-related family for cancer therapy is gaining increasing consideration.8 Koch et al observed a heterogeneous expression pattern among key proteins involved in the mitochondrial apoptosis pathway in 8 subtypes of TCL. Whereas the abundance of the antiapoptotic BCL2 family members based on immunoblotting or transcript levels correlated poorly with the activity of the BH3 mimetics, BH3 profiling reliably predicted sensitivity to BH3 mimetics in vitro and in vivo. Koch et al also observed significant correlation between BH3-profiling sensitivities and vulnerability scores obtained from previously existing CRISPR/Cas9 dropout screens performed on TCL-derived cell lines. Further, their studies demonstrated that most TCL-derived cell lines exhibited selective dependence on MCL1. Targeting MCL1 for cancer therapy is emerging as an attractive vulnerability target.9 The use of CHOP+MCL1 inhibition as a novel therapeutic regimen for TCLs has not been well explored and could improve the poor outcomes observed in TCLs. Accordingly, they focused the study on targeting of MCL1 and specifically evaluated the therapeutic efficacy of an MCL1 inhibitor in TCL contexts using TCL-derived cell lines and PDX models. The studies indicate that MCL1 inhibition by the selective inhibitor AZD5991 in patient-derived mouse xenografts significantly reduced tumor size (60%). However, the occurrence of tumor regrowth following drug withdrawal rationalized exploration of augmentative effects when combined with conventional chemotherapy. Accordingly, the authors went on to show tumor reduction following treatment with the MCL1 inhibitor (AZD5991) combined with CHOP, resulting in better survival compared with outcomes observed with either regimen alone. Importantly, this combination therapeutic approach (AZD5991+CHOP) was selective in efficacy and did not produce significant response in tumors that were not addicted to MCL1.
Although a notable major contribution of this article is demonstration of the utility of a functional biomarker-based strategy for identification of the most efficacious drugs or combinations for targeted therapy of TCL, it is worth noting that the principles underlying this strategy are generalizable in many scenarios in precision oncology. Nevertheless, limitations include the reliance on in vitro techniques, which, by their nature, exclude the contribution of the microenvironment. Further, the timelines for generation of PDXs and the immunodeficient mouse backgrounds in which they are propagated represent potential challenges with operationalization in clinically relevant settings. Notwithstanding, the utilization of functional biomarker-driven strategies for the selection of drug(s) with efficacy represents a major advance in the practice of precision oncology.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal