Abstract
Anticoagulant therapy is often refrained from out of fear of hemorrhagic complications. The most frequent type of major bleeding is gastrointestinal, but intracranial hemorrhage has the worst prognosis. Management of these complications in patients on anticoagulants should follow the same routines as for nonanticoagulated patients, as described here with the previously mentioned bleeds as examples. In addition, for life-threatening or massive hemorrhages, reversal of the anticoagulant effect is also crucial. Adequate reversal requires information on which anticoagulant the patient has taken and when the last dose was ingested. Laboratory data can be of some help, but not for all anticoagulants in the emergency setting. This is reviewed here for the different types of anticoagulants: vitamin K antagonists, heparins, fondaparinux, thrombin inhibitors and factor Xa inhibitors. Specific antidotes for the latter are becoming available, but supportive care and nonspecific support for hemostasis with antifibrinolytic agents or prothrombin complex concentrates, which are widely available, should be kept in mind.
Introduction
Bleeding is the dominant adverse event of treatment with any anticoagulant.1 Major bleeding complications such as intracranial hemorrhage (ICH) or massive gastrointestinal bleeding deter many patients and physicians from initiating treatment with anticoagulants. In a survey, Australian family physicians were more prone to select an antiplatelet agent or no treatment than an anticoagulant for patients with 2 falls in the past year, or with a history of peptic ulcer bleeding but now on a proton pump inhibitor, or with nosebleeds every 2 months, despite a very high risk of ischemic stroke.2 Clinically relevant nonmajor bleeds (CRNMBs) may generate a perception of reduced quality of life3 and thereby decrease the persistence of the patient on anticoagulant treatment. CRNMBs are time-consuming to manage and result in cost increments.4 The oral factor Xa inhibitors have been reported to cause menorrhagia in up to 30% of young women, significantly more than with warfarin,5 and it appears to be a class effect.6 This has resulted in increased unscheduled physician contacts and medical and surgical interventions. This complication can be managed with tranexamic acid, concomitant estrogen medication,7 or change to warfarin. The search for new anticoagulants with a lower risk of bleeding is therefore important, not only for improved safety, but also for better acceptance and persistence.
We will review here the epidemiology of anticoagulant-related bleeding and its management according to major types of bleeding and specifically for different anticoagulants.
Definitions of bleeding
It is crucial to be able to compare major bleeds and CRNMBs when analyzing study results, particularly in systematic reviews and meta-analyses. The International Society on Thrombosis and Haemostasis definition for major bleeding8 has been widely adopted during the past decade, not only in clinical trials, but also in observational studies. In cardiovascular trials, the Thrombolysis in Myocardial Infarction criteria are frequently used.9,10 These and other commonly used definitions are shown in Table 1. In a large trial comparing apixaban with warfarin for patients with atrial fibrillation, 3 of these definitions were used and the outcome, favoring apixaban, was the same with each definition.11 Because most of these definitions have a drop in hemoglobin as a criterion, they are not ideal for retrospective claims database analyses. Some authors have found it useful to also specify life-threating bleedings. CRNMBs, similar to the terms moderate bleeding or minor bleeding, have also been defined (Table 1). The remainder of this review will focus on major bleeding, which poses the greatest challenge in management.
Definitions for characterization of severity of bleeding
| Classification . | ISTH8,75,76 . | TIMI (non-CABG)9,10 . | GUSTO77 . | BARC78 . |
|---|---|---|---|---|
| Nonsurgical hemorrhages | ||||
| Major bleed | Fatal bleed and/or symptomatic plus critical organ or Hgb ↓ ≥2 g/dL or transfusion of ≥2 U red cells/whole blood | Fatal bleeding or intracranial or overt bleed with Hgb ↓ ≥5 g/dL or Hct ↓ ≥15% absolute | Severe or life-threatening: bleed that is intracranial or with hemodynamic compromise and requires intervention | Type 5a: probable fatal bleed |
| Type 5b: definite (confirmed) fatal bleed | ||||
| Type 4: CABG-related (see next) | ||||
| Type 3a: overt bleeding + Hgb ↓ 3-<5 g/dL, or any transfusion | ||||
| Type 3b: overt bleed + Hgb ↓ ≥5 g/dL, or cardiac tamponade, or requiring surgery, or intravenous vasopressors | ||||
| Type 3c: intracranial or intraocular bleed with compromised vision | ||||
| Clinically relevant nonmajor | Bleed requiring medical intervention by health care professional, or hospitalization/increased level of care, or face-to-face evaluation | Minor: clinically overt bleed + Hgb ↓ 3-<5 g/dL or Hct ↓ ≥10% No observed blood loss: Hgb ↓ ≥4 g/dL or Hct ↓ ≥12% Overt bleed requiring intervention, or leading to or prolonging hospitalization | Moderate: requires blood transfusion but no hemodynamic compromise | Type 2: any overt, actionable sign of hemorrhage + |
| (1) requiring nonsurgical, medical intervention by a health care professional, | ||||
| (2) leading to hospitalization or increased level of care, or | ||||
| (3) prompting evaluation | ||||
| Minimal | Not defined | Overt bleeding event that does not meet the criteria presented Clinically overt bleed + Hgb ↓ <3 g/dL or Hct ↓ <10% | Mild: bleeding that does not meet criteria for either severe or moderate bleeding | Type 1: bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalization, or treatment by a health care professional; may include episodes leading to self-discontinuation of medical therapy by the patient without consulting a health care professional |
| Hemorrhages related to surgery | ||||
| Major | Fatal bleed and/or bleeding in critical organ, or extrasurgical site bleed with Hgb ↓ ≥2 g/dL or transfusion of ≥2 U red cells/whole blood Surgical site bleed requiring second intervention, or that is unexpected and prolonged and/or causes hemodynamic instability, in combination with Hgb ↓ ≥2 g/dL or transfusion of ≥2 U | CABG-related: fatal bleed, or perioperative intracranial bleed, or reoperation after closure of sternotomy for control of bleeding, or chest tube output ≥2 L/24 h | Type 4, CABG-related: perioperative intracranial bleed within 48 h, or reoperation after closure of sternotomy for control of bleeding, or transfusion of >5 U whole blood or packed cells, or chest tube output ≥2 L/24 h |
| Classification . | ISTH8,75,76 . | TIMI (non-CABG)9,10 . | GUSTO77 . | BARC78 . |
|---|---|---|---|---|
| Nonsurgical hemorrhages | ||||
| Major bleed | Fatal bleed and/or symptomatic plus critical organ or Hgb ↓ ≥2 g/dL or transfusion of ≥2 U red cells/whole blood | Fatal bleeding or intracranial or overt bleed with Hgb ↓ ≥5 g/dL or Hct ↓ ≥15% absolute | Severe or life-threatening: bleed that is intracranial or with hemodynamic compromise and requires intervention | Type 5a: probable fatal bleed |
| Type 5b: definite (confirmed) fatal bleed | ||||
| Type 4: CABG-related (see next) | ||||
| Type 3a: overt bleeding + Hgb ↓ 3-<5 g/dL, or any transfusion | ||||
| Type 3b: overt bleed + Hgb ↓ ≥5 g/dL, or cardiac tamponade, or requiring surgery, or intravenous vasopressors | ||||
| Type 3c: intracranial or intraocular bleed with compromised vision | ||||
| Clinically relevant nonmajor | Bleed requiring medical intervention by health care professional, or hospitalization/increased level of care, or face-to-face evaluation | Minor: clinically overt bleed + Hgb ↓ 3-<5 g/dL or Hct ↓ ≥10% No observed blood loss: Hgb ↓ ≥4 g/dL or Hct ↓ ≥12% Overt bleed requiring intervention, or leading to or prolonging hospitalization | Moderate: requires blood transfusion but no hemodynamic compromise | Type 2: any overt, actionable sign of hemorrhage + |
| (1) requiring nonsurgical, medical intervention by a health care professional, | ||||
| (2) leading to hospitalization or increased level of care, or | ||||
| (3) prompting evaluation | ||||
| Minimal | Not defined | Overt bleeding event that does not meet the criteria presented Clinically overt bleed + Hgb ↓ <3 g/dL or Hct ↓ <10% | Mild: bleeding that does not meet criteria for either severe or moderate bleeding | Type 1: bleeding that is not actionable and does not cause the patient to seek unscheduled performance of studies, hospitalization, or treatment by a health care professional; may include episodes leading to self-discontinuation of medical therapy by the patient without consulting a health care professional |
| Hemorrhages related to surgery | ||||
| Major | Fatal bleed and/or bleeding in critical organ, or extrasurgical site bleed with Hgb ↓ ≥2 g/dL or transfusion of ≥2 U red cells/whole blood Surgical site bleed requiring second intervention, or that is unexpected and prolonged and/or causes hemodynamic instability, in combination with Hgb ↓ ≥2 g/dL or transfusion of ≥2 U | CABG-related: fatal bleed, or perioperative intracranial bleed, or reoperation after closure of sternotomy for control of bleeding, or chest tube output ≥2 L/24 h | Type 4, CABG-related: perioperative intracranial bleed within 48 h, or reoperation after closure of sternotomy for control of bleeding, or transfusion of >5 U whole blood or packed cells, or chest tube output ≥2 L/24 h |
BARC, Bleeding Academic Research Consortium; CABG, coronary artery bypass graft; GUSTO, Global Utilization of Streptokinase and Tissue Plasminogen Activator for Occluded Coronary Arteries; Hct, hematocrit; Hgb, hemoglobin; ISTH, International Society on Thrombosis and Haemostasis; TIMI, Thrombolysis in Myocardial Infarction.
Epidemiology of anticoagulant-associated major bleeding
In 23 randomized clinical trials using vitamin K antagonists (VKAs) in patients with atrial fibrillation, the median rate of major bleeding was 2.1 per 100 patient years.12 In 40 observational studies, the median rate was quite similar at 2.1 per 100 patient years.12 With direct oral anticoagulants (DOACs), the risk of major bleeding is ∼30% lower than with VKAs.13 In a nationwide Danish health care database study, this benefit was replicated, but with a lower risk of major bleeding for apixaban and dabigatran compared with rivaroxaban.14 In a systematic review of observational studies in atrial fibrillation, the relative risk reduction for major bleeding with apixaban was 38% compared with warfarin, 35% compared with dabigatran, and 46% compared with rivaroxaban.15 The corresponding absolute risk reductions were 1.7%, 1.4%, and 1.2%, respectively. Until there are studies comparing different DOACs head-to-head, these differences need to be considered with caution and the absolute risk differences are likely to be small.
Patients with venous thromboembolism (VTE) differ from the population with atrial fibrillation regarding risk factors for bleeding: younger age and infrequent comedication with antiplatelet agents are lower risk, but higher prevalence of cancer and more intensive initial anticoagulation are higher risk. The bleeding risk in patients receiving unfractionated heparin (UFH) depends on the patient’s baseline characteristics (age and number of comorbidities), heparin dose, concomitant use of other antithrombotic agents, and a recent history of trauma or surgery.16 Randomized controlled trials comparing fondaparinux with low-molecular-weight heparin (LMWH) or UFH have reported a similar risk of major bleeding of about 1% to 2% of patients.17,18
The most common type of major bleeding with oral anticoagulants is from the gastrointestinal tract (Table 2). In trials in patients with atrial fibrillation, this type of hemorrhage was more frequent in patients taking DOACs than VKAs, and that difference was due to a higher risk for major gastrointestinal bleeding with dabigatran and rivaroxaban (but not with apixaban or edoxaban). The most feared major bleeding, ICH, occurred in a higher proportion of patients treated for atrial fibrillation than for VTE, probably because of the older age in the former group.
Most common types of major bleeds in trials comparing VKAs with DOACs
| Indication . | No. of trials . | Type of major bleed . | VKA . | DOAC . |
|---|---|---|---|---|
| Atrial fibrillation | 4 | All major bleeds | 1769 | 2091 |
| Gastrointestinal | 583 (33%) | 1005 (48%) | ||
| Intracranial | 425 (24%) | 272 (13%) | ||
| VTE treatment | 7 | All major bleeds | 263 | 161 |
| Gastrointestinal | 83 (32%) | 50 (31%) | ||
| Intracranial | 45 (17%) | 17 (11%) |
| Indication . | No. of trials . | Type of major bleed . | VKA . | DOAC . |
|---|---|---|---|---|
| Atrial fibrillation | 4 | All major bleeds | 1769 | 2091 |
| Gastrointestinal | 583 (33%) | 1005 (48%) | ||
| Intracranial | 425 (24%) | 272 (13%) | ||
| VTE treatment | 7 | All major bleeds | 263 | 161 |
| Gastrointestinal | 83 (32%) | 50 (31%) | ||
| Intracranial | 45 (17%) | 17 (11%) |
Only phase 3 trials are included. The number of patients included was higher in the DOAC group than in the VKA group for the studies in atrial fibrillation because the studies with dabigatran and edoxaban included 2 DOAC dose regimens.
There is an ∼50% reduction of ICH and of fatal bleeds with DOACs compared with VKAs,13,19 although the absolute reduction is limited to 2 intracranial bleeds and 1 fatal bleed per 1000 patients per year. The outcome with respect to neurological function after an intracranial bleed does not seem to differ between the 2 groups of oral anticoagulants.20,21 Whereas there is no clear association between low body weight and risk of bleeding on the DOACs,22 there is an increased risk for bleeding with decreasing renal function reported for dabigatran and VKAs.
Management of major hemorrhage
Supportive care
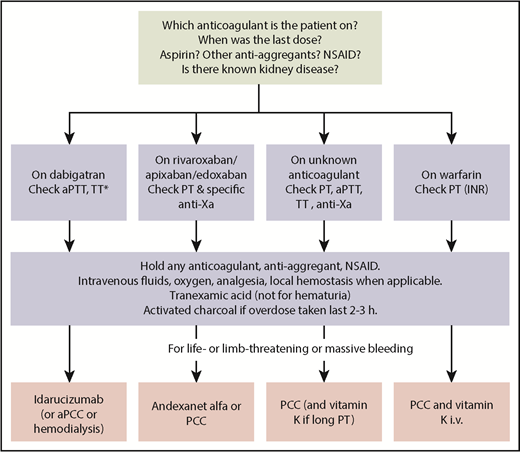
Before starting any anticoagulant-drug specific treatment and in parallel with starting supportive care, critical information should be obtained from the patient or family members. (1) What anticoagulant is the patient taking? (2) When was the last dose taken? (3) Is the patient also taking aspirin or other drugs that inhibit platelet function? (4) Is there known kidney disease (Figure 1)?
Algorithm for management of anticoagulant-associated major bleeding. aPCC, activated prothrombin complex concentrate; dTT, dilute thrombin time; iv, intravenous; NSAID, nonsteroid anti-inflammatory agent; *If dilute TT is available, it is the preferred test.
Algorithm for management of anticoagulant-associated major bleeding. aPCC, activated prothrombin complex concentrate; dTT, dilute thrombin time; iv, intravenous; NSAID, nonsteroid anti-inflammatory agent; *If dilute TT is available, it is the preferred test.
The basic management of anticoagulant-associated hemorrhages follows the same principles as for bleeds with other etiologies (Figure 1). Immediate actions that are available to stop or reduce the bleeding include local hemostasis (compression of accessible arterial bleeding, tamponade of nasal cavity, insertion of Sengstaken-Blakemore tube for esophageal variceal bleeding if urgent endoscopy is unavailable) and mitigation of the effects of blood loss (oxygen, intravenous fluids, other hemodynamic support, blood transfusion). Tranexamic acid should be used for trauma-related bleeding, based on the reduction of death from bleeding in nonanticoagulated patients with trauma.23 Tranexamic acid is contraindicated for hematuria because of the risk of clot formation in the ureter and hydronephrosis. Treatment with any anticoagulant, antiplatelet agent, or nonsteroid anti-inflammatory agent must be interrupted. Activated charcoal increases elimination of all DOACs and can, therefore, be used within a few hours in case of bleeding after overdose or accidental ingestion.
Estimation of the level of anticoagulant effect is helpful for optimal management of hemorrhage. In some cases, the medication intake may have been stopped for several days with the disappearance of the anticoagulant effect; the treatment should then be focused on the source of bleeding. Conversely, if the patient has developed acute kidney injury, there could be a substantial delay in the elimination of any DOAC. For VKAs, this estimation is quick and precise with point-of-care instruments for the international normalized ratio (INR). For DOACs, global assays such as thrombin time (TT), prothrombin time (PT) or activated partial thromboplastin time (aPTT) can, at best, give a rough qualitative assessment of the effect, but less so for apixaban and edoxaban than for dabigatran (with TT or aPTT) or rivaroxaban (with PT), as recently reviewed.24 Whereas TT is very sensitive for and will identify dabigatran at low concentrations, it will not distinguish clinically relevant from toxic levels. aPTT for dabigatran and PT for rivaroxaban at therapeutic doses are usually prolonged, but their sensitivity varies between reagents. Quantitative assessment with dilute TT for dabigatran and specifically calibrated anti-factor Xa for each of apixaban, rivaroxaban, or edoxaban are available for rapid analysis in a few tertiary care hospitals only.
ICH
The 90-day mortality in a recent multicenter pooled analysis was 33% and 31% for DOAC-associated and VKA-associated intracerebral bleeds, respectively.20 This represents the highest case fatality after any type of major bleed and is also higher than for intracranial bleeds in patients without anticoagulants. Hematoma volume, Glasgow Coma Scale score, and patient age were independently associated with fatal outcome.20 For patients on warfarin, the degree of anticoagulation is also associated with risk of fatal outcome (odds ratio, 1.5 for INR <2.0, 2.0 for INR 2.0 to 3.0, and 3.7 for INR >3.0).25 Because the hematoma expands with the duration of bleeding, it can be surmised that very early intervention should result in better outcome. In a cohort study assessing the prognostic effect of the reversal of VKA, correction of the INR did not improve mortality or functional outcome, but the median time from onset of symptoms until treatment was 5 hour (interquartile range, 3-16 h).26 This might be too long to expect an effect and an expedited process, similar to current management of ischemic stroke to meet the window for thrombolysis, warrants evaluation. Emergency medical services must perform a prehospital assessment and have already asked the questions listed previously. It should then give the emergency department advance information to shorten the time to computed tomography scanning. There should be a plan for rapid transfer of the patient to a tertiary care hospital.
Evacuation of a subdural or intracerebral hematoma should only be performed after the anticoagulant effect has disappeared or been reversed; such invasive procedures should be supported by current guideline recommendations.27 Mechanical devices such as intermittent pneumatic compression should be applied to prevent VTE.27 In stable patients, a prophylactic dose of heparin or LMWH can be started 2 to 4 days after the onset of bleeding.28 The decision regarding resumption of full anticoagulation must include an estimation of the risk of thromboembolic complications without resumption vs the risk of recurrent intracranial bleeding with resumption. The optimal timing for resumption after intracerebral bleeding, which has been studied with VKAs only, is in general about 8 weeks,29 shorter for traumatic intracerebral hemorrhage30 and longer for subdural hematoma (which has a higher risk of recurrence), rebleeding, or amyloid angiopathy. For patients with warfarin-associated ICH it seems reasonable to switch to a DOAC, in view of the overall lower risk of ICH, albeit not demonstrated yet for the risk of recurrent bleeds. Neither has it been established whether DOACs can be safely resumed at an earlier time than warfarin. For patients with ICH while on a DOAC, resumption at the reduced dose should be considered. The class I recommendations, in which there is evidence for and general agreement that the procedure or treatment is useful and effective with level of evidence A (multiple randomized clinical trials or meta-analyses) or B (1 randomized trial or observational studies), for management of ICH27 are summarized in Table 3.
Class I recommendations with level of evidence A or B for management of ICH
| Recommendation . | Class . | Level of evidence . |
|---|---|---|
| A baseline severity score should be performed as part of the initial evaluation of patients with ICH | I | B |
| Rapid neuroimaging with CT or MRI is recommended to distinguish ischemic stroke from ICH | I | A |
| Patients with ICH should have intermittent pneumatic compression for prevention of venous thromboembolism beginning the day of hospital admission | I | A |
| For ICH patients presenting with SBP between 150 and 220 mm Hg and without contraindication to acute BP treatment, acute lowering of SBP to 140 mm Hg is safe | I | A |
| Initial monitoring and management of ICH patients should take place in an intensive care unit or dedicated stroke unit with physician and nursing neuroscience acute care expertise | I | B |
| Clinical seizures should be treated with antiseizure drugs | I | A |
| A formal screening procedure for dysphagia should be performed in all patients before the initiation of oral intake to reduce the risk of pneumonia | I | B |
| Patients with cerebellar hemorrhage who are deteriorating neurologically or who have brainstem compression and/or hydrocephalus from ventricular obstruction should undergo surgical removal of the hemorrhage as soon as possible | I | B |
| BP should be controlled in all ICH patients. Measures to control BP should begin immediately after ICH onset | I | A |
| Given the potentially serious nature and complex pattern of evolving disability and the increasing evidence for efficacy, it is recommended that all patients with ICH have access to multidisciplinary rehabilitation | I | A |
| Recommendation . | Class . | Level of evidence . |
|---|---|---|
| A baseline severity score should be performed as part of the initial evaluation of patients with ICH | I | B |
| Rapid neuroimaging with CT or MRI is recommended to distinguish ischemic stroke from ICH | I | A |
| Patients with ICH should have intermittent pneumatic compression for prevention of venous thromboembolism beginning the day of hospital admission | I | A |
| For ICH patients presenting with SBP between 150 and 220 mm Hg and without contraindication to acute BP treatment, acute lowering of SBP to 140 mm Hg is safe | I | A |
| Initial monitoring and management of ICH patients should take place in an intensive care unit or dedicated stroke unit with physician and nursing neuroscience acute care expertise | I | B |
| Clinical seizures should be treated with antiseizure drugs | I | A |
| A formal screening procedure for dysphagia should be performed in all patients before the initiation of oral intake to reduce the risk of pneumonia | I | B |
| Patients with cerebellar hemorrhage who are deteriorating neurologically or who have brainstem compression and/or hydrocephalus from ventricular obstruction should undergo surgical removal of the hemorrhage as soon as possible | I | B |
| BP should be controlled in all ICH patients. Measures to control BP should begin immediately after ICH onset | I | A |
| Given the potentially serious nature and complex pattern of evolving disability and the increasing evidence for efficacy, it is recommended that all patients with ICH have access to multidisciplinary rehabilitation | I | A |
Table adapted from Hemphill et al.27 Class I recommendations are for when there is evidence for and general agreement that the procedure or treatment is useful and effective. Level of evidence A is based on multiple randomized clinical trials or meta-analyses; level of evidence B is based on 1 randomized trial or observational studies.
BP, blood pressure; CT, computed tomography; ICH, intracranial hemorrhage; MRI, magnetic resonance imaging; SBP, systolic blood pressure.
Gastrointestinal hemorrhage
The gastrointestinal canal is the most common source of anticoagulant-related major bleeding and can also serve as a red flag for malignancy. Endoscopic examination is therefore paramount both for diagnosis of the cause and often also to stop the bleeding. The latter can be achieved by local injection of epinephrine, cauterization, ablation, hemoclips for very deep ulcers with a visible blood vessel, argon plasma coagulation in case of angiodysplasia or gastric antral vascular ectasia, sclerotherapy, and band ligation for esophageal varices.31,32 The American College of Gastroenterology has published separate guidelines for management of variceal bleeding,32 gastric ulcer bleeding,33 small bowel bleeding,34 and lower gastrointestinal bleeding.35 For gastric ulcer bleeding, a proton pump inhibitor should be started intravenously. Nonsteroid anti-inflammatory agents need to be avoided both after upper and lower gastrointestinal bleeds; if such treatment has to be resumed, a selective inhibitor of cyclooxygenase 2 at the lowest dose should be given. The ideal time point for resumption of anticoagulation is difficult to define, but 1 study concluded that it is after 3 to 6 weeks after upper gastrointestinal bleeding.36 Earlier resumption can be considered if the thromboembolic risk is high.
For all major bleeds on anticoagulant treatment, a multidisciplinary approach is generally recommended, with the involvement of the specialist for the bleeding organ, hemostasis specialists, neurologist, and/or cardiologist to optimize the hemostatic treatment and the timing of prophylaxis or secondary prophylaxis for thromboembolism.
Reversal of anticoagulant agents
The reversal of anticoagulant effect must be individualized in each case by balancing the risk of thromboembolic events and considering the indication for anticoagulation vs the severity of bleeding, the urgency. and the requirement for a complete reversal. Table 4 summarizes the reversal strategies for different anticoagulants.
Reversal strategies for different anticoagulants
| Anticoagulant type . | Target . | Half-life, h . | Route of elimination . | Reversal strategy . | Laboratory investigation . |
|---|---|---|---|---|---|
| Vitamin K antagonists | Vitamin K–dependent coagulation factors | 20-60 (warfarin) | Liver metabolism; metabolites primarily eliminated in the urine (warfarin) | Vitamin K, PCC, plasma | INR |
| UFH | Antithrombin, factor IIa, factor Xa | 1-2 | Therapeutic dose: nonrenal elimination; very high doses: possible renal contribution | Protamine sulfate | aPTT |
| LMWH | Factor Xa | 3-7 | Renal | Protamine sulfate: partial reversal; rFVIIa: life-threatening bleeding | Chromogenic anti-Xa assay |
| Fondaparinux | Factor Xa | 17-21 | Renal | rFVIIa (high dose, 90 mcg/kg): life-threatening bleeding | Chromogenic anti-Xa assay |
| Dabigatran | Factor IIa | 12-17 | Renal (80%) | Idarucizumab, aPCC | aPTT (if normal, it excludes above on-therapy dabigatran levels but does not exclude the therapeutic range; TT (if normal, it excludes the presence of dabigatran); dTT; ECA |
| Apixaban | Factor Xa | 8-15 | Renal (25%) | 4F-PCC, andexanet alfa | Chromogenic anti-Xa assay |
| Betrixaban | Factor Xa | 19-27 | Renal (11%) | 4F-PCC, andexanet alfa | Chromogenic anti-Xa assay |
| Edoxaban | Factor Xa | 9-11 | Renal (35%) | 4F-PCC, andexanet alfa | PT (may be elevated, although a normal PT does not exclude clinically relevant levels); chromogenic anti-Xa assay |
| Rivaroxaban | Factor Xa | 9-13 | Renal (66%) | 4F-PCC, andexanet alfa | PT (may be elevated, although a normal PT does not exclude clinically relevant levels); chromogenic anti-Xa assay |
| Anticoagulant type . | Target . | Half-life, h . | Route of elimination . | Reversal strategy . | Laboratory investigation . |
|---|---|---|---|---|---|
| Vitamin K antagonists | Vitamin K–dependent coagulation factors | 20-60 (warfarin) | Liver metabolism; metabolites primarily eliminated in the urine (warfarin) | Vitamin K, PCC, plasma | INR |
| UFH | Antithrombin, factor IIa, factor Xa | 1-2 | Therapeutic dose: nonrenal elimination; very high doses: possible renal contribution | Protamine sulfate | aPTT |
| LMWH | Factor Xa | 3-7 | Renal | Protamine sulfate: partial reversal; rFVIIa: life-threatening bleeding | Chromogenic anti-Xa assay |
| Fondaparinux | Factor Xa | 17-21 | Renal | rFVIIa (high dose, 90 mcg/kg): life-threatening bleeding | Chromogenic anti-Xa assay |
| Dabigatran | Factor IIa | 12-17 | Renal (80%) | Idarucizumab, aPCC | aPTT (if normal, it excludes above on-therapy dabigatran levels but does not exclude the therapeutic range; TT (if normal, it excludes the presence of dabigatran); dTT; ECA |
| Apixaban | Factor Xa | 8-15 | Renal (25%) | 4F-PCC, andexanet alfa | Chromogenic anti-Xa assay |
| Betrixaban | Factor Xa | 19-27 | Renal (11%) | 4F-PCC, andexanet alfa | Chromogenic anti-Xa assay |
| Edoxaban | Factor Xa | 9-11 | Renal (35%) | 4F-PCC, andexanet alfa | PT (may be elevated, although a normal PT does not exclude clinically relevant levels); chromogenic anti-Xa assay |
| Rivaroxaban | Factor Xa | 9-13 | Renal (66%) | 4F-PCC, andexanet alfa | PT (may be elevated, although a normal PT does not exclude clinically relevant levels); chromogenic anti-Xa assay |
dTT: dilute thrombin time; ECA: ecarin chromogenic assay; 4F-PCC, 4 factor prothrombin complex concentrate.
Reversal of VKAs
VKAs inhibit vitamin K epoxide reductase, resulting in reduced production of functional vitamin K, which is a cofactor for vitamin K–dependent carboxylase, the enzyme that carboxylates the glutamic acid residues of factors II, VII, IX, and X, and anticoagulant proteins C, S, and Z.37 The glutamic acid residues mediate calcium-dependent binding of the coagulation factors to phospholipids.38 The anticoagulant effect of VKAs can be reversed in the setting of major bleeding via replacement of vitamin K, enhancing the hepatic production of γ-carboxylated coagulation factors, or by replacement of coagulation factors directly using plasma or prothrombin complex concentrates (PCCs).
Vitamin K
Vitamin K can be administered intravenously, orally, or subcutaneously, although the former 2 routes of administration have been shown to have more reproducible bioavailability than the subcutaneous route.38 In addition, even though both the oral and intravenous forms of vitamin K are equally effective in correcting the elevated INR to a similar degree after 24 hours,38,39 the intravenous form has the advantage of achieving this within 6 to 8 hours.39 Therefore, the American College of Chest Physicians guidelines recommend the intravenous route using doses of 5 to 10 mg of vitamin K as a slow infusion (over 30 minutes) for reversal of VKA-associated major bleeding, along with a rapid reversal agent such as PCC.40 The reason for the combined use is that PCC administration only provides a transient INR correction because of the short half-lives of the vitamin K–dependent coagulation factors, especially factor VII (6 hours). The administration of vitamin K is required for a more sustained INR correction by restoring the hepatic synthesis of these coagulation factors. The downside of using such high doses of vitamin K is a transient VKA resistance state with difficulties achieving therapeutic INRs.
Plasma vs PCC
Plasma contains all the vitamin K–dependent coagulation factors at a theoretical concentration of 1 U/mL and is readily available worldwide.41 However, ∼2 L of plasma is required to replace the coagulation factors, a volume that is difficult to transfuse rapidly, especially in elderly patients at risk of volume overload. In addition, plasma must be thawed and matched for blood group. Furthermore, transfusion of plasma is associated with complications such as acute lung injury, acute transfusion reactions, and a small risk of infections.42 In contrast to plasma, PCC does not need to be matched for blood group or thawed and can be administered in volumes 1/25th of plasma.43 There are 2 forms of nonactivated PCC available: 3-factor PCC and 4F-PCC, with the former having a much lower concentration of factor VII. Therefore, if 3-factor PCC is used for the acute reversal of warfarin anticoagulation, plasma can be added to provide a source of factor VII.44 A meta-analysis of 13 studies (5 randomized trials and 8 observational studies) that compared plasma vs PCC in patients requiring an urgent reversal of warfarin because of major bleeding or before an emergent surgery reported that PCC was associated with a significantly greater reduction of all-cause mortality, a more rapid INR correction, and less posttransfusion volume overload.45 Effective hemostasis was achieved in a higher proportion of patients that received PCC compared with those who received plasma, albeit not statistically significantly.45 Furthermore, there was no difference in the risk of thromboembolic events between the 2 groups.45 In accordance with these results, the American College of Chest Physicians guidelines recommend PCC over plasma for a rapid correction of warfarin-related bleeding.40
Reversal of heparins
UFH binds to antithrombin resulting in its conformational change, thereby enhancing antithrombin-mediated inhibition of thrombin and factor Xa. In contrast, LMWHs mainly inactivate factor Xa via antithrombin.16
Protamine sulfate
Protamine sulfate is a positively charged alkaline protein derived from fish sperm. It completely reverses the anticoagulant effects of UFH46 via forming a complex with the highly acidic and negatively charged heparin47 at a dose of 1 mg/100 U.47 Protamine sulfate has a very short half-life (about 7 minutes); therefore, repeated doses may be required for complete reversal of UFHs, although it is important to note that the maximum dose is 50 mg.16 The aPTT can be used to monitor the protamine-mediated UFH reversal.16 Protamine sulfate only partially reverses the anti-Xa activity of LMWHs (∼60%-80%).48 Therefore, even though the aPTT may normalize after protamine administration, it is important to also measure the anti-Xa activity.48
There is a black box warning about the potential for protamine sulfate causing uncommon hypersensitivity reactions, including anaphylaxis in patients with fish allergy, those with previous exposure to protamine or protamine-containing drugs such as NPH insulin, or those who receive high doses, rapid administration, or repeated doses of protamine sulfate.16 Patients with known protamine sulfate allergy can be pretreated with steroids and antihistamines.16 Protamine sulfate-related thrombocytopenia has also been reported, especially in patients undergoing cardiac surgery.49,50
rFVIIa
Recombinant factor VIIa (rFVIIa) has been shown in an ex vivo study to reverse the anticoagulant effects of heparin and enoxaparin51 and to control the heparin- and LMWH-related bleeding in animal studies52 and case reports.53,54 However, given the lack of evidence showing the effectiveness and a possible risk of thromboembolic complication with off-label use of rFVIIa,55 its use should be limited to management of heparin- or LMWH-related severe bleeding not responding to other reversal measures.
Reversal of pentasaccharide anticoagulants
Fondaparinux is currently the only pentasaccharide anticoagulant in use for VTE treatment and prevention. It binds to antithrombin and inhibits factor Xa only.56 Fondaparinux does not have a specific antidote. Protamine is not effective in reversing the anticoagulant effects of fondaparinux.
aPCC
In an animal model, aPCC corrected the endogenous thrombin potential and reduced the duration of fondaparinux-related bleeding.57 In an in vitro study involving nonbleeding healthy volunteers, PCC, aPCC, and rFVIIa were compared in regards to correction of thrombin generation.58 Low-dose aPCC (20 U/kg) completely corrected the thrombin generation, whereas rFVIIa partially corrected the thrombin generation.56 These results are limited to the reversal effects seen in vitro, which may not translate to in vivo efficacy.
rFVIIa
In addition to reversing the anticoagulant effect of fondaparinux in vitro,59 rFVIIa at high doses (90 mcg/kg) has been shown to normalize endogenous thrombin potential, aPTT, and PT in healthy volunteers given therapeutic doses of fondaparinux (10 mg).59,60 Furthermore, a case series on the use of rFVIIa at a 90 mcg/kg dose for reversal of fondaparinux-related life-threatening bleeding reported successful management of bleeding in 4 of 8 patients.61 Therefore, rFVIIa can be used for reversal of fondaparinux-related critical bleeding, although the increased risk of thromboembolic events with its off-label use should be considered.55
Reversal of DTIs
Direct thrombin inhibitors (DTIs) inhibit thrombin independent of antithrombin.62 Bivalent DTIs inhibit thrombin at both the active site and exosite 1 and include hirudin and bivalirudin, whereas argatroban and dabigatran are univalent DTIs that bind to the active site only.62 Except for dabigatran, which is an oral DTI, all DTIs are parenteral anticoagulants.62 There is no direct reversal agent available for the parenteral DTIs, although bleeding may be managed by discontinuing agents, given their short half-lives.62
Hemodialysis
Because of the low plasma protein binding of dabigatran, hemodialysis may be used for its removal. A systematic review of 22 studies involving 35 patients who underwent renal replacement therapy (RRT) for reversal of dabigatran-related bleeding reported that hemostasis was achieved in 24 (71%) of patients.63 Dabigatran plasma concentration was also significantly reduced following RRT; however, there was a rebound increase in the dabigatran plasma concentration in 12 patients (57%) after cessation of RRT.63 In addition, the logistic issue of insertion of a dialysis catheter in a fully anticoagulated patient with active bleeding makes dialysis a challenging reversal option.
PCC
In a phase 1 study of 6 healthy volunteers receiving dabigatran vs placebo, 4F-PCC was unable to correct aPTT, ecarin clotting time, or TT.64 In contrast, 4F-PCC completely reversed PT and endogenous TT in healthy volunteers receiving rivaroxaban.64 Therefore, 4F-PCC has not been investigated further for the reversal of the anticoagulant effects of dabigatran.
aPCC
A prospective cohort study of 14 patients with dabigatran-related major bleeding examined the effectiveness and safety of aPCC at a dose of 50 U/kg.65 The effectiveness of aPCC was evaluated as good in 9 (64%) and moderate in the rest (36%). There were no thromboembolic complications. In countries that have no access to idarucizumab, aPCC may be an alternative for management of dabigatran-associated major bleeding.
Idarucizumab
Idarucizumab, a specific reversal agent for dabigatran, is a monoclonal Fab antibody fragment that binds to dabigatran and completely reverses its anticoagulant effect within minutes.66 The final report of the Reversal Effects of Idarucizumab on Active Dabigatran trial analyzed outcomes in 503 patients who received idarucizumab for reversal of dabigatran in the setting of major bleeding (301 patients in group A) or urgent surgery (202 patients in group B).66 Of the 203 evaluable patients with major bleeding, 134 (67.7%) had complete cessation of bleeding within 24 hours (the median time to hemostasis was 2.5 hours after idarucizumab administration). Of the 197 patients undergoing urgent surgery, hemostasis was assessed as normal in 184 (93%), mildly abnormal in 10 (5%), and moderately abnormal in 3 (2%). Thromboembolic complications occurred in 24 of the 503 patients (4.8%) within 30 days and in 34 patients (6.8%) within 90 days. The antidote should be given as a bolus dose of 5 g intravenously and has been approved for use in case of major bleeding as well as for emergency surgery to prevent bleeding.
Reversal of direct factor Xa inhibitors
Apixaban, betrixaban, edoxaban, and rivaroxaban directly inhibit factor Xa independently of antithrombin. Options for the management of direct factor Xa inhibitors are similar to those for dabigatran-related bleeding, except direct factor Xa inhibitors are highly protein bound and are unlikely to be removed from the circulation via hemodialysis.
PCC
Two prospective cohort studies evaluated the effectiveness and safety of 4F-PCC for the management of major bleeding in patients taking apixaban or rivaroxaban.67,68 A Swedish study of 84 patients who received a median 4F-PCC dose of 2000 U reported that it was effective in 58 (69%) patients and ineffective in 26 (30.9%).67 Most of the patients in the latter group (61.5%) had ICH. Two patients had ischemic stroke during a 30-day follow-up. In a Canadian study, 66 patients received 4F-PCC for management of major bleeding (54 had 4F-PCC at a fixed dose of 2000 U as part of a hospital protocol).68 The management of major bleeding was assessed as effective in 45 (68%) and ineffective in 21 patients (32%). Five patients (8%) had a major thromboembolic event and 9 patients (14%) died during a 30-day follow-up. Seven of these deaths were adjudicated as a result of the initial ICH.
Andexanet alfa
Andexanet alfa is an inactive form of factor Xa that acts as a “decoy” via binding and sequestering the factor Xa inhibitors, which also include fondaparinux and LMWH.69 The interim report of the Prospective, Open-Label Study of Andexanet Alfa in Patients Receiving a Factor Xa Inhibitor Who Have Acute Major Bleeding study examined the efficacy and safety of andexanet alfa in managing major bleeding in 228 patients who were taking a factor Xa inhibitor.69 Of 132 patients adjudicated for efficacy, good or excellent hemostasis was achieved at 12 hours in 109 patients (83%, 95% confidence interval, 75-89). At 30 days, 24 patients (11%) had a thrombotic event and 27 (12%) died.69 The US Food and Drug Administration has recently approved the antidote, but it will initially be available at a limited number of hospitals only. The antidote has only been studied for reversal in patients with major bleeding and not for emergency surgery. It is reasonable to use 4F-PCC for management of Xa inhibitor-associated major bleeds in centers that lack access to andexanet alfa. The use of 4F-PCC for the latter indication has been compared with andexanet alfa in Table 5.
Comparison of 4F-PCC and andexanet alfa for the reversal of direct factor Xa inhibitors
| Reversal agent . | 4F-PCC . | Andexanet alfa . |
|---|---|---|
| Mechanism of action | Reverses PT and endogenous thrombin generation; indirect, nonspecific pro-hemostatic | Acts as a “decoy” via binding and sequestering the factor Xa inhibitors Direct specific antidote |
| Dose | 50 U/kg for major bleeding or a fixed dose of 2000 U used for simplicity | Dose depends on the dose and timing of the last dose of the FXa inhibitor* Low dose: 400 mg intravenous bolus at 30 mg/min, followed by infusion of 480 mg at 4 mg/min for up to 2 h High dose: 800 mg intravenous bolus at 30 mg/min, followed by infusion of 960 mg 8 mg/min for up to 2 h |
| Availability | Readily available at most hospitals | Limited availability |
| Rate of effective management of major bleeding (95% CI) | With the ISTH criteria: 69% (61%-76%) Not using the ISTH criteria: 75% (53%-97%)70 | 83% (75%-89%)69 |
| Rate of thromboembolic complications (95% CI) | 5% (1%-9%)70 | 11% (NA)69 |
| Cost | $1.27† per unit71 For 2000 U 4F-PCC $2540 | $3300 per vial of 100 mg72 Low dose (9 vials) $29 700, high dose (18 vials) $59 400 |
| Reversal agent . | 4F-PCC . | Andexanet alfa . |
|---|---|---|
| Mechanism of action | Reverses PT and endogenous thrombin generation; indirect, nonspecific pro-hemostatic | Acts as a “decoy” via binding and sequestering the factor Xa inhibitors Direct specific antidote |
| Dose | 50 U/kg for major bleeding or a fixed dose of 2000 U used for simplicity | Dose depends on the dose and timing of the last dose of the FXa inhibitor* Low dose: 400 mg intravenous bolus at 30 mg/min, followed by infusion of 480 mg at 4 mg/min for up to 2 h High dose: 800 mg intravenous bolus at 30 mg/min, followed by infusion of 960 mg 8 mg/min for up to 2 h |
| Availability | Readily available at most hospitals | Limited availability |
| Rate of effective management of major bleeding (95% CI) | With the ISTH criteria: 69% (61%-76%) Not using the ISTH criteria: 75% (53%-97%)70 | 83% (75%-89%)69 |
| Rate of thromboembolic complications (95% CI) | 5% (1%-9%)70 | 11% (NA)69 |
| Cost | $1.27† per unit71 For 2000 U 4F-PCC $2540 | $3300 per vial of 100 mg72 Low dose (9 vials) $29 700, high dose (18 vials) $59 400 |
CI, confidence interval; NA, not available.
Low dose is used for all patients receiving apixaban and for those receiving rivaroxaban ≥8 h ago. High dose is used for those receiving rivaroxaban <8 h ago or at an unknown time.
Price is in USD. Cost of 4F-PCC is lower in Canada and Europe.
Antidotes vs supportive care
Antidotes are an appreciated addition to the armamentarium for management of anticoagulant-associated bleeds, but their importance should not be overestimated. None of the antidotes or PCC have been studied in a randomized controlled trial. It is therefore impossible to know how much of the reported improvement can be ascribed to the supportive management, to the endogenous elimination of the anticoagulant drug, or simply to the natural course of the disease. In an analysis of the major bleeds in treatment trials with dabigatran, supportive care was associated with good effectiveness in 91% of patients on dabigatran.73 The antidotes are expensive and should therefore be used with discretion. It is unclear what proportion of the reported thromboembolic events can be attributed to the reversal agents. However, the underlying procoagulant condition, activation of coagulation by bleeding/surgery, and delay in start of thromboprophylaxis or resumption of anticoagulation certainly contribute to these events.
Future developments
New anticoagulants targeted at coagulation factors XI and XII are under development.74 As opposed to the currently available DOACs, several different strategies to target these factors are explored. These include antibodies, antisense oligonucleotides, aptamers, polyanion antagonists, and the more traditional small molecules that bind to the active site. Although the main objective of this development is to retain the thromboprophylactic effect and to further reduce the risk of bleeding vs present-day anticoagulants, hemorrhage can never be eliminated when resulting from trauma, ruptured blood vessels, or need for emergent major surgery. It will probably become a requirement for approval of any new anticoagulant to have an effective reversal tool available that will differ for the various strategies listed in this article.
Authorship
Contribution: S.P. and S.S. wrote the paper.
Conflict-of-interest disclosure: S.S. has received honoraria from Boehringer Ingelheim, Bayer HealthCare, Daiichi Sankyo, and Sanofi, and research support from Boehringer Ingelheim and Octapharma. The remaining author declares no competing financial interests.
Correspondence: Sam Schulman, Thrombosis Service, HHS-General Hospital, 237 Barton St E, Hamilton, ON, L8L 2X2, Canada; e-mail: schulms@mcmaster.ca.