Key Points
A biomarker staging system for AL amyloidosis using thresholds of BNP >81 pg/mL and TnI >0.1 ng/mL strongly correlates with NT-proBNP-based systems.
This new BNP-based staging system predicts overall survival in patients with AL amyloidosis.
Abstract
Immunoglobulin light chain amyloidosis (AL amyloidosis) is caused by misfolded light chains that form soluble toxic aggregates that deposit in tissues and organs, leading to organ dysfunction. The leading determinant of survival is cardiac involvement. Current staging systems use N-terminal pro-brain natriuretic peptide (NT-proBNP) and cardiac troponins T and I (TnT and TnI) for prognostication, but many centers do not offer NT-proBNP. We sought to derive a new staging system using brain natriuretic peptide (BNP) that would correlate with the Mayo 2004 staging system and be predictive for survival in AL amyloidosis. Two cohorts of patients were created: a derivation cohort of 249 consecutive patients who had BNP, NT-proBNP, and TnI drawn simultaneously to create the staging system and a complementary cohort of 592 patients with 10 years of follow-up to determine survival. In the derivation cohort, we found that a BNP threshold of more than 81 pg/mL best associated with Mayo 2004 stage and also best identified cardiac involvement. Three stages were developed based on a BNP higher than 81 pg/mL and a TnI higher than 0.1 ng/mL and compared with Mayo 2004 with high concordance (κ = 0.854). In the complementary cohort, 25% of patients had stage I, 44% had stage II, 15% had stage III, and 16% had stage IIIb disease with a median survival not reached in stage I, 9.4 years in stage II, 4.3 years in stage III, and 1 year in stage IIIb. This new Boston University biomarker scoring system will allow centers without access to NT-proBNP the ability to appropriately stage patients with AL amyloidosis. This trial was registered at www.clinicaltrials.gov as #NCT00898235.
Medscape Continuing Medical Education online

In support of improving patient care, this activity has been planned and implemented by Medscape, LLC and the American Society of Hematology. Medscape, LLC is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.00 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 75% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 286.
Disclosures
CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, owns stock, stock options, or bonds from Pfizer. Associate Editor Laurie H. Sehn served as an advisor or consultant for AbbVie, Amgen, Celgene, Janssen, Karyopharm Therapeutics, Merck, Roche/Genentech, Seattle Genetics, and TG Therapeutics and received grants for clinical research from Roche/Genentech. The authors declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Assess a new brain natriuretic peptide (BNP)-based biomarker staging system for light chain (AL) amyloidosis and its correlation with N-terminal pro-BNP-based systems, based on a retrospective cohort study
Determine the predictive value of this BNP-based staging system for overall survival in AL amyloidosis, based on a retrospective cohort study
Evaluate the clinical implications of findings regarding this BNP-based staging system for AL amyloidosis, based on a retrospective cohort study
Release date: January 17, 2019; Expiration date: January 17, 2020
Introduction
Systemic light-chain (AL) amyloidosis is characterized by the overproduction of immunoglobulin light chains secreted by clonal bone marrow plasma cells that misfold and form soluble toxic aggregates. These aggregates deposit as amyloid fibrils in multiple organs, causing widespread damage and dysfunction.1 Progressive amyloid deposition results in organ failure, which is especially catastrophic when the heart is affected, as cardiac involvement is the primary driver of death in AL disease.1 Cardiac involvement has been noted in approximately 70% of patients with systemic AL amyloidosis.2,3 Myocardial amyloid deposition in cardiac tissues causes dysfunction through both architectural damage as well as through direct myocardial toxicity and oxidative damage by amyloidogenic light chains, possibly via interactions with mitochondrial proteins.4 Amyloidogenic light chains purified from patients with amyloid cardiomyopathy induce p38 mitogen-activated protein kinase signaling, resulting in oxidative stress, impaired excitation-contraction coupling, and eventually cardiomyocyte death.5,6 Evidence of myocardial toxicity and cellular injury can be determined clinically by measurement of circulating cardiac-specific biomarkers: N-terminal pro-brain natriuretic peptide (NT-proBNP), brain natriuretic peptide (BNP), and cardiac troponin T (TnT) or troponin I (TnI).7,8 Interestingly, light chain induced mitogen-activated protein kinase signaling also induces BNP and NT-proBNP precursor protein synthesis, and thus natriuretic peptide-based staging systems in particular have garnered unique attention as indices of both cardiac injury and amyloid disease activity.9
Given the central role cardiac involvement plays in the survival of patients with AL amyloidosis, biomarker staging systems were developed on the basis of circulating markers of cardiac, renal, and clonal disease specifically using serum concentrations of NT-proBNP, cardiac TnT, and the concentration of circulating amyloidogenic free light chains (FLCs).10 The Mayo Clinic group first established a simple and reliable staging system in 2004 based on NT-proBNP and cardiac troponins, which was modified by European investigators (NT-proBNP >8500 pg/mL) to improve discrimination of very high risk patients.11,12 This cardiac staging system is the system most widely used for clinical trial design and patient management. It was then determined that clonal burden, assessed by bone marrow plasma cell infiltration and dFLC (difference between involved and uninvolved circulating FLCs), afforded additional information regarding survival. Participants who have a very low (<50 mg/L) dFLC level had a significantly better outcome across different stages.13-15 The Mayo Clinic group incorporated the dFLC level (>180 mg/L) in their revised staging system in 2012.10
Although measurement of NT-proBNP is incontrovertibly useful, many medical facilities do not possess assays for NT-proBNP, and instead use the cleaved hormone, BNP. BNP and NT-proBNP are both derived from the same precursor peptide (preproBNP) that is secreted by cardiac myocytes in response to distension and stretching caused by volume expansion and/or pressure overload.16 PreproBNP is then cleaved to proBNP, which is again cleaved to the active hormone BNP (amino acids 77-108) and the biologically inactive NT-proBNP (amino acids 1-76).17,18 Although BNP and NT-proBNP are secreted at a 1:1 equimolar basis, they are differentially subjected to renal clearance, and circulating NT-proBNP has a longer half-life than BNP.17,18 For these reasons, measurements of NT-proBNP and BNP are not readily interchangeable.
Less well studied than NT-proBNP, BNP has also been shown to be predictive of mortality in AL cardiac amyloidosis, but the marker has not been included in staging systems.10,19 In fact, in the setting of advanced renal disease, BNP may even be superior to NT-proBNP for survival prognostication.20 With these considerations, we retrospectively developed a staging system using BNP that might align with an established NT-proBNP staging paradigm, identify cardiac involvement, and predict survival among a large cohort of patients with AL amyloidosis.
Methods
Patient population
Two separate cohorts of patients were identified retrospectively. The derivation cohort consisted of 250 consecutive patients with AL amyloidosis who were evaluated at the Amyloidosis Center of Boston University/Boston Medical Center from April 2016 through September 2016. This time frame was chosen as both NT-proBNP and BNP were measured in all patients at the time of evaluation. From this cohort, 2 analyses were performed. First, we determined the optimal BNP value to develop a staging system that matched the Mayo 2004 system using NT-proBNP (while maintaining a common cutoff TnI of 0.1 ng/mL in both systems), and second, we determined the optimal BNP value to develop a staging system that identified cardiac involvement. A second complementary cohort consisted of 1073 patients with AL amyloidosis studied on initial visit to the center between 2004 and 2014. The complementary cohort was developed to determine the prognostic utility of the BNP-based staging system that was determined from the derivation cohort analysis. Similar to the Mayo staging system, patients were classified as stage I if both biomarkers were below threshold, stage II if 1 biomarker was above threshold, and stage III if both biomarkers were above threshold. To subclassify patients with stage III as stage IIIb, a BNP cutoff was determined via receiver operating characteristic (ROC) analysis of survival at 1 year. Clinical and laboratory data were obtained from review of the electronic health records and from the prospectively maintained database of the Amyloidosis Center. The presence of cardiac involvement was determined by the following criteria, in order of preference: endomyocardial biopsy or cardiac magnetic resonance imaging (MRI) consistent with cardiac amyloidosis, intraventricular septal end diastole (IVSd) thickness of at least 12 mm obtained on transthoracic echocardiography without other cause of wall thickening (consistent with established consensus criteria),21 and IVSd at least 11 mm in men or at least 10 mm in women with no history of hypertension or valvular disease (consistent with current reference ranges, as established by the American Society of Echocardiography).22 Renal involvement was defined by the consensus criteria of the International Society of Amyloidosis.21 All patients provided written informed consent to have their records used for research studies in accordance with the Institutional Review Board’s requirements, in accordance with the Declaration of Helsinki and the Health Insurance Portability and Accountability Act guidelines.
Data collection
All laboratory values were obtained at time of initial clinic visit and were recorded in the electronic health record. Laboratory testing was performed in the clinical laboratory of Boston Medical Center. BNP has a minimum concentration of detection of 10 pg/mL and a threshold of heart failure established at 100 pg/mL. NT-proBNP has a minimum concentration of detection of 10 pg/mL and an age-adjusted threshold for heart failure that begins at 300 pg/mL. TnI has a minimum concentration of detection of 0.006 ng/mL and is considered abnormal at 0.013 ng/mL in women and 0.033 ng/mL in men. Estimated glomerular filtration rate (eGFR) was calculated using the chronic kidney disease (CKD)–epidemiology collaboration creatinine equation based on creatinine and age at the time of visit. Echocardiographic variables, including interventricular septal thickness and left ventricular ejection fraction, were collected from the electronic health record. The primary outcome was overall survival (OS).
Statistical analysis
Baseline characteristics were presented as either percentage of total for categorical variables or median and interquartile range for continuous variables. Categorical variables were tested for significance using either χ2 or Fisher exact test, as appropriate. Continuous variables were compared using the Mann-Whitney U test. ROC analyses were determined for BNP and NT-proBNP against the presence of cardiac involvement. Youden’s numbers, area under the curve (AUC), and confidence intervals (CIs) were determined. Concordance analysis between the Mayo 2004 staging system (TnI > 0.1 ng/mL; NT-proBNP > 332 pg/mL) and a staging system using TnI > 0.1 ng/mL and the derived value for BNP was determined using linear weighted κ statistics. To test for significance between sensitivity and specificity, McNemar’s test was performed on 2 × 2 tables comparing Mayo biomarker stage (NT-proBNP) and Boston University (BU) biomarker stage (BNP), using patients with or without cardiac involvement.23 In the complementary cohort, survival was calculated from the time of diagnosis. BNP cutoff for stage IIIb was determined via ROC analysis of survival at 1 year. Kaplan-Meier with log rank analysis between groups was performed to determine significant differences in survival between the 4 biomarker stages. Differences in patient characteristics among complementary cohort stages were determined using the same statistical methods as the baseline characteristics for the derivation cohort. All statistics were performed with MedCalc Statistical Software version 18.2.1 (MedCalc Software bvba, Ostend, Belgium).
Results
Patient characteristics of derivation cohort
Two hundred fifty consecutive patients with AL amyloidosis were evaluated from April 2016 through September 2016. One patient was excluded from analysis as a result of not having a full set of cardiac biomarkers available, rendering a final derivation cohort of 249 patients. Baseline clinical and laboratory characteristics of the patients in the derivation cohort are presented in Table 1. On the basis of the criteria for cardiac amyloidosis identification summarized here, 116 (47%) patients had evidence of cardiac involvement and 133 (53%) patients did not. Cardiac involvement was determined by endomyocardial biopsy in 22 (19%) patients, by cardiac MR in the context of positive extracardiac biopsy for light-chain amyloidosis in 18 (15%) patients, and by echocardiographic criteria in 76 (66%) patients, of whom 82% had IVSd of at least 12 mm. The median age of both groups, with and without cardiac involvement, was 65 years (interquartile range, 59-71 years). There were no significant differences in renal involvement or history of atrial fibrillation, diabetes, or coronary artery disease between these 2 groups. Functional status was significantly worse in patients with cardiac involvement, with 54% of patients having NYHA class II and III symptoms compared with 17% of patients without cardiac involvement. As expected, BNP, NT-proBNP, and TnI levels were significantly elevated in patients with cardiac involvement (P < .001). Median IVSd was 3 mm greater in patients with cardiac involvement (P < .001), whereas there were no differences in left ventricular ejection fraction observed. Patients with cardiac amyloidosis were more likely to have a λ typic plasma cell dyscrasia (P < .001), and also more likely to have autonomic neuropathy (P = .001). Similar differences between groups were observed when excluding the 14 patients who were adjudicated to have cardiac involvement with an IVSd smaller than 12 mm and no history of positive cardiac biopsy or cardiac MRI.
Baseline differences between patients with and without evidence of cardiac involvement in the derivation cohort
| . | Absence of cardiac involvement (n = 133) . | Presence of cardiac involvement (n = 116) . | P . | ||
|---|---|---|---|---|---|
| Number (%) . | Median (25%-75%) . | Number (%) . | Median (25%-75%) . | ||
| General characteristics | |||||
| Male sex | 68 (51) | 68 (59) | .2 | ||
| Age | 65 (59-71) | 65 (60-70) | .051 | ||
| BMI, kg/m2 | 28.0 (22.3.6-32.6) | 26.3 (23.5-29.8) | .03 | ||
| Amyloidosis involvement | |||||
| Chain type, κ/λ* | 44/88 | 15/101 | <.001 | ||
| Peripheral nervous system involvement | 8 (6) | 15 (13) | .048 | ||
| Autonomic nervous system involvement | 11 (8) | 26 (22) | .001 | ||
| Kidney involvement | 86 (64) | 74 (63) | .9 | ||
| Medical history | |||||
| Atrial fibrillation | 15 (11) | 21 (18) | .1 | ||
| Hypertension | 73 (54) | 38 (32) | <.001 | ||
| Diabetes | 12 (9) | 12 (10) | .7 | ||
| Coronary artery disease | 10 (9) | 16 (14) | .1 | ||
| Stroke | 2 (2) | 8 (7) | .03 | ||
| Functional status | |||||
| NYHA class | <.001 | ||||
| I | 111 (83) | 53 (46) | |||
| II | 18 (14) | 43 (37) | |||
| III | 4 (3) | 20 (17) | |||
| Laboratory studies | |||||
| FLC-diff | 13.4 (5.1-34.9) | 28.4 (6.3-93.8) | .008 | ||
| BNP, pg/mL | 54 (26-103) | 181 (94-529) | <.001 | ||
| NT-proBNP, pg/mL | 181 (74-604) | 1313 (451-4193) | <.001 | ||
| Troponin I, ng/mL | 0.006 (0.006-0.015) | 0.041 (0.012-0.116) | <.001 | ||
| eGFR, mL/min/1.73 m2 | 69 (37-86) | 60 (38-81) | .3 | ||
| Transthoracic echocardiography | |||||
| IVSd, mm | 9 (8-10) | 12 (11-14) | <.001 | ||
| Ejection fraction, % | 65 (60-69) | 60 (50-65) | .003 | ||
| . | Absence of cardiac involvement (n = 133) . | Presence of cardiac involvement (n = 116) . | P . | ||
|---|---|---|---|---|---|
| Number (%) . | Median (25%-75%) . | Number (%) . | Median (25%-75%) . | ||
| General characteristics | |||||
| Male sex | 68 (51) | 68 (59) | .2 | ||
| Age | 65 (59-71) | 65 (60-70) | .051 | ||
| BMI, kg/m2 | 28.0 (22.3.6-32.6) | 26.3 (23.5-29.8) | .03 | ||
| Amyloidosis involvement | |||||
| Chain type, κ/λ* | 44/88 | 15/101 | <.001 | ||
| Peripheral nervous system involvement | 8 (6) | 15 (13) | .048 | ||
| Autonomic nervous system involvement | 11 (8) | 26 (22) | .001 | ||
| Kidney involvement | 86 (64) | 74 (63) | .9 | ||
| Medical history | |||||
| Atrial fibrillation | 15 (11) | 21 (18) | .1 | ||
| Hypertension | 73 (54) | 38 (32) | <.001 | ||
| Diabetes | 12 (9) | 12 (10) | .7 | ||
| Coronary artery disease | 10 (9) | 16 (14) | .1 | ||
| Stroke | 2 (2) | 8 (7) | .03 | ||
| Functional status | |||||
| NYHA class | <.001 | ||||
| I | 111 (83) | 53 (46) | |||
| II | 18 (14) | 43 (37) | |||
| III | 4 (3) | 20 (17) | |||
| Laboratory studies | |||||
| FLC-diff | 13.4 (5.1-34.9) | 28.4 (6.3-93.8) | .008 | ||
| BNP, pg/mL | 54 (26-103) | 181 (94-529) | <.001 | ||
| NT-proBNP, pg/mL | 181 (74-604) | 1313 (451-4193) | <.001 | ||
| Troponin I, ng/mL | 0.006 (0.006-0.015) | 0.041 (0.012-0.116) | <.001 | ||
| eGFR, mL/min/1.73 m2 | 69 (37-86) | 60 (38-81) | .3 | ||
| Transthoracic echocardiography | |||||
| IVSd, mm | 9 (8-10) | 12 (11-14) | <.001 | ||
| Ejection fraction, % | 65 (60-69) | 60 (50-65) | .003 | ||
One patient did not have light chain typing performed.
Derivation cohort analysis: BNP threshold determination for Mayo stage concordance
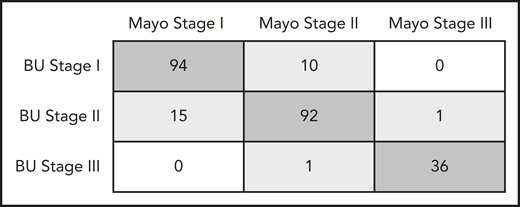
Using the derivation cohort, we developed a staging system that best matched the categorization of the Mayo 2004 scheme (TnI > 0.1 ng/mL; NT-proBNP > 332 pg/mL). We elected to maintain the TnI threshold higher than 0.1 ng/mL and then varied stage assignments by different BNP values with the goal of finding a BNP threshold that would best predict having an NT-proBNP higher than 332 pg/mL. We compared the results of our new BNP-based system with the Mayo 2004 staging, using linear weighted κ statistics. The optimal agreement between the 2 staging systems occurred with a BNP threshold higher than 81 pg/mL (κ = 0.854). A matrix of Mayo vs BU (BNP-based) biomarker staging using the BNP threshold higher than 81 pg/mL is illustrated in Figure 1. This threshold of 81 pg/mL was identical to the value obtained through ROC analysis for cardiac involvement (see following). Using a BNP threshold higher than 81 pg/mL and TnI higher than 0.1 ng/mL, the new staging system resulted in 89.2% of all patients being placed in identical categories as under Mayo 2004 staging.
Concordance between Mayo 2004 stage and BU stage. There is an 89.2% agreement between staging systems (κ = 0.854).
Concordance between Mayo 2004 stage and BU stage. There is an 89.2% agreement between staging systems (κ = 0.854).
Derivation cohort analysis: BNP threshold determination for cardiac involvement
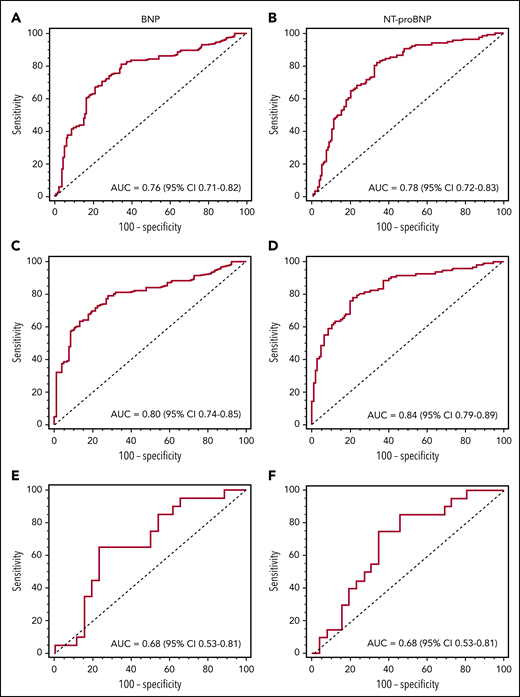
Next, ROC plots were created to determine optimal BNP threshold values for cardiac involvement in patients with and without advanced CKD. Advanced CKD was defined as an eGFR of less than 30 mL/min/1.73 m2, or stages IV to V, based on the National Kidney Foundation’s published staging system.24 To determine whether a BNP-based system would be superior to the Mayo 2004 system, we also constructed ROC curves based on NT-proBNP (Figure 2). Both BNP and NT-proBNP-based systems were effective in adjudicating cardiac involvement, as suggested by statistically significant AUC (P < .05), regardless of the presence or absence of advanced CKD. Among patients without any CKD, NT-proBNP (AUC, 0.84; 95% confidence interval [CI], 0.79-0.89) proved to be superior to BNP (AUC, 0.80; 95% CI, 0.74-0.85; P = .006) for identification of cardiac amyloidosis (Figure 2C-D). When patients with advanced CKD were included, however, there was no statistical difference between BNP or NT-proBNP (Figure 2A-B,E-F) for cardiac amyloidosis identification. For both BNP and NT-proBNP, the optimal threshold value was the same when considering all patients or only patients without CKD. For BNP, this value was 81 pg/mL (Youden’s J = 0.47 in all patients and J = 0.51 in non-CKD patients) and for NT-proBNP, it was 288 pg/mL (Youden’s J = 0.48 in all patients and J = 0.57 in non-CKD patients). It is notable that this NT-proBNP threshold for cardiac amyloidosis adjudication is different than the Mayo 2004 threshold of 332 pg/mL, which was derived from survival analysis. As anticipated, biomarker threshold values to identify cardiac involvement were significantly greater in patients with advanced CKD with a BNP threshold of 472 pg/mL (Youden’s J = 0.42) and an NT-proBNP cutoff of 2930 pg/mL (Youden’s J = 0.40).
ROC curves based on presence of cardiac involvement. (A) BNP in all patients (n = 249) with a threshold higher than 81 pg/mL. (B) NT-proBNP in all patients (n = 249) with a threshold higher than 288 pg/mL. (C) BNP in patients with eGFR at least 30 mL/min/1.73 m2 (n = 203) with a threshold higher than 81 pg/mL. (D) NT-proBNP in patients with eGFR at least 30 mL/min/1.73 m2 (n = 203) with a threshold higher than 288 pg/mL. (E) BNP in patients with eGFR less than 30 mL/min/1.73 m2 (n = 46) with a threshold higher than 472 pg/mL. (F) NT-proBNP in patients with eGFR less than 30 mL/min/1.73 m2 (n = 46) with a threshold higher than 2930 pg/mL.
ROC curves based on presence of cardiac involvement. (A) BNP in all patients (n = 249) with a threshold higher than 81 pg/mL. (B) NT-proBNP in all patients (n = 249) with a threshold higher than 288 pg/mL. (C) BNP in patients with eGFR at least 30 mL/min/1.73 m2 (n = 203) with a threshold higher than 81 pg/mL. (D) NT-proBNP in patients with eGFR at least 30 mL/min/1.73 m2 (n = 203) with a threshold higher than 288 pg/mL. (E) BNP in patients with eGFR less than 30 mL/min/1.73 m2 (n = 46) with a threshold higher than 472 pg/mL. (F) NT-proBNP in patients with eGFR less than 30 mL/min/1.73 m2 (n = 46) with a threshold higher than 2930 pg/mL.
Derivation cohort analysis: CKD
To further clarify whether a BNP- or NT-proBNP-based staging system (as opposed to the natriuretic peptide alone) was superior for identification of patients with cardiac involvement in the presence of advanced CKD, we constructed 2 × 2 tables based on eGFR (Table 2). For the purposes of this analysis, patients in stage II or III were defined to have cardiac involvement, whereas patients in stage I were defined to have no cardiac involvement. This categorization based on biomarker stage was compared with cardiac involvement as defined in this study (by biopsy, cardiac MRI, or echocardiography parameters). There was no significant difference in sensitivity between the Mayo 2004 staging system and the BU staging system at any eGFR for cardiac amyloidosis identification. Similarly, there was no difference in specificity when analyzing all patients; however, in patients with an eGFR lower than 30 mL/min/1.73 m2, the difference approached significance (P = .063). Consistent with the data presented here, the Mayo 2004 staging system did show superior specificity over the BNP-based system when excluding patients with advanced CKD (P = .023), which correlates with the ROC analysis finding of superiority using an NT-proBNP based system in this population.
Sensitivity and specificity of staging systems depending on eGFR in the derivation cohort
| . | Sensitivity (95% CI) . | P . | Specificity (95% CI) . | P . |
|---|---|---|---|---|
| All patients (n = 249) | ||||
| BU | 0.83 (0.78-0.88) | 1 | 0.63 (0.57-0.69) | .481 |
| Mayo | 0.82 (0.77-0.87) | 0.66 (0.60-0.72) | ||
| eGFR ≥ 30 mL/min/1.73 m2(n = 203) | ||||
| BU | 0.80 (0.75-0.86) | .688 | 0.70 (0.64-0.76) | .023 |
| Mayo | 0.78 (0.72-0.84) | 0.79 (0.73-0.84) | ||
| eGFR < 30 mL/min/1.73 m2(n = 46) | ||||
| BU | 0.95 (0.89-1) | 1 | 0.35 (0.21-0.48) | .063 |
| Mayo | 1 (1-1) | 0.15 (0.05-0.26) |
| . | Sensitivity (95% CI) . | P . | Specificity (95% CI) . | P . |
|---|---|---|---|---|
| All patients (n = 249) | ||||
| BU | 0.83 (0.78-0.88) | 1 | 0.63 (0.57-0.69) | .481 |
| Mayo | 0.82 (0.77-0.87) | 0.66 (0.60-0.72) | ||
| eGFR ≥ 30 mL/min/1.73 m2(n = 203) | ||||
| BU | 0.80 (0.75-0.86) | .688 | 0.70 (0.64-0.76) | .023 |
| Mayo | 0.78 (0.72-0.84) | 0.79 (0.73-0.84) | ||
| eGFR < 30 mL/min/1.73 m2(n = 46) | ||||
| BU | 0.95 (0.89-1) | 1 | 0.35 (0.21-0.48) | .063 |
| Mayo | 1 (1-1) | 0.15 (0.05-0.26) |
Complementary cohort analysis: survival with a BNP-based staging system
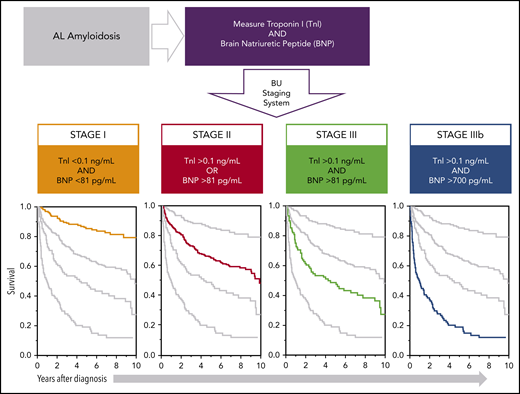
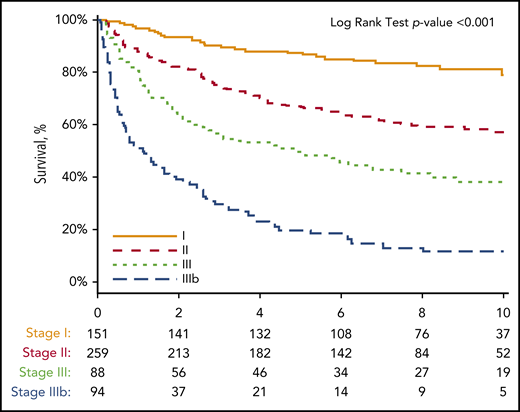
Having demonstrated that a BNP threshold higher than 81 pg/mL effectively identified both cardiac involvement and stratified patients to a concordant Mayo 2004 biomarker stage, further analysis was performed to determine whether this novel BNP-based staging system would be predictive of OS. Because the derivation cohort had only 2 years of follow-up available, a second cohort of 1073 patients with AL amyloidosis evaluated at an initial visit between 2004 and 2014 was identified (NT-proBNP was not measured at our center before 2014). A total of 592 patients (55%) had both BNP and TnI drawn at the initial visit, and thus were able to be assigned a biomarker stage, comprising the final complementary cohort. Of these 592 patients, 151 patients (25.5%) were assigned to stage I, 259 patients (43.6%) were assigned to stage II, and 182 patients (30.7%) were assigned to stage III. The median time from diagnosis to initial visit at our center was 2.3 months. Of 592 patients, 149 (25.2%) received high-dose melphalan and stem cell transplantation (37.1% with stage I, 25.9% with stage II, and 14.3% with stage III), 203 (34.3%) received proteasome inhibitor-based treatment regimens, 67 (11.3%) received immunomodulatory agent-based regimens, and 45 (7.6%) received alkylating agent-based regimens. Eighteen patients (10.0%) with stage III disease and could not receive any proposed treatment because of early deaths. Treatment regimens were not available for 110 (18.6%) patients as a result of being administered by referring physicians. A comparison of patient characteristics organized by stage is illustrated in Table 3. As expected, biomarker and echocardiographic features all significantly worsened as the stage assignment increased. To create a stage IIIb, a BNP threshold of 700 pg/mL was derived by ROC analysis for survival at 1 year among the 182 patients with stage III disease (AUC, 0.73; 95% CI, 0.66-0.81). The median OS from the time of diagnosis was 1.0 year for stage IIIb, 4.3 years for stage III, 9.4 years for stage II, and not reached for stage I patients (Figure 3; HR, 2.6; 95% CI, 1.7-3.9; P < .001). 43.6% of patients with stage IIIb disease died within 6 months.
Patient characteristics of complementary cohort depending on BU stage
| . | Stage I (n = 151) . | Stage II (n = 259) . | Stage III (n = 182) . |
|---|---|---|---|
| General characteristics | |||
| Age | 59 (52-67) | 63 (54-68)* | 62 (56-68)* |
| Male, n (%) | 83 (55) | 154 (60) | 124 (68)* |
| Treatment | |||
| HDM/SCT, n (%) | 56 (37) | 67 (26)* | 26 (14)**,† |
| Proteasome inhibitor, n (%) | 48 (32) | 92 (36) | 63 (35) |
| Immunomodulatory agent, n (%) | 14 (9) | 35 (14) | 18 (10) |
| Alkylators, n (%) | 4 (2) | 19 (7)* | 22 (12)* |
| Laboratory data | |||
| BNP, pg/mL | 36 (23-57) | 210 (130-410)** | 719 (394-1401)**,†† |
| Troponin I, ng/mL | 0.011 (0.006-0.020) | 0.035 (0.018-0.062)** | 0.218 (0.138-0.399)**,†† |
| eGFR, mL/min/1.73 m2 | 84 (54-99) | 64 (39-87)** | 56 (33-80)**,† |
| Transthoracic echocardiography | (n=136) | (n=242) | (n=174) |
| IVSd, mm | 10 (8-12) | 12 (10-14)** | 14 (12-16)**,†† |
| Ejection fraction, % | 64 (60-65) | 61 (55-65)** | 52 (41-60)**,†† |
| . | Stage I (n = 151) . | Stage II (n = 259) . | Stage III (n = 182) . |
|---|---|---|---|
| General characteristics | |||
| Age | 59 (52-67) | 63 (54-68)* | 62 (56-68)* |
| Male, n (%) | 83 (55) | 154 (60) | 124 (68)* |
| Treatment | |||
| HDM/SCT, n (%) | 56 (37) | 67 (26)* | 26 (14)**,† |
| Proteasome inhibitor, n (%) | 48 (32) | 92 (36) | 63 (35) |
| Immunomodulatory agent, n (%) | 14 (9) | 35 (14) | 18 (10) |
| Alkylators, n (%) | 4 (2) | 19 (7)* | 22 (12)* |
| Laboratory data | |||
| BNP, pg/mL | 36 (23-57) | 210 (130-410)** | 719 (394-1401)**,†† |
| Troponin I, ng/mL | 0.011 (0.006-0.020) | 0.035 (0.018-0.062)** | 0.218 (0.138-0.399)**,†† |
| eGFR, mL/min/1.73 m2 | 84 (54-99) | 64 (39-87)** | 56 (33-80)**,† |
| Transthoracic echocardiography | (n=136) | (n=242) | (n=174) |
| IVSd, mm | 10 (8-12) | 12 (10-14)** | 14 (12-16)**,†† |
| Ejection fraction, % | 64 (60-65) | 61 (55-65)** | 52 (41-60)**,†† |
All values presented as median (25-75%) unless otherwise specified.
HDM/SCT, high-dose melphalan and stem cell transplantation.
P value compared with stage I is <.05.
P value compared with stage I is <.001.
P value compared with stage II is <.05.
P value compared with stage II is <.001.
Kaplan-Meier curve for OS from the time of diagnosis in 592 patients based on BU staging system. Thresholds used: BNP higher than 81 pg/mL and TnI higher than 0.1 ng/mL with staging system as follows: no thresholds reached, stage I; 1 threshold reached, stage II; 2 thresholds reached, stage III; BNP higher than 700 pg/mL and stage III, stage IIIb. Number at risk for each stage at each point is shown below the x-axis. Stage I did not reach median survival through 12 years of follow-up. Stage II median survival was 9.4 years. Stage III median survival was 4.3 years. Stage IIIb median survival was 1.0 year.
Kaplan-Meier curve for OS from the time of diagnosis in 592 patients based on BU staging system. Thresholds used: BNP higher than 81 pg/mL and TnI higher than 0.1 ng/mL with staging system as follows: no thresholds reached, stage I; 1 threshold reached, stage II; 2 thresholds reached, stage III; BNP higher than 700 pg/mL and stage III, stage IIIb. Number at risk for each stage at each point is shown below the x-axis. Stage I did not reach median survival through 12 years of follow-up. Stage II median survival was 9.4 years. Stage III median survival was 4.3 years. Stage IIIb median survival was 1.0 year.
Discussion
In this large, single-center retrospective cohort analysis of patients with AL amyloidosis, we developed a novel BNP-based cardiac biomarker staging categorization scheme, the BU biomarker score, to both identify cardiac involvement and predict OS. There is a need for a validated BNP-based staging system, as many centers worldwide use this natriuretic peptide in their clinical chemistry laboratories, while often NT-proBNP is unavailable. We demonstrate that a staging system using a BNP threshold of more than 81 pg/mL in conjunction with a TnI threshold higher than 0.1 ng/mL obtained at the index visit accurately identified cardiac involvement and stratified patients in concordance with the established Mayo 2004 staging scheme, using NT-proBNP higher than 332 pg/mL. We elected to not use the updated Mayo 2012 system that incorporates dFLC in addition to NT-proBNP higher than 1800 pg/mL, as Mayo 2004 is simpler, widely used, and may potentially be superior in staging patients with AL amyloidosis.25
We demonstrate that the BU biomarker score also conferred similar prognostic information with respect to OS as Mayo 2004. In the original Mayo 2004 report, median survival for stage I, II, and III was 26.4, 10.5, and 3.5 months, respectively, while using an NT-proBNP and TnT model, and was 27.2, 11.1, and 4.1 months, respectively, while using an NT-proBNP and TnI model.8 Our TnI-based system, similar to Mayo 2004 staging, demonstrates poorer survival in stage III disease compared with stage I and II disease. However, the median OS for each stage is greatly increased compared with Mayo 2004, with median survival not reached in stage I, 9.4 years in stage II, 4.3 years in stage III, and 1.0 years in stage IIIb. This impressive increase in survival outcomes is attributable to the administration of contemporary antiplasma cell therapies in our group of patients (2004-2014), as Mayo 2004 staging included patients from 1979 to 2000. Importantly, 70.1% of patients in our complementary cohort received high-dose melphalan and stem cell transplantation and/or novel agents, and perhaps these contemporary therapies led to the improvement in OS compared with the Mayo 2004 staging. Notably, our data provide further external validation that use of these biomarkers in staging of AL amyloidosis affords information regarding survival, even with contemporary treatment approaches. Comparing the 2 staging schemes, it is also striking to note the similarity in survival trajectory. Both our system and Mayo 2004 demonstrate that nearly 40% to 45% of deaths in stage III disease occur within the first year of diagnosis, with survival curves after 1 year inscribing more horizontal slopes. This reiterates the observation that even despite contemporary treatments, advanced cardiac involvement in AL amyloidosis can be rapidly fatal in a sizable proportion of patients, with 43.6% of stage IIIb patients dying within 6 months. However, one can also conclude that survival to 1 year for those with advanced stage III disease is an important milestone, for our data also show that 20% of stage III patients can survive up to 10 years after diagnosis.
It is also important to emphasize that the Mayo scheme was developed to predict survival, and not necessarily to identify cardiac involvement. Our finding of a different NT-proBNP threshold for cardiac involvement identification (288 pg/mL) in the derivation cohort as compared with that derived from the Mayo 2004 survival analysis (332 pg/mL) supports this point. This difference is one of the justifications for not including renal function in the Mayo 2004 scheme, as elevated biomarkers, irrespective of mechanism (cardiac involvement vs impaired renal clearance), connote worse survival. However, it is certainly reasonable to postulate that elevated markers most likely reflect some degree of cardiac impairment. For this reason, we sought to determine whether our BNP-based scheme might identify cardiac involvement as well as align with Mayo 2004, and thus prognosis. In separate analyses, we showed that the identical BNP threshold higher than 81 pg/mL aligned both with Mayo 2004 and the presence of cardiac involvement, as defined by endomyocardial biopsy, cardiac MRI, or wall thickness criteria. These results substantiate this BNP threshold. As a corollary, we identified a smaller proportion of cardiac amyloidosis involvement (47%) than in prior studies, but this was precisely because we excluded biomarkers in the adjudication. If one considered biomarker stage II or III as defining some degree of cardiac involvement, 75% of our complementary cohort would then be adjudicated as meeting this threshold, similar to prior studies.
Given NT-proBNP’s strict reliance on renal clearance, we had hoped to establish that a BNP-based system would be superior for detecting cardiac involvement in patients with advanced CKD. Our data suggest that in CKD stage IV or V, a BNP-based system may be superior (with a specificity that approaches significance); however, because of a relatively low number of patients with advanced CKD, we were unable to conclusively prove this. Interestingly, NT-proBNP was found to have superior specificity among patients without advanced CKD, but that this difference fell from significance when all patients were considered. This finding would suggest that the strength in a BNP system overall results from its reduced reliance on renal clearance. During ROC analysis, we found significantly higher cutoffs for both biomarkers (BNP > 472 pg/mL and NT-proBNP > 2930 pg/mL) in patients with advanced CKD. Given the marked difference between these values and our proposed cutoff for BNP and the Mayo 2004 cutoff for NT-proBNP, this raises the question of whether different models and larger patient numbers are needed to predict survival in patients with advanced CKD.
In addition, in this report, we used a slightly expanded definition for cardiac involvement in AL amyloidosis because of changing practice patterns. The current definition for cardiac involvement as described in the 2005 consensus opinion is a mean wall thickness of at least 12 mm with no other causes (ie, hypertension and/or valvular heart disease).18 However, since the introduction of high sensitivity cardiac biomarker assays and the resultant staging systems, this narrow definition is now inadequate and in need of revision. Many patients have biochemical evidence of cardiac involvement but wall thickness thinner than 12 mm, which leads to many being treated as having cardiac involvement while not officially meeting echocardiographic criteria. To address this deficiency and to capture those patients with wall thicknesses above the established normal range, but below the consensus threshold of 12 mm, we used the American Society of Echocardiography guidelines, which state that the abnormal range for women begins with IVSd higher than 10 mm and for men with IVSd higher than 11 mm.19 This decision was further supported by the fact that of the 28 patients with IVSd less than 12 mm who were adjudicated to have cardiac involvement, 14 (50%) had either characteristic cardiac MRI findings with diffuse delayed gadolinium enhancement or endomyocardial biopsy with Congo red staining. We note that because all patients did not have endomyocardial biopsy or cardiac MRI, the BNP threshold we defined may miss subtle cardiac involvement that does not result in wall thickening. Further follow-up imaging analysis is beyond the scope of this report. As noted, the BNP cutoff for cardiac involvement may be even lower if every patient were to have MRI or/and biopsy for evaluation of cardiac involvement.
This study has a number of important limitations. First, as a retrospective analysis, we could have been subject to selection bias. We attempted to avoid this by considering all sequential patients seen at our center who had both BNP and TnI (complementary cohort) or BNP, NT-proBNP, and TnI (derivation cohort). Second, we chose to not redefine the TnI staging threshold so as to simplify the analysis and also to rely on well-established data supporting this threshold. Although it is possible that BNP interacts with TnI differently than NT-proBNP, as BNP and NT-proBNP are cleaved from the same pro-hormone, we thought reasonable to not redefine TnI. Third, we have not explored the use of BNP in evaluating response to treatment and using more than 30% reduction in NT-proBNP remains the gold standard for standardized assessment of organ response following treatment.
In summary, we have created a staging system that uses a combination of BNP higher than 81 pg/mL and TnI higher than 0.1 ng/mL that is predictive for survival in patients with AL amyloidosis and also accurately identifies cardiac involvement. Through strong correlation with the well-validated and well-studied Mayo 2004 scheme, institutions without access to NT-proBNP could instead use BNP with reasonable confidence for application in stratifying and prognosticating patients with AL amyloidosis. Furthermore, we propose that this staging system may be used for patient selection (or exclusion) for clinical trials for AL amyloidosis if NT-proBNP is unavailable. In addition, our system will allow clinicians at centers without NT-proBNP to hold well-informed discussions about survival and treatment plans with patients with AL amyloidosis. Confirmatory studies using other independent data sets are now required to externally validate these findings.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Amyloid Research Fund of Amyloidosis Center at Boston University School of Medicine. R.M. is the recipient of the David C. Seldin research training award.
Authorship
Contribution: B.L. designed the study, performed research, analyzed the data, performed statistical analysis, and wrote the manuscript; F.L.R. designed the study, performed research, analyzed the data, and wrote the manuscript; R.M. performed research; G.D. performed statistical analysis; and V.S. designed the study, analyzed the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Vaishali Sanchorawala, 820 Harrison Ave, FGH 1007, Boston, MA 02118; e-mail: vaishali.sanchorawala@bmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal