Key Points
WPBs contain CD63+ intraluminal vesicles that are released during secretagogue-evoked exocytosis.
Cryo-electron microscopy of intact vitrified endothelial cells reveals intraluminal vesicles as novel structural features of WPBs.
Abstract
Weibel-Palade bodies (WPBs) are secretory granules that contain von Willebrand factor and P-selectin, molecules that regulate hemostasis and inflammation, respectively. The presence of CD63/LAMP3 in the limiting membrane of WPBs has led to their classification as lysosome-related organelles. Many lysosome-related organelles contain intraluminal vesicles (ILVs) enriched in CD63 that are secreted into the extracellular environment during cell activation to mediate intercellular communication. To date, there are no reports that WPBs contain or release ILVs. By light microscopy and live-cell imaging, we show that CD63 is enriched in microdomains within WPBs. Extracellular antibody recycling studies showed that CD63 in WPB microdomains can originate from the plasma membrane. By cryo-electron tomography of frozen-hydrated endothelial cells, we identify internal vesicles as novel structural features of the WPB lumen. By live-cell fluorescence microscopy, we directly observe the exocytotic release of EGFP-CD63 ILVs as discrete particles from individual WPBs. WPB exocytosis provides a novel route for release of ILVs during endothelial cell stimulation.
Introduction
Endothelial cells regulate hemostasis and inflammation through direct cell–cell contacts, secretion of soluble or membrane-associated mediators, and the release of small bioactive lipid vesicles (extracellular vesicles [EVs]). Many of the soluble secreted molecules, such as the adhesive glycoprotein von Willebrand factor (VWF), are stored and released in a regulated fashion from specialized secretory granules called Weibel-Palade bodies (WPBs).1 EVs can arise by several distinct mechanisms: (1) exocytosis of late endosomes (LEs)/multivesicular bodies (MVBs) to release intraluminal vesicles (ILVs; termed exosomes upon secretion), (2) budding from the plasma membrane (shedding microvesicles or ectosomes), or (3) plasma membrane blebbing during programmed cell death (apoptotic bodies). EVs contain a variety of signaling molecules that modulate gene expression and function of target cells and are now widely viewed as important mediators of intercellular communication and control.2
WPBs form at the trans-Golgi network (TGN) through pH- and Ca2+-dependent condensation of VWF and the VWF-propolypeptide (VWFpp) to form helical tubule structures.3-5 VWF-VWFpp tubules account for the majority of the protein content of WPBs and give the organelle its distinctive morphology.3 The leukocyte adhesion molecule P-selectin is also stored in the WPB limiting membrane; upon release into the plasma membrane, it mediates the tethering and rolling of leukocytes on the vessel wall prior to extravasation at sites of inflammation. Efficient P-selectin–mediated leukocyte capture requires the tetraspanin CD63 (also called LAMP3) that is also present in the limiting membrane of WPBs and coreleased to the plasma membrane during exocytosis.6-8 P-selectin enters WPBs during their formation at the TGN; however, CD63 is delivered to WPBs at a later stage through a poorly defined interaction with LEs/MVBs9,10 requiring the endosomal sorting complex AP-3 and annexin 8.7,10
The interaction of WPBs with endosomal components, their acidic luminal pH, and acquisition of CD63/LAMP3 have led to the classification of WPBs as lysosomal-related organelles (LROs),11 a functionally diverse set of compartments containing different cargoes that nonetheless share certain features or components with lysosomes. LRO biogenesis is complex and organelle specific: some form by remodeling/maturation of endosomal compartments (eg, MVBs, secretory lysosomes, melanosomes), some originate from the TGN (WPBs), and others may involve contributions from both pathways (eg, lytic granules, platelet granules).11
LROs that undergo fusion with the plasma membrane to release their contents include the major histocompatibility complex class II–enriched compartment of B lymphocytes, lytic granules of cytotoxic T cells, platelet dense core and α-granules, basophilic granules, lamellar bodies of lung epithelia cells, osteoclast granules, sperm acrosomes, and WPBs.12,13 In most cases, the delivery of these organelles to the plasma membrane, as well as their exocytosis, is regulated by “secretory Rab proteins” and their effector molecules. For WPBs, these include Rab27A, MyRIP, Slp4-a, and Munc13-4.14-17 Some LROs contain ILVs that can be released during fusion with the plasma membrane,12,18,19 and, unsurprisingly, several key regulators of WPB and other LRO exocytosis (eg, Rab27A, Slp4-a) also control exosome release.20,21 Despite their classification as LROs and sharing a common set of molecular components regulating exocytosis, it is not known whether WPBs contain or release ILVs.
Using live-cell imaging and high-resolution cryo-electron tomography of vitrified endothelial cells, we identify and characterize ILVs in WPBs. We directly demonstrate the exocytotic release of EGFP-CD63–enriched ILVs from individual WPBs during hormone stimulation. This is a new route for EV release from endothelial cells and extends the range of signaling modalities through WPBs.
Methods
Endothelial cell culture, transfections, immunocytochemistry, antibodies, DNA constructs, and reagents
Human umbilical vein endothelial cells (HUVECs) or human heart microvasculature endothelial cells (HHMECs) were purchased, cultured, nucleofected, and processed for immunocytochemistry, as previously described.15,22 VWF–monomeric red fluorescent protein (mRFP), VWF-mCherry, VWFpp-mRFP, VWFpp–monomeric enhanced green fluorescent protein (mEGFP), mRFP-Rab27A, and enhanced green fluorescent protein (EGFP)-CD63 have been described previously (see Bierings et al15 and references therein). Rabbit anti-VWF (A0082; 1:10 000 dilution) was from Dako (Ely, United Kingdom), rabbit anti-VWFpp (1:500) is described in Hewlett et al,23 mouse anti-lysobisphosphatidic acid (LBPA) (Z-PLBPA, 1:1000) was from tebu-bio (Peterborough, United Kingdom), mouse anti–P-selectin (clone AK6; 1:50) was from Serotec (Kidlington, United Kingdom), rabbit anti-VPS2B (ab33174; 1:300) and mouse anti-Alix (ab117600; 1:300) were from Abcam (Cambridge, United Kingdom), rabbit anti-syntenin (133003; 1:100) was from Synaptic Systems (Göttingen, Germany), mouse anti-TSG101 (GTX70255) and rat anti-HSP70 (GTX191366) were from GeneTex (Irvine, CA), mouse anti-CD9 (clone HI9a; 1:1000) and anti-CD81 (clone 5A6; 1:1000) were from BioLegend (London, United Kingdom), mouse anti-CD63 (clone H5C6; 1:200) was from the Developmental Studies Hybridoma Bank, normal mouse immunoglobulin G1 (SC-3877; 1:55) and mouse anti-CD63–tetramethylrhodamine isothiocyanate (TRITC; SC-5275; 1:55) were from Insight Biotechnology (Wembley, United Kingdom). Secondary antibodies coupled to fluorophores (1:200) were from Jackson ImmunoResearch. All other reagents were from Sigma-Aldrich, unless otherwise stated.
Cell culture on electron microscopy grids, electron cryomicroscopy and analysis of images, tilt series and tomograms
HUVECs or HHMECs were grown on carbon film on gold grid supports for microscopy, as previously described.3 Gold grids with cells on were washed briefly in phosphate buffered saline (PBS), and 4 µL of 40% protein A–conjugated 10-nm gold colloid (BBI Life Sciences) in PBS was added between washing and freezing, to act as fiducial markers. WPBs were imaged in cells that were left unstimulated or following stimulation with PBS containing 100 µM histamine dihydrochloride or ionomycin (300 nM or 1 µM ionomycin, Streptomyces conglobatus).
Grids were frozen by plunging into liquid ethane using a manual plunge-freezer or an FEI Vitrobot Mark III (FEI Company) at room temperature and humidity (manual) or at 22°C and room humidity (Vitrobot; humidifier switched to off). Frozen grids were stored in liquid N2.
Frozen grids were imaged using a Spirit TWIN microscope (FEI Company) operating at 120 kV and equipped with an Eagle 2k camera (FEI Company) using a Gatan 626 cryotomography holder or were imaged with an LN2 cooled Polara microscope (FEI Company) operating at 200 kV and equipped with an F224 HD CCD camera (TVIPS). TIA (FEI Company) and SerialEM image acquisition software were used, and low-dose procedures were used in both packages. SerialEM was used to collect whole-grid montages at ∼140× magnification, which were used for locating areas of interest for further imaging using low-dose procedures.
Single-axis tilt-series were collected automatically using SerialEM, with an angular range of −60° to +60° and increment of 2° or 3°. Total dose for tilt-series was limited to 50 to 70 e−/Å2, giving individual images with a dose of 1.2 to 1.7 e−/Å2. The dose per image was kept constant for each tilt angle in a series. The target defocus was set at −8 µm. Tomographic tilt series were aligned with the help of fiducial markers using Etomo from IMOD software.24 Projection images in aligned tilt series were normalized based on their histograms, reconstructed to 3-dimensional volumes, and analyzed as previously described.3
Image and volume analysis
Simple image-processing tasks, such as crop, pad, and rotate, were performed in Ximdisp, and fast Fourier transform calculations were performed using Ximdisp and FF trans from the MRC suite. Figures were prepared using Photoshop CS4 (Adobe). Amira (FEI Visualization Sciences Group) and IMOD were used to generate 3-dimensional models.
VWF tubules were manually traced using IMOD. Tomograms were segmented using the Amira “Segmentation”' tool. Membranes and tubules were rendered and displayed in Amira.
Live-cell fluorescence imaging, confocal FRAP, and analysis
Nucleofected cells were plated at confluent density in culture medium onto 35-mm-diameter poly-d-lysine–coated glass glass-bottom culture dishes (MatTek, Ashland, MA) or 25-mm-diameter glass coverslips (#1.0, 0.15 mm; VWR International, Lutterworth, United Kingdom). Glass coverslips (25-mm diameter) were mounted in Rose chambers containing physiological saline: NaCl 140 mM, KCl 5 mM, MgSO4 1 mM, CaCl2 2 mM, glucose 10 mM, HEPES 20 mM, pH 7.3 (adjusted with NaOH). High-speed dual-color epifluorescence imaging was carried out on an Olympus IX71 inverted microscope equipped with an Olympus UPLSAPO 100× 1.40NA oil objective, a 1.6× magnifier, and an iXon3 EMCCD camera operated in frame-transfer mode at full gain and cooled to −70°C (Andor, Belfast, United Kingdom). Full-frame images were acquired at 30 frames per second. High-speed single or sequential dual-color excitation wavelength switching (470 nm ± 40 nm and 572 nm ± 35 nm) was by OptoLED (Cairn Research, Faversham, United Kingdom); the excitation filter set consisted of a GFP/DsRed dual band dichroic mirror (Chroma part 51019) and a GFP/DsRed dual band emitter. Image capture and wavelength switching were synchronized using WinFluor software (John Dempster, Strathclyde University, Glasgow, United Kingdom). The microscope was housed within an environmental chamber maintained at 36°C, and cells were stimulated with histamine (100 µM). Confocal fluorescence recovery after photo bleaching (FRAP) experiments were carried out using Leica Microsystems (Mannheim, Germany) TCS SP2 or SP5 (8 kHz resonant scanner) confocal microscopes equipped with a Leica HCX PL APO 63× 1.32NA (SP2) or HCX PL APO CS 100× oil immersion objective with NA of 1.40 (SP2) or 1.46 (SP5), as previously described.15,25,26 Excitation (bidirectional “fly” FRAP mode) was at 488 nm (EGFP) and 561 nm (mRFP). Emission windows for single wavelength (EGFP) were 495 to 620 nm and emission windows for dual color (simultaneous “fly” mode excitation) were 500 to 545 nm (EGFP) and 585 to 750 nm (mRFP). Images from SP2 were collected at 512 × 128 (or 64) pixels and at zooms between 20 and 32, and images from SP5 were collected at 512 × 300 pixels and at zooms between 19.1 and 38.8. FRAP imaging and regions of interest were set as previously described,15,25,26 and single- or dual-color bleaching was applied during 2 to 10 consecutive frames acquired at 0.344 seconds (SP2) or between 0.792 and 0.962 seconds (SP5). Images were background subtracted and analyzed using custom-made macros implemented in ImageJ (http://rsb.info.nih.gov/ij/).26 Image montages and AVI video clips (jpeg compression) were made in ImageJ2/Fiji. Data plots were made in Origin 9.2 (OriginLab, Northampton, MA). Results are expressed as mean ± standard error of the mean (SEM).
Results
Enrichment of CD63 in discrete microdomains within WPBs
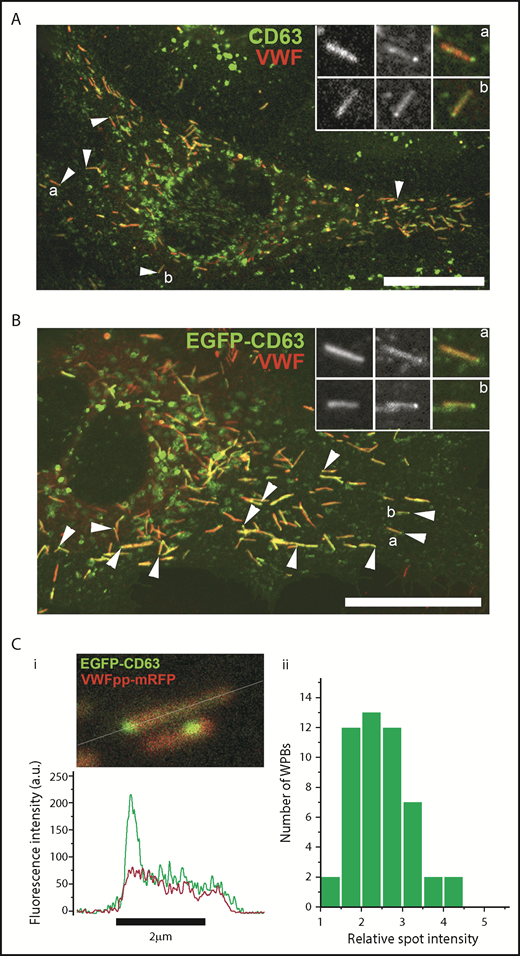
Tetraspanins, including the ubiquitously expressed CD63, are among the most common endosomal components enriched in secreted ILVs27,28 (http://www.exocarta.org/), and CD63, in particular, is implicated in cargo sorting to exosomes.29 Consequently, CD63 is widely used as a marker to identify, visualize, and track ILVs/exosomes within and between cells.30 To determine whether WPBs contained CD63-enriched regions, we first analyzed the pattern of endogenous CD63 in WPBs by immunocytochemistry (Figure 1A). Consistent with previous studies, CD63 immunoreactivity was seen on WPBs and other endomembrane compartments.31 Close inspection revealed discrete bright “microdomains” of CD63 immunoreactivity associated with some WPBs, often near the ends of the organelle but also at intermediate points up to midbody (supplemental Figure 1A, available on the Blood Web site). Expression of EGFP-CD63 produced similar features (Figure 1B), and, crucially, live-cell fluorescence imaging showed that the EGFP-CD63 microdomains were connected to and moved with (but not within) the WPB (supplemental Figure 1A; supplemental Videos 1-2). Measurement of WPB EGFP-CD63 fluorescence intensity in live cells showed the microdomains to be stable in intensity and up to 4 to 5 times brighter than the bulk signal in the WPB membrane (Figure 1C), reminiscent of the enrichment reported for CD63 in ILVs of LEs/MVBs and exosomes.32 Further immunofluorescence analysis showed that other WPB membrane proteins (Rab27A, P-selectin) were present in the limiting membrane of the granule but were not concentrated in CD63-rich microdomains (supplemental Figure 1B).
CD63 is enriched in microdomains on WPBs. Confocal images of a single fixed HUVEC immunolabeled with specific antibodies to CD63 (green) and VWF (red) (A) or expressing exogenous EGFP-CD63 (green) and immunolabeled for VWF (red) (B). Scale bars, 10 µm. Arrowheads indicate bright regions of CD63 (A) or EGFP-CD63 (B) closely associated with individual WPBs. Insets show, on expanded scales, the fluorescence, in grayscale, for VWF (left panels) and CD63 (middle panels) and the color merge image (right panels; VWF in red, CD63 in green) for WPBs indicated by a and b. (A-B) Images were taken at room temperature using a Leica SP2 confocal microscope (and software) equipped with a PL APO 100× 1.4NA objective. (Ci) Image from a live-cell confocal fluorescence experiment of an EGFP-CD63 (green) and VWFpp-mRFP (red) coexpressing HUVEC showing 2 WPBs containing discrete bright microdomains of EGFP-CD63 fluorescence. Intensity plots through the long axis of the upper WPB (white line) are shown in the line graph below (green: CD63, red VWFpp). (Cii) Histogram of the fold increase in mean EGFP fluorescence intensity in microdomains compared with nonmicrodomain regions (bulk WPB membrane) for 50 WPBs. Mean microdomain EGFP intensity was 2.5- ± 0.7-fold (n = 49 WPBs; range, 1.4-4.1) that in the bulk membrane of the corresponding WPB. (C) Images were taken at 37°C using a Leica SP5 with an HCX PL APO CS 100× 1.46NA oil objective, pinhole (airy) 1.5, zoom 30 to 35.5, scan speed 1400 Hz in xyt acquisition mode.
CD63 is enriched in microdomains on WPBs. Confocal images of a single fixed HUVEC immunolabeled with specific antibodies to CD63 (green) and VWF (red) (A) or expressing exogenous EGFP-CD63 (green) and immunolabeled for VWF (red) (B). Scale bars, 10 µm. Arrowheads indicate bright regions of CD63 (A) or EGFP-CD63 (B) closely associated with individual WPBs. Insets show, on expanded scales, the fluorescence, in grayscale, for VWF (left panels) and CD63 (middle panels) and the color merge image (right panels; VWF in red, CD63 in green) for WPBs indicated by a and b. (A-B) Images were taken at room temperature using a Leica SP2 confocal microscope (and software) equipped with a PL APO 100× 1.4NA objective. (Ci) Image from a live-cell confocal fluorescence experiment of an EGFP-CD63 (green) and VWFpp-mRFP (red) coexpressing HUVEC showing 2 WPBs containing discrete bright microdomains of EGFP-CD63 fluorescence. Intensity plots through the long axis of the upper WPB (white line) are shown in the line graph below (green: CD63, red VWFpp). (Cii) Histogram of the fold increase in mean EGFP fluorescence intensity in microdomains compared with nonmicrodomain regions (bulk WPB membrane) for 50 WPBs. Mean microdomain EGFP intensity was 2.5- ± 0.7-fold (n = 49 WPBs; range, 1.4-4.1) that in the bulk membrane of the corresponding WPB. (C) Images were taken at 37°C using a Leica SP5 with an HCX PL APO CS 100× 1.46NA oil objective, pinhole (airy) 1.5, zoom 30 to 35.5, scan speed 1400 Hz in xyt acquisition mode.
At the plasma membrane, tetraspanins can form enriched areas or microdomains that appear as long-lived “spot-like” structures in which contributing tetraspanins and associated proteins are in dynamic exchange with the bulk plasma membrane on a time scale of seconds.33 To examine whether EGFP-CD63 in the WPB limiting membrane was in diffusional equilibrium with CD63 microdomains, we used single WPB FRAP analysis in EGFP-CD63 and VWF-mRFP coexpressing HUVECs.25,26 Consistent with our previous studies,25 EGFP-CD63 was freely mobile in the WPB limiting membrane, undergoing rapid and complete recovery, by lateral membrane diffusion, after each period of bleaching (supplemental Figure 2; supplemental Video 3). The core protein VWF-mRFP was used to confirm the organelle’s identity and was completely immobile, showing no recovery after bleaching, as previously reported.25 Our FRAP analysis showed that EGFP-CD63 in microdomains did not contribute to recovery of EGFP-CD63 fluorescence within the limiting membrane nor did microdomains reaccumulate fluorescence from the WPB limiting membrane when selectively bleached (supplemental Figure 2). The results indicate that EGFP-CD63 in microdomains is not in diffusional equilibrium with EGFP-CD63 in the WPB limiting membrane.
Intraluminal vesicles in WPBs revealed by cryo-electron tomography
The presence of microdomains containing the membrane tetraspanin CD63, but topologically separated from the WPB membrane, suggested that these were ILVs. To test this, we applied high-resolution electron cryomicroscopy to image the thin edge of plunge-frozen whole-mount HUVECs, an approach that we have previously shown to reveal the high-resolution architecture of organelles without chemical fixation or staining.3 In 2-dimensional projection images, WPBs appear as rod-shaped granules, which are denser than the surrounding cytoplasm (Figure 2) and contain tubules of VWF that are the source of the signature helical pattern in their Fourier transforms (Figure 2B, right panel). We can identify ILVs in these images (arrows). In fact, 12% of 535 2-dimensional images show evidence for ≥1 and up to 3 ILVs per granule. To clearly identify the internal vesicles in the context of granule architecture without the ambiguity of overlap in the 2-dimensional image, we performed electron cryotomography and volume reconstruction. Figure 3 and supplemental Video 4 show a tomogram section containing WPBs, other vesicular organelles, cytoskeletal filaments, ribosomes, and other particles. ILVs within WPBs are indicated by arrowheads. The lumen of WPB ILVs was less electron dense than the surrounding VWF tubules, being similar in density to the lumen of ILVs of MVBs and to regions of cell cytosol.
Electron cryomicroscopy of frozen-hydrated HUVECs showing WPBs in cytoplasm. (A-C) Two-dimensional images of WPBs show internal density for VWF tubules. ILVs are indicated by arrowheads. Fourier transform of WPB interior in panel B shows helical layer lines for VWF (arrows). Scale bars, 200 nm.
Electron cryomicroscopy of frozen-hydrated HUVECs showing WPBs in cytoplasm. (A-C) Two-dimensional images of WPBs show internal density for VWF tubules. ILVs are indicated by arrowheads. Fourier transform of WPB interior in panel B shows helical layer lines for VWF (arrows). Scale bars, 200 nm.
Section of an electron cryotomogram of a frozen-hydrated endothelial cell showing region with WPBs. ILVs within WPBs are indicated by arrowheads. Scale bar, 200 nm.
Section of an electron cryotomogram of a frozen-hydrated endothelial cell showing region with WPBs. ILVs within WPBs are indicated by arrowheads. Scale bar, 200 nm.
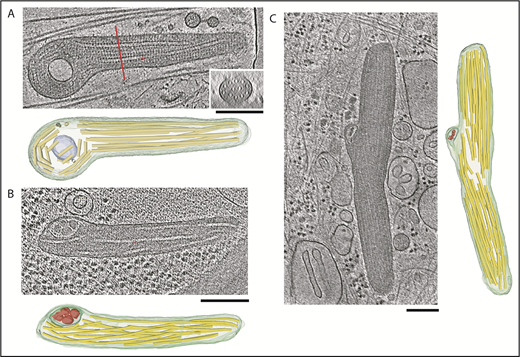
We built structural models for 22 ILVs from 15 tomograms (Figures 4 and 5; supplemental Figure 3). ILVs were not confined to regions close to the ends of WPBs but could be seen at any point up to midbody. In many cases, the membrane of the ILV was in close apposition to the WPB limiting membrane and sometimes associated with a bulge in the WPB limiting membrane. ILVs were often nonspherical in shape, appearing compressed between the smooth limiting membrane of the WPB and VWF tubules. As previously observed, WPBs contained paracrystals of helical VWF (shown in cross-section in Figure 4A; supplemental Video 5). A prominent membrane-bound ILV can be seen where the paracrystalline packing is disrupted, giving the granule a club-shape, a common morphology for WPBs. WPB ILVs were also observed in adult HHMECs (Figure 4B; supplemental Figure 3C), confirming that these structures are not specific to HUVECs but represent a general feature of endothelial WPBs.
Tomogram sections of WPBs containing ILVs along with structural models. (A-C) Tomogram section with structural model consisting of WPB limiting model (green), ILV membrane (blue-green), VWF tubules (yellow), and ILV internal content (red). (A) Inset shows tomogram cross-section at the location of the red line. (B) WPB from an HHMEC with an ILV in the left side of the granule. The ILV contains internal content similar to cytoplasmic granules, as shown in supplemental Figure 4. (C) WPB in a HUVEC with a kink in the middle where tubules have disrupted paracrystalline order. The ILV contains densities similar in size to cytosolic densities visible throughout the tomogram section. Scale bars, 200 nm.
Tomogram sections of WPBs containing ILVs along with structural models. (A-C) Tomogram section with structural model consisting of WPB limiting model (green), ILV membrane (blue-green), VWF tubules (yellow), and ILV internal content (red). (A) Inset shows tomogram cross-section at the location of the red line. (B) WPB from an HHMEC with an ILV in the left side of the granule. The ILV contains internal content similar to cytoplasmic granules, as shown in supplemental Figure 4. (C) WPB in a HUVEC with a kink in the middle where tubules have disrupted paracrystalline order. The ILV contains densities similar in size to cytosolic densities visible throughout the tomogram section. Scale bars, 200 nm.
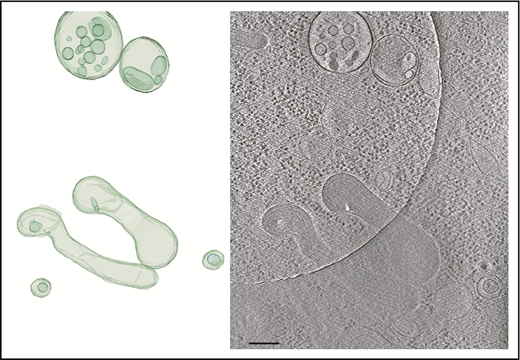
Tomogram section showing WPBs and MVBs. Tomogram section (right panel) shows WPBs and MVBs containing ILVs, as indicated in the structural model (left panel). Scale bar, 200 nm.
Tomogram section showing WPBs and MVBs. Tomogram section (right panel) shows WPBs and MVBs containing ILVs, as indicated in the structural model (left panel). Scale bar, 200 nm.
We observed many WPB ILVs of HUVECs or HHMECs to contain densities and structures resembling cytoplasmic components (Figure 4B-C; supplemental Figure 4; supplemental Video 6). Measurements of WPB ILVs, ILVs of single-vesicular bodies and MVBs, and single internal vesicle bodies in tomograms (Figure 5) showed them to have a similar size distribution and to include some large outliers (eg, ILV in WPB; supplemental Figure 3A). The majority of ILVs in WPBs (mean [± SEM] volume, 147 292 ± 41 225 nm3; n = 15 measurements) are similar in size to the small vesicles within the MVBs (mean [± SEM] volume, 165 286 ± 30 664 nm3; n = 25 measurements; supplemental Video 7).
WPB ILVs contain CD63 derived from the endocytic pathway but may differ in composition from ILVs in MVBs
Because endogenous CD63 cycles from the plasma membrane to WPBs via the endocytic pathway,7,9,10 we next examined whether CD63 in WPB ILVs was also derived from this trafficking route by monitoring the accumulation of an extracellularly applied TRITC-labeled mouse anti-human CD63 antibody in WPBs, as previously described7 (Figure 6). The TRITC-anti-CD63 antibody, but not a nontargeting mouse immunoglobulin G1 control antibody (supplemental Figure 5), was readily trafficked to WPBs (Figure 6A) and was enriched within WPB ILVs in both control and EGFP-CD63–expressing HUVECs (Figure 6B-C), confirming that WPB ILV CD63 is of endosomal origin. We next looked for evidence of other components in WPB-ILVs that are reported to be present in ILVs of endosomal origin. Biochemical studies have identified cholesterol as one of the lipids enriched in exosomes.34 Localization of cholesterol-rich regions by filipin staining in HUVECs showed abundant labeling of endosomal/lysosomal structures but no labeling of WPBs (supplemental Figure 6C). Some,35 but not all,36 studies suggest that LEs/MVBs and their ILVs are enriched in LBPA. Immunostaining of HUVECs for endogenous LBPA showed a striking punctate enrichment within LEs/MVBs but no labeling of WPBs (supplemental Figure 6Ai), a result consistent with a previous study.9 The fluorescent phosphatidyl ethanolamine (PE) analog, N-Rh-PE (1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-[lissamine rhodamine B sulfonyl]), is taken up from the plasma membrane, trafficked to LEs/MVBs, and incorporated into exosomes.37,38 Incubation of HUVECs with N-Rh-PE revealed no WPB staining (supplemental Figure 6Bi-ii). Other common exosomal markers, including CD9, CD81, and several endosomal sorting complexes required for transport (ESCRT) components (including Alix, TSG101, VSP2/Chmp2B, HSP70, and the autophagy marker LC3) were not detected in WPBs (supplemental Figures 7 and 8). However, we did detect the PDZ domain containing adapter protein, syntenin, associated with WPBs (supplemental Figure 8B). Together, the results suggest that WPB ILVs have a distinct composition.
CD63 in WPB ILVs is of endosomal origin. (A) Confocal fluorescence image of HUVECs incubated live in the presence of an extracellular TRITC-conjugated mouse anti-CD63 antibody (red; 1:55 dilution; 24 hours). The cells were fixed and immunolabeled with a specific antibody to VWFpp (green). Scale bar, 20 µm. The region in the white box is shown as grayscale insets to illustrate the accumulation of extracellularly applied TRITC–anti-CD63 in WPBs. Examples of the accumulation of extracellularly applied TRITC-anti-CD63 in microdomains in WPBs in control (B; mock transfected) or EGFP-CD63–transfected (C) HUVECs incubated live with the TRITC–anti-CD63 antibody (red) and subsequently immunolabeled for VWFpp (blue) and EGFP-CD63 (anti-GFP antibody, green). TRITC–anti-CD63 can be seen in bright microdomains on WPBs that colocalized with EGFP-CD63 microdomains. Scale bars, 2 µm.
CD63 in WPB ILVs is of endosomal origin. (A) Confocal fluorescence image of HUVECs incubated live in the presence of an extracellular TRITC-conjugated mouse anti-CD63 antibody (red; 1:55 dilution; 24 hours). The cells were fixed and immunolabeled with a specific antibody to VWFpp (green). Scale bar, 20 µm. The region in the white box is shown as grayscale insets to illustrate the accumulation of extracellularly applied TRITC–anti-CD63 in WPBs. Examples of the accumulation of extracellularly applied TRITC-anti-CD63 in microdomains in WPBs in control (B; mock transfected) or EGFP-CD63–transfected (C) HUVECs incubated live with the TRITC–anti-CD63 antibody (red) and subsequently immunolabeled for VWFpp (blue) and EGFP-CD63 (anti-GFP antibody, green). TRITC–anti-CD63 can be seen in bright microdomains on WPBs that colocalized with EGFP-CD63 microdomains. Scale bars, 2 µm.
Direct observation of ILV secretion during WPB exocytosis
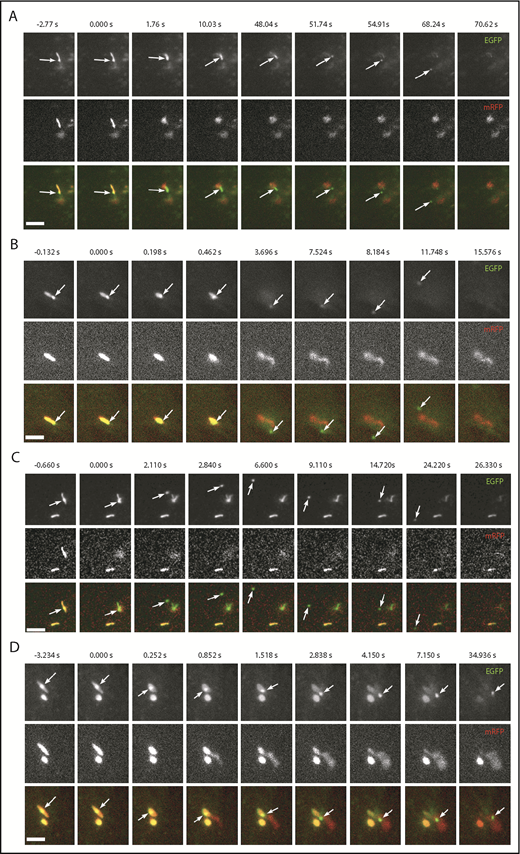
Having established that WPBs do contain ILVs, we next asked whether these could be released during exocytosis. If so, we predicted that WPB-associated EGFP-CD63 microdomains (ILVs) would be released as discrete fluorescent particles during WPB exocytosis. To test this, we monitored histamine-evoked WPB exocytosis in live HUVECs coexpressing VWF-mCherry and EGFP-CD63 using high-speed epifluorescence imaging. EGFP-CD63–labeled ILVs were released as discrete particles from individual WPBs during histamine stimulation (Figure 7). Figure 7 shows image sequences taken from supplemental Videos 8-11, respectively. In Figure 7A-C, the EGFP-CD63 particles (arrows) escape rapidly into the bulk solution and are lost from view. Figure 7D shows an example of the particle becoming trapped within the extracellular patch of VWF secreted during WPB exocytosis. In addition to direct release into the bulk solution, the particles were secreted into the narrow 2-dimensional plane between the cell and the glass coverslip. In these cases, the extracellular diffusion of the particles could be visualized for long periods before the particles eventually encountered the cell edge and escaped into the bulk media (eg, supplemental Video 12). Consistent with our ultrastructural data, we also observed release of multiple ILVs from single WPBs (supplemental Video 13). Thus, CD63-containing ILVs are released from the interior of the WPB to the extracellular medium, along with VWF, during exocytosis.
WPB EGFP-CD63 microdomains are secreted as discrete particles during exocytosis. (A-D) Examples of image montages taken from dual-color live-cell videos (supplemental Videos 8-11, respectively) of WPBs containing EGFP-CD63 (top panels; green in color merge) and VWFpp-mRFP (middle panels) prior to (frame 1) and during (frames 2-9) exocytosis evoked by histamine stimulation (100 µM). Scale bars, 2 µm. In each case, the WPB indicated by the arrow undergoes a morphological transition from rod to spheroid shape, accompanied by expulsion of VWFpp-EGFP and release of the EGFP-CD63 ILV as a discrete particle. (A-C) The EGFP-CD63 particle diffuses out of the field of view. (D) The particle remains trapped within the patch of secreted VWF. Images were acquired sequentially on a wide-field microscope equipped with an Olympus UPLSAPO 100× 1.4NA objective, an OptoLED epifluorescence excitation system, and an Andor iXon3 EMCCD camera operating at 30 frames per second.
WPB EGFP-CD63 microdomains are secreted as discrete particles during exocytosis. (A-D) Examples of image montages taken from dual-color live-cell videos (supplemental Videos 8-11, respectively) of WPBs containing EGFP-CD63 (top panels; green in color merge) and VWFpp-mRFP (middle panels) prior to (frame 1) and during (frames 2-9) exocytosis evoked by histamine stimulation (100 µM). Scale bars, 2 µm. In each case, the WPB indicated by the arrow undergoes a morphological transition from rod to spheroid shape, accompanied by expulsion of VWFpp-EGFP and release of the EGFP-CD63 ILV as a discrete particle. (A-C) The EGFP-CD63 particle diffuses out of the field of view. (D) The particle remains trapped within the patch of secreted VWF. Images were acquired sequentially on a wide-field microscope equipped with an Olympus UPLSAPO 100× 1.4NA objective, an OptoLED epifluorescence excitation system, and an Andor iXon3 EMCCD camera operating at 30 frames per second.
Discussion
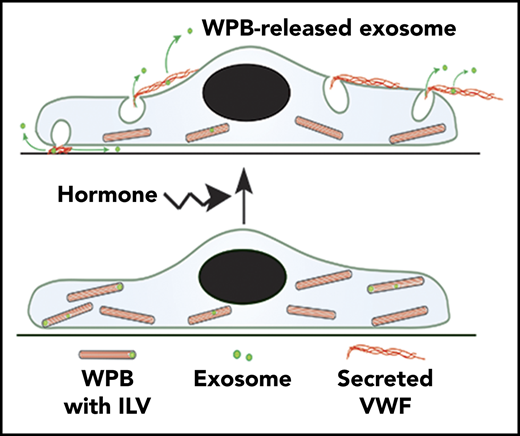
Here, we demonstrate that WPBs contain CD63+ ILVs and release them during secretagogue-evoked exocytosis. Shedding of plasma membrane–derived vesicles and MVB–plasma membrane fusion have been visualized in live cells21,39,40 ; however, direct imaging of ILV release from individual regulated secretory granules during exocytosis has not been reported previously. To indicate their specific origin, we refer to the secreted vesicles described here as WPB-released exosomes.
Our cryomicroscopy studies of the thin periphery of endothelial cells show that mature WPBs contain ILVs that are embedded within and distort the paracrystalline assemblies of VWF tubules or are squeezed between the VWF paracrystal and the tightly wrapped granule-limiting membrane. This accounts for their immobility within the granule.
During exocytosis, WPB-released exosomes are secreted into the surrounding medium, although they may initially be entangled by the secreted VWF. In other systems, tethering has been proposed as a mechanism of restricting exosomes to local target sites.41 WPB exocytic events involve complex structural changes in the granule,1 which may selectively release small molecules to the bloodstream, as well as CD63 to the plasma membrane, without releasing VWF.42 ILV release adds an additional signaling diversity to these exocytic events.
The identification of CD63-rich ILVs within the WPB lumen extends the features that WPBs share with other LROs. Many LROs, such as melanosomes and lytic granules, release CD63-rich vesicles. Our observations draw further attention to the similarity between WPBs and platelet α-granules, which originate as MVBs containing ILVs but become filled with dense material, including VWF and P-selectin, during maturation. In addition, our immunofluorescence data show that WPB ILVs are enriched in CD63, but lack CD9 or CD81, which is also the case with the ILVs of platelet α-granules.18
In contrast to platelet α-granules, WPBs form by the polymerization of VWF in nascent granules at the TGN. Protrusive clathrin-coated membrane buds are a feature of nascent WPBs, reflecting the active sorting away of material not destined for storage in the mature WPB.43 Mature WPBs lack bilayer coats,3,43,44 and our cryo-electron microscopy images of vitrified endothelial cells show a tight, almost shrink-wrapped, limiting membrane surrounding the paracrystalline core of VWF and associated ILVs. Newly forming WPBs emerging from the TGN lack CD63, but soon after acquire the tetraspanin through a poorly defined interaction with endosomal components9,10 that involves the adapter protein AP3 and annexin 8.7,10 These observations (on WPBs and platelet α-granules) contribute to the view that the LRO possesses a mixture of endosomal and regulated secretory features that depends on the organelle’s biogenesis and specialization.
In this study, we have shown that the CD63 in WPB ILVs is trafficked via the endosomal system. CD63 is a ubiquitously expressed integral membrane protein found on the plasma membrane and endosomal compartments of all cells.45 In endosomal compartments, CD63 is enriched in a subset of ILVs and is present on exosomes secreted during MVB fusion with the plasma membrane.34,46,47 CD63 delivery to WPBs could occur through small vesicles formed by endosomal membrane budding.48 Such vesicles can contain AP3,49 the adapter protein implicated in CD63 delivery to WPBs.10 Alternatively, direct fusion and content transfer between LEs/MVBs and lysosomal compartments are well documented,50 and a similar process could account for diffusional transfer of CD63 to the limiting membrane of the maturing WPB, as well as direct MVB to WPB ILV transfer. The absence in WPBs of several markers reported to be enriched in ILVs/exosomes of LEs/MVBs, LPBA,9 cholesterol,36 CD9, and CD81,32,45 as well as the exogenous marker reported to accumulate in LE/MVB ILVs, N-Rh-PE,37 indicates that WPB ILVs may represent a distinct population with similarities to platelet α-granules.
We looked for the presence of ESCRT components and associated proteins known to be involved in MVB exosome formation and found syntenin localized to WPBs. Syntenin is a cytosolic PDZ domain protein that acts as an intracellular adapter involved in many processes, including exosome biogenesis and secretion.51 Syntenin binds directly to CD6352 and regulates the formation of CD63-containing exosomes.51,53 The localization of CD63 and syntenin on WPBs, as well as the presence of cytoplasmic components within WPB ILVs, may indicate that WPB ILVs form by inward budding of the WPB limiting membrane during organelle maturation.
Growing evidence suggests that endothelial-derived exosomes provide an important route for the exchange of proteins, lipids, and nucleic acids that contribute to intercellular communication and regulation of the immune and cardiovascular systems.54,55 Endothelial cell–derived exosomes are reported to directly modulate many target cells47,56,57 ; in turn, endothelial cells are a target for exosomes released from other cells.58-61 For example, angiopoietin-2, an important regulator of vascular network formation, is secreted from endothelial cells on the outer surface of CD63+ exosomes.47 Although the etiology of these secreted exosomes has been assumed to be LEs/MVBs, angiopoietin-2 can be stored in the lumen of WPBs for regulated secretion,62 raising the intriguing possibility that some of these vesicles may be released through WPB exocytosis.
Endothelial cells accumulate hundreds of WPBs under resting conditions and may contain similar numbers of MVBs.1,21 During Ca2+ stimulation, WPB exocytosis is rapid in onset, peaking 5 to 10 seconds after stimulation, involves up to 50% of the stored organelles, and is largely complete within 1 or 2 minutes of stimulation.63 Ca2+-stimulated MVB fusion is reported to be slower in onset (2-6 minutes), involves a subpopulation of CD63+ MVBs,21,40,64 and is estimated to result in only a small fraction (∼3%) of CD63+ MVB ILVs being released as exosomes.21 Therefore, WPBs are well placed for exosome release following acute cell activation and prior to exosome release by MVB fusion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The anti-CD63 monoclonal antibody H5C6, developed by J. T. August and J. E. K. Hildreth, was obtained from the Developmental Studies Hybridoma Bank, created by the Eunice Kennedy Shriver National Institute of Child Health and Human Development of the National Institutes of Health and maintained at the Department of Biology at the University of Iowa.
T.C. was funded by the UK Medical Research Council under program grant MC_PC_13053. P.B.R. is supported by the Francis Crick Institute, which receives its core funding from Cancer Research UK (FC001143), the UK Medical Research Council (FC001143), and the Wellcome Trust (FC001143).
Authorship
Contribution: J.S., A.-V.F., J.T., N.I.K., L.K., and T.C performed research and analyzed data; and P.B.R. and T.C. designed the research and wrote the manuscript
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.S. is MRC-University of Glasgow Centre for Virus Research, Glasgow, United Kingdom.
N.I.K. is retired from MRC National Institute for Medical Research, The Ridgeway, London, United Kingdom.
The current affiliation for L.K. is Francis Crick Institute, London, United Kingdom.
Correspondence: Tom Carter, Molecular and Clinical Sciences Research Institute, St George’s University, Cranmer Ter, London SW17 0RE, United Kingdom; e-mail: tcarter@sgul.ac.uk; and Peter B. Rosenthal, Structural Biology of Cells and Viruses Laboratory, The Francis Crick Institute, 1 Midland Rd, London NW1 1AT, United Kingdom; e-mail: peter.rosenthal@crick.ac.uk.
REFERENCES
Author notes
J.S. and A.-V.F. contributed equally to this work.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal