Key Points
Cerebral metabolic stress is reduced in SCA patients receiving HU compared with untreated patients, but remains higher than patients receiving CTT.
HU reduces the volume of tissue with maximal metabolic stress in the internal border zone, a region at high risk for stroke.
Abstract
Chronic transfusion therapy (CTT) prevents stroke in selected patients with sickle cell anemia (SCA). We have shown that CTT mitigates signatures of cerebral metabolic stress, reflected by elevated oxygen extraction fraction (OEF), which likely drives stroke risk reduction. The region of highest OEF falls within the border zone, where cerebral blood flow (CBF) nadirs; OEF in this region was reduced after CTT. The neuroprotective efficacy of hydroxyurea (HU) remains unclear. To test our hypothesis that patients receiving HU therapy have lower cerebral metabolic stress compared with patients not receiving disease-modifying therapy, we prospectively obtained brain magnetic resonance imaging scans with voxel-wise measurements of CBF and OEF in 84 participants with SCA who were grouped by therapy: no disease-modifying therapy, HU, or CTT. There was no difference in whole-brain CBF among the 3 cohorts (P = .148). However, whole-brain OEF was significantly different (P < .001): participants without disease-modifying therapy had the highest OEF (median 42.9% [interquartile range (IQR) 39.1%-49.1%]), followed by HU treatment (median 40.7% [IQR 34.9%-43.6%]), whereas CTT treatment had the lowest values (median 35.3% [IQR 32.2%-38.9%]). Moreover, the percentage of white matter at highest risk for ischemia, defined by OEF greater than 40% and 42.5%, was lower in the HU cohort compared with the untreated cohort (P = .025 and P = .034 respectively), but higher compared with the CTT cohort (P = .018 and P = .029 respectively). We conclude that HU may offer neuroprotection by mitigating cerebral metabolic stress in patients with SCA, but not to the same degree as CTT.
Introduction
The neurologic consequences of sickle cell anemia (SCA) are significant, ranging from cognitive deficits affecting academic achievement to devastating strokes in the setting of cerebral vasculopathy. Historically, 11% of individuals with SCA experience an overt stroke by age 20 years.1 Screening with transcranial Doppler ultrasound (TCD) and initiation of chronic transfusion therapy (CTT) for elevated time-averaged mean blood flow velocities in the internal carotid or middle cerebral arteries decreases the relative risk for initial overt stroke by 92%.2-4 However, TCD is not predictive of silent cerebral infarcts (SCIs),5 which occur in from 30.8% to 39.1% of individuals before age 18 years without any apparent plateau in prevalence.6,7 In addition, patients with SCA who have not suffered from cerebral infarction still endure cognitive dysfunction8-11 that progresses with age,11,12 as well as aberrant development of gray and white matter.13
Red blood cell transfusion has long been established as an effective treatment of stroke prevention in SCA,4,14-17 whereas the role of hydroxyurea (HU) in the prevention of the neurologic consequences in SCA is still evolving. HU induces hemoglobin (Hb) F production, decreasing the severity of anemia in patients with SCA,18 and has become standard of care for children unaffected by overt stroke or elevated TCD.19 Accumulating evidence suggests HU may be neuroprotective, specifically preventing conversion to20-23 and secondary reversion to24 abnormal TCD velocities, but data do not support the use of HU for secondary stroke prevention when the resources for CTT are available.25 Similarly for SCIs, data suggest a likely benefit of HU in prevention of SCIs; however, a lack of randomized controlled trials leaves the exact role and degree of benefit of HU unclear.26-28
Both HU and CTT increase arterial oxygen content (CaO2), which is low in SCA, via an increase in Hb. The cerebral metabolic rate of oxygen use (CMRO2), a measure of oxygen demand within the brain, is the product of CaO2, cerebral blood flow (CBF) and oxygen extraction fraction (OEF).29 To maintain CMRO2 in the setting of low CaO2, both CBF30,31 and OEF32,33 are globally elevated in patients with SCA. The border zone regions of the brain, with relatively low CBF, demonstrate the greatest elevation in OEF in patients with SCA and colocalize with regions at greatest risk for infarction.33-35 CTT increases CaO2, which lowers CBF and OEF, thereby relieving ongoing cerebral metabolic stress and providing a likely mechanism for stroke risk reduction.36 The effect of HU on cerebral metabolic stress, however, has not been characterized. Studying oxygen metabolism in patients treated with CTT or HU helps define the relative effect of each intervention on ischemic physiology. Moreover, measures of cerebral oxygen metabolism hold promise to individualize therapies aimed at mitigating the neurologic consequences of SCA.
In this study, we prospectively obtained voxel-wise measurements of CBF and OEF in patients with SCA not receiving disease-modifying therapy, and in those receiving HU therapy or CTT. First, we hypothesized that patients receiving HU would have lower CBF and OEF compared with patients not receiving disease-modifying interventions. Second, we hypothesized that the volume of brain tissue with OEF elevation, suggestive of tissue at heightened risk for stroke, would be smaller in the HU cohort compared with the untreated cohort. If proven, these data would suggest that HU reduces metabolic stress and may aid in prevention of cerebral infarcts and other neurologic morbidity of SCA.
Methods
This study was approved by the Institutional Review Board at Washington University in St. Louis, Missouri. Informed consent was obtained from all participants or their legal guardians. Participants aged 5 years or older with SCA, Hb SS, or Hb S β thalassemia null disease, were recruited from St. Louis Children’s Hospital or Barnes Jewish Hospital at Washington University School of Medicine. Exclusion criteria include hypertension requiring more than 1 pharmacologic intervention, diabetes mellitus requiring insulin therapy, concomitant treatment with HU and CTT, history of stem cell transplant, neurologic condition other than SCA, and inability to tolerate a magnetic resonance imaging (MRI) scan without sedation. Institutional standard of care guidelines for annual screening with TCD and initiation of HU by 1 year of age were formalized in 2011 and 2014, respectively. Before this, practice varied on the basis of provider preference and TCD availability. Participants were grouped for analyses by their SCA therapy at the time of brain MRI: SCA without disease-modifying therapy, SCA receiving HU, and SCA receiving CTT. Details regarding HU dosing and CTT were obtained through review of the medical record and participant medication log on day of the MRI. Participants receiving CTT were scanned within a 24-hour period before the administration of their scheduled red blood cell transfusion. All participants underwent laboratory evaluation, including a complete blood count, Hb capillary gel electrophoresis, and Hb venous saturation for evaluation of dyshemoglobins and measurement of peripheral oxygen saturation (SpO2) with pulse oximetry, as part of the study evaluation. For the CTT cohort, pretransfusion laboratory values were used for all analyses, with the exception of dyshemoglobin measurements, which were drawn posttransfusion in 2 participants. Three participants did not have a Hb capillary gel electrophoresis obtained, excluding them from multivariate modeling including these variables. Five participants did not have Hb venous saturations drawn, and 1 participant had this laboratory evaluation performed 11 months after the study visit. Vasculopathy was defined as narrowing of the internal carotid artery or middle cerebral artery identified by magnetic resonance angiogram (MRA). Seven participants (2 in the subgroup not receiving disease-modifying therapy and 5 receiving HU) did not have a MRA as part of their study evaluation. These participants were evaluated for vasculopathy by review of a clinically obtained MRA or TCD within a mean of 32.9 ± 22.0 months of the study evaluation. Data from 54 participants included in this study have been previously reported by our research group.33,35,36
Imaging protocol and processing
Participants underwent brain MRI without sedation on a Siemens 3T Tim Trio or 3T Biograph mMR scanner (Erlangen, Germany) with a 12-channel head coil. Standard three-dimensional magnetization prepared rapid acquisition gradient echo (MP-RAGE) T1 (echo time/repetition time [TE/TR] = 2.95/1800 ms; inversion time = 1000 ms; flip angle = 8°; acquired voxel resolution 1 × 1 × 1 mm, 0.48 × 0.48 × 1 mm voxel resolution after in-plain interpolation), axial and coronal fluid attenuated inversion recovery (FLAIR, TE/TR, 93/9000 ms; inversion time, 2500 ms; 0.86 × 0.86 mm in-plane resolution, 5-mm slice thickness), and MRA sequences were acquired. A board-certified neuroradiologist reviewed images to classify the participants as having an infarction and/or vasculopathy, and a board-certified neurologist (A.L.F. or K.P.G.) manually delineated infarcts on the FLAIR images. Regions of infarction were excluded from image analysis. We used a pseudocontinuous arterial spin labeling sequence (TE/TR, 12/3780 ms; in-plane voxel resolution, 3 × 3 mm; slice thickness, 5 mm, 22 slices; number of averages, 80; labeling duration, 2000 ms; postlabel delay, 1500 ms) to measure CBF.37 Blood T1 was measured individually in the superior sagittal sinus with an inversion-recovery echo planar imaging sequence with an adiabatic nonselective inversion pulse, or modeled by participant’s absolute Hb A and Hb S if a measured value was unavailable to improve CBF reproducibility.38,39 Thirty-four participants did not have a pseudocontinuous arterial spin labeling sequence obtained with a 1.5-second postlabel delay because of a change in study protocol, and were excluded from CBF analyses. Voxel-wise, OEF was measured with an asymmetric spin echo sequence.40 Two subjects did not have an asymmetric spin echo sequence obtained as part of their MRI scan. Quantification of CBF and OEF have been previously described.33,36
FMRIB’s Automated Segmentation Tool was used to segment individual MP-RAGE images into gray and white matter,41 and FMRIB’s Integrated Registration and Segmentation Tool was used to segment subcortical nuclei.42 To limit partial volume effects, voxels with a probability less than 0.7 of being classified correctly as gray or white matter were removed, and a 1-voxel morphological erosion of gray and white matter was applied to segmented images. FMRIB’s Linear Image Registration Tool coregistered images obtained within a scan session.43,44
Participant OEF maps and segmentations coregistered to the MP-RAGE were transformed to the International Consortium for Brain Mapping template with Advanced Normalization Tools45 and averaged to obtain per cohort OEF maps. OEF maps containing only white matter voxels with acceptable fitting errors33 were averaged by cohort and thresholded to identify regions of the average cohort map with OEF greater than 40%, 42.5%, and 45%. Three thresholds were evaluated to ensure results were not specific to a single threshold, with 40% being the median whole-brain OEF for the HU cohort. Individual participant white matter OEF maps were also thresholded at OEF values greater than 40%, 42.5%, and 45% to permit volumetric comparisons between cohorts. To adjust for differences in whole-brain volume between participants, we normalized the individual thresholded volumes by individual total white matter volume before comparison. To investigate the regional prevalence of elevated OEF, voxel-wise population heat maps within the white matter were computed by dividing the number of participants with high OEF (exceeding thresholds of 40%, 42.5%, or 45%) by the total number of participants within each cohort who had OEF values with acceptable fitting errors per voxel.
Statistical analysis
Data are described with median (interquartile range). Comparisons between the 3 cohorts were made with Kruskal-Wallis test, χ-squared test, or Fisher’s exact test, with significant differences followed by pairwise comparisons with Mann-Whitney U test. Bivariate correlations between whole-brain CBF and OEF with cohort, age, sex, total Hb, SpO2, percentage Hb A (%Hb A), % Hb F, % Hb S, ratio of gray to white matter volume, and presence of SCI, overt stroke, or vasculopathy were performed with Spearman’s ρ (ρ). General linear modeling with stepwise entry was used for multivariate analyses of whole-brain CBF and OEF. A univariate P < .3 was required for entry into, and P = .05 for retention within the general linear model. Patients found to be statistical outliers in invalid regressions, with Studentized residuals ≥3 and Cook’s distance values ≥1, were removed from model, and naive regression was repeated. Final multivariate models were valid without collinearity and with normal residuals. Raw P values are reported with significance specified as a P < .05 after correction by the Benjamini-Hochberg procedure for a final, overall family-wise error rate of .05. Statistical analyses were performed with SAS (version 9.2, SAS Institute Inc, Cary, NC).
Results
Brain MRIs were obtained from 84 participants divided into 3 cohorts: no disease-modifying therapy (N = 23), HU (N = 38), and CTT (N = 23). There were no significant differences in age, sex, genotype, or SpO2 among the 3 cohorts (Table 1). The HU cohort was taking a median HU dose of 24.6 (interquartile range, 18.3-30.8) mg/kg/day.
Description of the study population
| . | No disease-modifying therapy (N = 23) . | Hydroxyurea therapy (N = 38) . | Chronic transfusion therapy (N = 23) . | P . |
|---|---|---|---|---|
| Age, median (IQR), y | 11.0 (8.0-21.0) | 11.5 (9.0-23.0) | 15.0 (10.0-20.0) | .940 |
| Sex, female (%) | 11 (47.8) | 17 (44.7) | 14 (60.9) | .460 |
| Genotype | .661 | |||
| Hb SS, n (%) | 20 (87.0) | 33 (86.8) | 22 (95.7) | |
| HbSβ0, n (%) | 3 (13.0) | 5 (13.2) | 1 (4.3) | |
| SpO2, median (IQR), % | 96.0 (94.0-99.0) | 99.0 (97.0-100.0) | 97.0 (96.0-98.0) | .035 |
| Hematologic parameters | ||||
| Hb, median (IQR), g/dL | 7.8 (7.1-8.1)*,† | 8.7 (7.8-9.9)* | 9.1 (8.5-9.8)† | .001‡ |
| Hb A, median (IQR), % | 0.0 (0.0-0.0)† | 0.0 (0.0-0.0)¶ | 52.2 (43.0-63.1)†,¶ | <.001‡ |
| Hb F, median (IQR), % | 7.9 (3.4-14.4)*,† | 17.7 (12.2-25.5)*,¶ | 2.2 (0.0-4.5)†,¶ | <.001‡ |
| Hb S, median (IQR), % | 82.4 (75.1-89.9)† | 78.4 (70.2-83.4)¶ | 41.8 (31.7-49.4)†,¶ | <.001‡ |
| Carboxyhemoglobin, median (IQR), % | 2.7 (2.3-3.7)† | 2.4 (1.8-3.1) | 2.0 (1.9-2.6)† | .023‡ |
| Methemoglobin, median (IQR), % | 2.2 (1.9-2.5) | 2.3 (1.9-2.6) | 1.8 (1.6-2.4) | .206 |
| Neurologic disease | ||||
| Silent infarct, n (%) | 10 (43.5)† | 15 (39.5)¶ | 19 (82.6)†,¶ | .003‡ |
| Overt stroke, n (%) | 0 (0)† | 2 (5.3)¶ | 11 (47.8)†,¶ | <.001‡ |
| Vasculopathy, n (%) | 1 (4.4)† | 2 (5.3)¶ | 10 (43.5)†,¶ | <.001‡ |
| . | No disease-modifying therapy (N = 23) . | Hydroxyurea therapy (N = 38) . | Chronic transfusion therapy (N = 23) . | P . |
|---|---|---|---|---|
| Age, median (IQR), y | 11.0 (8.0-21.0) | 11.5 (9.0-23.0) | 15.0 (10.0-20.0) | .940 |
| Sex, female (%) | 11 (47.8) | 17 (44.7) | 14 (60.9) | .460 |
| Genotype | .661 | |||
| Hb SS, n (%) | 20 (87.0) | 33 (86.8) | 22 (95.7) | |
| HbSβ0, n (%) | 3 (13.0) | 5 (13.2) | 1 (4.3) | |
| SpO2, median (IQR), % | 96.0 (94.0-99.0) | 99.0 (97.0-100.0) | 97.0 (96.0-98.0) | .035 |
| Hematologic parameters | ||||
| Hb, median (IQR), g/dL | 7.8 (7.1-8.1)*,† | 8.7 (7.8-9.9)* | 9.1 (8.5-9.8)† | .001‡ |
| Hb A, median (IQR), % | 0.0 (0.0-0.0)† | 0.0 (0.0-0.0)¶ | 52.2 (43.0-63.1)†,¶ | <.001‡ |
| Hb F, median (IQR), % | 7.9 (3.4-14.4)*,† | 17.7 (12.2-25.5)*,¶ | 2.2 (0.0-4.5)†,¶ | <.001‡ |
| Hb S, median (IQR), % | 82.4 (75.1-89.9)† | 78.4 (70.2-83.4)¶ | 41.8 (31.7-49.4)†,¶ | <.001‡ |
| Carboxyhemoglobin, median (IQR), % | 2.7 (2.3-3.7)† | 2.4 (1.8-3.1) | 2.0 (1.9-2.6)† | .023‡ |
| Methemoglobin, median (IQR), % | 2.2 (1.9-2.5) | 2.3 (1.9-2.6) | 1.8 (1.6-2.4) | .206 |
| Neurologic disease | ||||
| Silent infarct, n (%) | 10 (43.5)† | 15 (39.5)¶ | 19 (82.6)†,¶ | .003‡ |
| Overt stroke, n (%) | 0 (0)† | 2 (5.3)¶ | 11 (47.8)†,¶ | <.001‡ |
| Vasculopathy, n (%) | 1 (4.4)† | 2 (5.3)¶ | 10 (43.5)†,¶ | <.001‡ |
HbSβ0, Hb S β thalassemia null disease.
Significant difference between cohort not receiving disease-modifying therapy vs cohort receiving HU.
Significant difference between cohort not receiving disease-modifying therapy vs cohort receiving CTT.
Statistically significant.
Significant difference between cohort receiving HU vs cohort receiving CTT.
Although Hb was significantly lower in the cohort not receiving disease-modifying therapy vs the other 2 cohorts, there was no significant difference in Hb between the cohorts receiving HU and CTT therapy (P = .162). As expected, the cohort receiving CTT had a significantly higher Hb A percentage compared with the other 2 cohorts, and the cohort receiving HU had a significantly higher Hb F percentage compared with the other 2 cohorts. The CTT cohort had a significantly lower Hb S percentage compared with the other 2 cohorts. The CTT cohort had a significantly higher prevalence of SCI, overt stroke, and vasculopathy, whereas there was not a difference in prevalence between the cohort without disease-modifying therapy and those receiving HU (Table 1).
CBF was compared among the 3 cohorts to evaluate potential effects of HU on global and regional CBF. Whole-brain (P = .148), gray matter (P = .312), and white matter (P = .274) CBF were not significantly different between the 3 cohorts (Table 2; Figure 1). Forty-five participants (53.6%) had a measured T1, and T1 was calculated in 39 (46.4%) participants. There was not a significant difference between measured and calculated T1 values (measured T1, 1881.9 [1705.0-2013.5] ms; calculated T1, 1824.4 [1737.9-1909.6] ms; P = .302). There was not a difference in whole-brain (P = .152), gray matter (P = .287), or white matter (P = .499) CBF between participants with a measured or calculated T1. To investigate factors influencing CBF, we explored the relationships among CBF; treatment cohort, age, sex, SpO2, Hb, % Hb A, % Hb F, % Hb S, the ratio between gray and white matter volume, and presence of SCI, overt stroke, and vasculopathy. On univariate analysis, whole-brain CBF increased with younger age (ρ = −0.350; P = .013) and lower Hb (ρ = −0.392; P = .005), but was not significantly correlated with SpO2 (P = .223), % Hb A (P = .910), % Hb F (P = .170), % Hb S (P = .148), or the ratio of gray to white matter volume (P = .053). Two participants were found to be statistical outliers and were removed from the final multivariate model. In multivariate analysis, total Hb (β = −5.30; 95% confidence interval [CI], −8.23 to −2.37; P < .001), presence of vasculopathy (β = −14.23; 95% CI, −22.56 to −5.89; P = .001), % Hb F (β = −0.01; 95% CI, −0.01 to −0.00; P = .003), age (β = −0.55; 95% CI, −0.91 to −0.18; P = .004), and male sex (β = −7.41; 95% CI, −14.29 to −0.52; P = .036) remained independent predictors of whole-brain CBF (R2 = 0.581; P < .001).
Whole brain and segmented CBF and OEF between cohorts
| . | No disease-modifying therapy, median (IQR) . | Hydroxyurea therapy, median (IQR) . | Chronic transfusion therapy, median (IQR) . | P . |
|---|---|---|---|---|
| Cerebral blood flow | (N = 11) | N = 25 | N = 14 | |
| Whole brain CBF, mL/100 g/min | 99.8 (82.7-117.7) | 90.8 (74.2-100.2) | 87.0 (75.9-96.4) | .148 |
| Gray matter CBF, mL/100 g/min | 112.5 (93.4-130.1) | 97.4 (86.7-109.8) | 94.3 (87.7-112.8) | .312 |
| White matter CBF, mL/100 g/min | 60.5 (46.1-81.5) | 55.5 (46.6-66.6) | 51.1 (48.7-55.9) | .274 |
| OEF | N = 22 | N = 38 | N = 22 | |
| Whole brain OEF, % | 42.9 (39.1-49.1)*,† | 40.7 (34.9-43.6)*,¶ | 35.3 (32.2-38.9)†,¶ | <.001‡ |
| Gray matter OEF, % | 44.3 (39.2-49.2)*,† | 41.2 (36.3-44.4)*,¶ | 35.0 (33.2-37.7)†,¶ | <.001‡ |
| White matter OEF, % | 42.6 (38.1-48.7)*,† | 39.8 (33.8-43.5)*,¶ | 34.1 (30.8-39.2)†,¶ | <.001‡ |
| . | No disease-modifying therapy, median (IQR) . | Hydroxyurea therapy, median (IQR) . | Chronic transfusion therapy, median (IQR) . | P . |
|---|---|---|---|---|
| Cerebral blood flow | (N = 11) | N = 25 | N = 14 | |
| Whole brain CBF, mL/100 g/min | 99.8 (82.7-117.7) | 90.8 (74.2-100.2) | 87.0 (75.9-96.4) | .148 |
| Gray matter CBF, mL/100 g/min | 112.5 (93.4-130.1) | 97.4 (86.7-109.8) | 94.3 (87.7-112.8) | .312 |
| White matter CBF, mL/100 g/min | 60.5 (46.1-81.5) | 55.5 (46.6-66.6) | 51.1 (48.7-55.9) | .274 |
| OEF | N = 22 | N = 38 | N = 22 | |
| Whole brain OEF, % | 42.9 (39.1-49.1)*,† | 40.7 (34.9-43.6)*,¶ | 35.3 (32.2-38.9)†,¶ | <.001‡ |
| Gray matter OEF, % | 44.3 (39.2-49.2)*,† | 41.2 (36.3-44.4)*,¶ | 35.0 (33.2-37.7)†,¶ | <.001‡ |
| White matter OEF, % | 42.6 (38.1-48.7)*,† | 39.8 (33.8-43.5)*,¶ | 34.1 (30.8-39.2)†,¶ | <.001‡ |
Significant difference between cohort not receiving disease-modifying therapy vs cohort receiving HU.
Significant difference between cohort not receiving disease-modifying therapy vs cohort receiving CTT.
Statistically significant.
Significant difference between cohort receiving HU vs cohort receiving CTT.
Whole-brain and segmented CBF and OEF between cohorts. Although there was no difference in CBF among the 3 cohorts in the whole brain, gray matter, or white matter (A), there was a significant decrease in whole brain, gray matter and white matter OEF in the cohort receiving HU therapy (blue) compared with those not receiving disease-modifying therapy (red), but not to the extent of those receiving CTT (green) (B). *Statistically significant after Benjamini-Hochberg step-up procedure used to correct for multiple comparisons.
Whole-brain and segmented CBF and OEF between cohorts. Although there was no difference in CBF among the 3 cohorts in the whole brain, gray matter, or white matter (A), there was a significant decrease in whole brain, gray matter and white matter OEF in the cohort receiving HU therapy (blue) compared with those not receiving disease-modifying therapy (red), but not to the extent of those receiving CTT (green) (B). *Statistically significant after Benjamini-Hochberg step-up procedure used to correct for multiple comparisons.
We further evaluated effects of HU on global and regional OEF, finding that whole-brain (P < .001), gray matter (P < .001), and white matter (P < .001) OEF differed among the 3 cohorts. Pairwise comparisons found that OEF was significantly decreased in the cohort receiving HU therapy when compared with the cohort not receiving disease-modifying therapy. Moreover, the cohort receiving CTT had a significantly lower OEF than the other 2 cohorts in the whole brain, gray matter, and white matter (Table 2; Figure 1). To investigate potential mechanisms of OEF reduction, we modeled predictors of OEF including treatment cohort, age, sex, SpO2, total Hb, % Hb A, % Hb F, % Hb S, ratio of gray to white matter volume, and presence of SCI, overt stroke, or vasculopathy. On univariate analysis, whole-brain OEF increased with younger age (ρ = −0.253; P = .022), lower SpO2 (ρ = −0.371; P = .001), lower Hb (ρ = −0.845; P < .001), lower % Hb A (ρ = −0.370; P < .001), higher % Hb F (ρ = 0.224; P = .047), and higher % Hb S (ρ = 0.371; P < .001), but not the ratio of gray matter volume to white matter volume (P = .118). In multivariate analysis, Hb (β = −3.40; 95% CI, −3.99 to −2.81; P < .001), % Hb A (β = −0.00; 95% CI, −0.00 to −0.00; P < .001), age (β = −0.13; 95% CI, −0.21 to −0.06; P < .001), SpO2 (β = −0.42; 95% CI, −0.70 to −0.14; P = .004), and male sex (β = −1.42; 95% CI, −2.80 to −0.04; P = .044) remained independent predictors of whole-brain OEF (R2 = 0.780; P < .001).
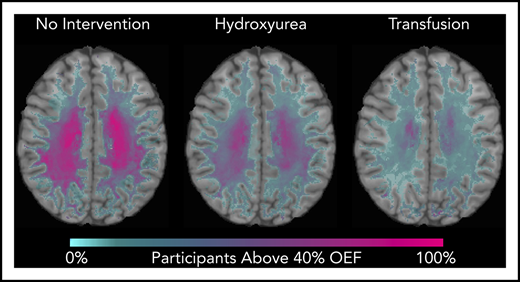
We identified that the region of greatest OEF was located in the deep white matter (Figure 2), where CBF is found to be at a nadir, consistent with prior reports.33,36 To determine the effect of HU on regional OEF, as an indicator of tissue at heightened risk for ischemia, we thresholded individual OEF maps to measure white matter volumes with OEF values greater than 40%, 42.5%, and 45%. The cohort receiving HU had a lower percentage white matter volume with OEF values greater than 40% (P = .025), 42.5% (P = .034), and 45% (P = .052) when compared with the cohort not receiving disease-modifying therapy. However, the HU cohort had a higher percentage white matter volume with OEF values greater than 40% (P = .018), 42.5% (P = .029), and 45% (P = .035) when compared with the CTT cohort (Table 3). We further quantified the percentage of patients within each voxel with high OEF, defined as greater than 40%, 42.5%, and 45%. Figure 3 illustrates the prevalence of patients with OEF above the 3 thresholds across the 3 cohorts. The highest prevalence of elevated OEF consistently falls within the internal border zone in all 3 cohorts, with a stepwise decrease in prevalence of regional peak OEF for the HU and CTT cohorts compared with the cohort not receiving disease-modifying therapy.
HU decreases the volume of brain tissue with elevated OEF in the border zone. The average white matter OEF map for each cohort was thresholded at 40% (dark teal), 42.5% (light teal), and 45% (white) OEF to identify the brain regions with peak oxygen metabolic stress, as a potential index of elevated stroke risk. The figure shows the average OEF map for each cohort (left), paired with the thresholded regions overlaid on a T1 atlas (right). There is a decrease in thresholded brain tissue in the cohort treated with HU when compared with those not receiving disease-modifying therapy across all thresholds, but the cohort receiving CTT has a significantly lower volume of at risk tissue compared with the HU cohort.
HU decreases the volume of brain tissue with elevated OEF in the border zone. The average white matter OEF map for each cohort was thresholded at 40% (dark teal), 42.5% (light teal), and 45% (white) OEF to identify the brain regions with peak oxygen metabolic stress, as a potential index of elevated stroke risk. The figure shows the average OEF map for each cohort (left), paired with the thresholded regions overlaid on a T1 atlas (right). There is a decrease in thresholded brain tissue in the cohort treated with HU when compared with those not receiving disease-modifying therapy across all thresholds, but the cohort receiving CTT has a significantly lower volume of at risk tissue compared with the HU cohort.
Comparison of brain volumes experiencing metabolic stress between cohorts
| . | No disease-modifying therapy, median (IQR) . | Hydroxyurea therapy, median (IQR) . | Chronic transfusion therapy, median (IQR) . | P . |
|---|---|---|---|---|
| White matter volume (%) | ||||
| OEF threshold >40% | 48.1 (21.9-64.3)*,† | 32.3 (6.3-47.3)*,¶ | 6.1 (2.5-25.5)†,¶ | <.001‡ |
| OEF threshold >42.5% | 36.0 (13.1-59.3)*,† | 20.4 (3.6-36.7)*,¶ | 3.9 (1.3-15.3)†,¶ | .001‡ |
| OEF threshold >45% | 22.1 (8.6-49.4)† | 11.2 (2.2-25.4)¶ | 2.1 (0.7-10.9)†,¶ | .002‡ |
| . | No disease-modifying therapy, median (IQR) . | Hydroxyurea therapy, median (IQR) . | Chronic transfusion therapy, median (IQR) . | P . |
|---|---|---|---|---|
| White matter volume (%) | ||||
| OEF threshold >40% | 48.1 (21.9-64.3)*,† | 32.3 (6.3-47.3)*,¶ | 6.1 (2.5-25.5)†,¶ | <.001‡ |
| OEF threshold >42.5% | 36.0 (13.1-59.3)*,† | 20.4 (3.6-36.7)*,¶ | 3.9 (1.3-15.3)†,¶ | .001‡ |
| OEF threshold >45% | 22.1 (8.6-49.4)† | 11.2 (2.2-25.4)¶ | 2.1 (0.7-10.9)†,¶ | .002‡ |
Significant difference between cohort not receiving disease-modifying therapy vs cohort receiving HU.
Significant difference between cohort not receiving disease-modifying therapy vs cohort receiving CTT.
Statistically significant.
Significant difference between cohort receiving HU vs cohort receiving CTT.
Percentage of patients with brain tissue experiencing increased metabolic stress decreases with HU. These population heat maps illustrate the percentage of participants within each white matter voxel that has an OEF exceeding the defined threshold overlaid on a T1 atlas (OEF threshold of 40%, left column; 42.5%, middle column; 45%, right column). The highest prevalence of elevated OEF consistently falls within the internal border zone in all 3 cohorts. The percentage of participants exceeding these thresholds is decreased in participants treated with HU compared with those not receiving disease-modifying therapy, but not to the extent of the participants receiving CTT.
Percentage of patients with brain tissue experiencing increased metabolic stress decreases with HU. These population heat maps illustrate the percentage of participants within each white matter voxel that has an OEF exceeding the defined threshold overlaid on a T1 atlas (OEF threshold of 40%, left column; 42.5%, middle column; 45%, right column). The highest prevalence of elevated OEF consistently falls within the internal border zone in all 3 cohorts. The percentage of participants exceeding these thresholds is decreased in participants treated with HU compared with those not receiving disease-modifying therapy, but not to the extent of the participants receiving CTT.
Discussion
We report that cerebral OEF is decreased in patients with SCA treated with HU compared with those not receiving disease-modifying therapy, but not to the same degree as those patients receiving CTT. Multivariate modeling of OEF within this study population shows that a change in total Hb and % Hb A, but not a manipulation of Hb F or Hb S, had greatest influence on OEF reduction. Last, we report that the volume of brain tissue with elevated OEF is decreased in participants treated with HU, and is further decreased in patients treated with CTT. Together, the data suggest that HU may provide neuroprotection by improving cerebral oxygen delivery, leading to a reduction in OEF. Moreover, the data also suggest that the reduction in OEF with HU is not as great as seen with CTT.
HU is accepted as the standard of care in clinical practice for children with SCA who do not require CTT.19 Yet, investigations thus far have not precisely defined the role of HU for primary and secondary stroke prevention. Previous studies suggest that treatment with HU can prevent the conversion of TCD from conditional to abnormal velocity.20-23 The TWiTCH trial reported that patients with an abnormal TCD, but normal MRA, can safely transition from CTT to HU without reversion of TCD to abnormal, allowing HU to be used for primary stroke prevention within a specific subgroup of patients with SCA.24 However, HU is not used for secondary stroke prevention if the resources for CTT are available after publication of the SWiTCH trial, showing that the combination of HU plus phlebotomy was inferior to CTT plus chelation therapy in patients with a history of overt stroke.25 Although CTT is beneficial in preventing recurrent SCIs and overt strokes,17 the role of HU in primary or secondary prevention of SCIs remains unclear. The HUSTLE study found patients treated with HU had slower SCI accumulation than untreated patients,26 which was corroborated by Rushton et al’s retrospective study.27 In contrast, Rigano et al reported an increase in detection and progression of SCIs in a cohort treated with HU when compared with the cohort’s pretreatment period in a multicenter, retrospective study evaluating clinical consequences of SCD in participants who were treated with HU.28 Our data provide physiologic evidence that patients with SCA treated with HU have lower OEF when compared with those not receiving disease-modifying therapy. As the OEF in patients treated with HU is approaching that of healthy control participants,33,36 we interpret this finding to support the clinical data that HU may provide neuroprotection in select clinical scenarios. However, other interpretations of a reduction in OEF are discussed here. We also found that OEF in HU-treated patients was higher than patients treated with CTT, suggesting that HU may not be a replacement for CTT, especially when stroke risk is known to be high.25
We have previously shown that the brain region with greatest OEF falls within the internal border zone where CBF nadirs,33 and this region of elevated OEF colocalizes with the region at greatest risk for infarction.33-35 In this study, we demonstrate that this region at risk, defined by elevated OEF, is smaller in patients treated with HU, but still larger than seen in those treated with CTT. Our findings illustrate that the volume of brain with high OEF decreased in patients treated with HU when compared with those not receiving disease-modifying therapy, regardless of the chosen threshold, suggesting that treatment reduces the amount of tissue potentially at risk for stroke. A planned future analysis is to follow patients with MRI to prospectively determine whether elevated OEF is predictive of stroke risk.
The most likely mechanism for OEF reduction with HU is an increase in arterial oxygen content (CaO2), primarily resulting from increased total Hb concentration. We found that total Hb was the strongest predictor of whole-brain OEF in the multivariate model, including all 3 cohorts, and have previously reported significant relationships between Hb and OEF.33,36 In support of these findings, lower Hb has previously been associated with increased stroke risk in patients with SCA.1,17,46 Moreover, cerebral infarction has been found in acutely anemic children without SCD.47 HU increases total Hb by inducing the synthesis of Hb F,18 which binds oxygen with higher affinity than Hb A and Hb S,48,49 leading to increased oxygen saturation. In addition, heterocellular expression of Hb F within red cells with HU retards polymerization of deoxygenated Hb S, resulting in reduced sickling and hemolysis in hypoxic tissue.50 Hence, Hb F affects OEF directly through an increase in Hb concentration and/or by altering red cell sickling. OEF in sickled vs normal red cells has not been studied, as it requires the examination of oxygen metabolism at a single-cell level.
The reduction in OEF in patients treated with HU and CTT could additionally be explained by a left shift in the oxygen-Hb dissociation curve, with Hb A, Hb F, and Hb S affecting OEF via differential oxygen binding.48,49 Despite similar total Hb concentrations, OEF was significantly different in the CTT cohort compared with the HU cohort, suggesting that Hb isoforms, in addition to Hb concentration, may influence the fraction of oxygen extracted from the blood. In our multivariate model, total Hb concentration and the Hb A percentage, only present through transfusion in this cohort, predicted OEF. In addition to providing an infusion of normal Hb A-containing red cells, CTT reduces Hb S percentage more than HU therapy does. Although our multivariate model did not demonstrate an effect of % Hb F, limited power may have precluded the detection of an effect. Further investigations will be required to elucidate the role of individual Hb isoforms, and left-shifting of the oxygen-Hb dissociation curve, on cerebral oxygen metabolism.
Finally, a reduction in OEF may be related to microvascular disease in SCA. Microvascular disease potentially increases capillary transit time heterogeneity, causing shunting across the capillary bed with resultant reduction in OEF.51 This phenomenon and its effect on OEF has not been measured in SCA, but capillary transit time heterogeneity has been modeled in other neurovascular insults.52,53 Although an increase in capillary transit time heterogeneity may reduce local OEF, other microvascular studies in patients with SCA have found a decrease in capillary-level red blood cell velocity, increased capillary tortuosity, and presence of red blood cell pauses within the microvasculature.54-57 With such competing influences, the net effect of microvascular disease on OEF in SCA is unclear. Further studies with novel technologies capable of measuring OEF at the capillary level are ultimately required to understand the effects of SCA on regional cerebral oxygen metabolism.
Although we see a significant difference in OEF between cohorts, CBF was not different between cohorts, nor was cohort a significant predictor of CBF in our multivariate analysis. Because of the smaller sample size of CBF data collected, these data may be underpowered to detect a difference between the cohorts, which is particularly relevant to CBF as it varies with age58 and severity of anemia.39,59 Furthermore, individuals with SCA demonstrate impaired cerebrovascular reserve60,61 and autoregulation.62 The current therapeutic options in SCA may be insufficient to provide adequate reduction in hemodynamic stress, while allowing for some degree of normalization of OEF in the setting of impaired cerebrovascular reserve and autoregulation. Although our data are limited by sample size, our results suggest that OEF may be a more sensitive biomarker of ongoing cerebral metabolic stress than CBF in SCA.
Strengths of our study include the application of a noninvasive MRI method, capable of measuring voxel-wise OEF in the border zone region at highest risk for stroke in SCA. However, our study has limitations. The cross-sectional design prevents one from determining causality; thus, longitudinal studies are needed to confirm whether HU leads to a reduction in OEF. The present analysis did not investigate regional OEF in the vascular distribution of stenotic vessels in participants with vasculopathy, which will be an essential future direction to understand the effect of large vessel vasculopathy on regional metabolic stress. Our analyses of CBF were limited by sample size; thus, they may have been underpowered to detect subtle differences. The recommendation to use a longer postlabel delay in children by the International Society for Magnetic Resonance in Medicine Perfusion Study Group and the European Consortium for ASL in Dementia was published in 2016,63 which was after data collection for this study began. Hence, fewer subjects had CBF data collected with a 1.5-second postlabel delay, but the current sample size was sufficient for model fitting. We also did not find a difference in CBF when comparing pre- and posttransfusion CBF measurements with those obtained in nontransfused patients with SCA.36 In addition, we used a single compartment model in the processing of CBF, which can potentially underestimate CBF when compared with phase contrast MRI resulting from labeling inefficiencies in the setting of increased blood velocity, inhomogeneities in labeling and venous outflow, or microvascular shunting in patients with anemia.64 A T1 value estimated by absolute Hbs A and Hbs S was used in the CBF calculation for participants for which an in vivo T1 measurement was not available. Estimated T1 values may be falsely elevated, which could lead to an underestimation of CBF.65 However, we did not find a significant difference between those participants with a measured vs calculated T1 value (P = .300).
We conclude that HU mitigates cerebral metabolic stress, as measured by a reduction in OEF, in patients with SCA, but not to the same degree as CTT. Through an increase in Hb, HU improves cerebral oxygen delivery, leading to a reduction in OEF. Although HU’s role in the prevention of overt stroke, SCIs, and cognitive decline in SCA warrants further investigation, we provide physiologic data to support that HU may be neuroprotective.
For original data, please contact the corresponding authors.
Presented in abstract form at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 11 December 2017.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Liam Comiskey and Rachel Shields for their assistance with project coordination.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (K23HL136904 [M.E.F.], 5K12 HL087107 [M.E.F.], R01 HL129241 [A.L.F., M.L.H.]), the National Institutes of Health, National Institute of Neurological Disorders and Stroke (1K23NS099472-01 [K.P.G.]), the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12HD04734 [K.P.G.]), the National Center for Advancing Translational Sciences (UL1TR002345 [M.E.F.]), the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (U54HD087011 [M.E.F., J.S.S.]), the National Center for Advancing Translational Sciences (UL1TR000448 [M.E.F., K.P.G., A.L.F.]), the American Society of Hematology (M.E.F.), the Doris Duke Charitable Foundation (M.E.F.), and the Child Neurology Foundation (K.P.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authorship
Contribution: M.E.F., K.P.G., J-.M.L., and A.L.F. designed the study; M.E.F., A.L.F., and J.-M.L. produced figures and wrote the manuscript; D.R., S.F., A.M., C.E., and Y.C. processed data; M.M.B. performed statistical analyses and interpreted data; H.A. developed and maintained the asymmetric spin echo MRI sequence used in the study; M.E.F., K.P.G., D.R., S.F., M.L.H., M.B., K.V., J.S.S., R.C.M., and H.A. collected and interpreted data; and all authors critically reviewed and approved the manuscript.
Conflict-of-interest disclosure: M.E.F. declares equity ownership in Proclara Biosciences. M.L.H. declares research funding from Global Blood Therapeutics; spouse employment at Pfizer, Inc.; and scientific advisory board membership in the Sickle Cell Transplant Alliance for Research. The remaining authors declare no competing financial interests.
Correspondence: Andria L. Ford, Department of Neurology, Washington University in St. Louis, 660 South Euclid, Campus Box 8111, St. Louis, MO, 63110; e-mail: forda@wustl.edu; and Jin-Moo Lee, Department of Neurology, Washington University in St. Louis, 660 South Euclid, Campus Box 8111, St. Louis, MO, 63110; e-mail: leejm@wustl.edu.
REFERENCES
Author notes
M.E.F. and K.P.G. contributed equally to this study and are designated as joint first authors.
J.-M.L. and A.L.F. contributed equally to this study and are designated as joint last authors.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal