Abstract
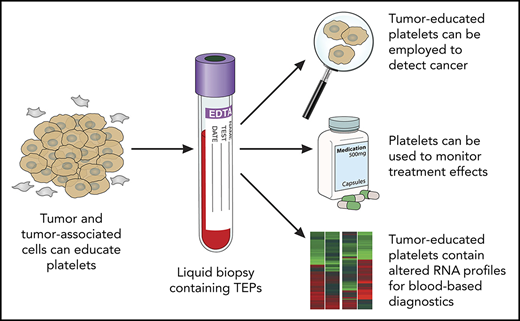
Liquid biopsies have been considered the holy grail in achieving effective cancer management, with blood tests offering a minimally invasive, safe, and sensitive alternative or complementary approach for tissue biopsies. Currently, blood-based liquid biopsy measurements focus on the evaluation of biomarker types, including circulating tumor DNA, circulating tumor cells, extracellular vesicles (exosomes and oncosomes), and tumor-educated platelets (TEPs). Despite the potential of individual techniques, each has its own advantages and disadvantages. Here, we provide further insight into TEPs.
Tumor-educated platelets (TEPs) as a liquid biopsy biosource
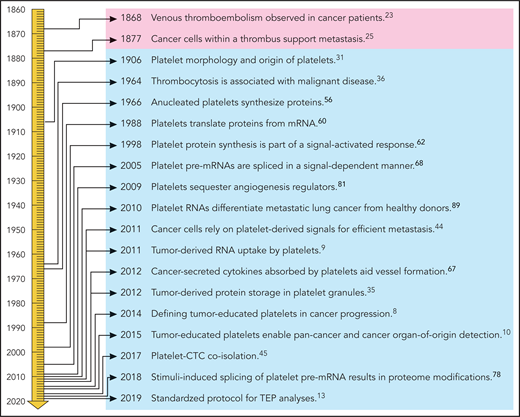
Liquid biopsies could provide a potential revolution in cancer diagnostics as a minimally invasive method for detecting and monitoring cancer. Liquid biopsies may provide an accurate and comprehensive snapshot of the tumor and its microenvironment on multiple levels.1 Liquid biopsies enable early detection (screening), as well as prognosis for the individual patient for tumor stage and identification of new targets for personalized treatment. In addition, blood tests can be employed for pretreatment classification for personalized therapy, early-therapy response monitoring, and “real-time” assessment of treatment effectiveness, as well as follow-up and early detection of disease recurrence. Multiple blood-based biosources are currently evaluated (reviewed in Heitzer et al,2 Babayan and Pantel,3 Aravanis et al,4 Best et al,5 and Wan et al6 ), including TEPs. Platelets, originating as anucleate cells from megakaryocytes, have emerged as central players in the systemic and local responses to tumor growth.5,7,8 In the past few years, methods for isolation and analysis of spliced TEP messenger RNA (mRNA) were developed to detect cancer with high accuracy.9-13 The development of TEPs as a potential liquid biopsy biosource, however, is a consequence of many studies on the role of platelets in cancer over the past few centuries (Figure 1).
Historical timeline of platelets in relation to cancer. Professional illustration by Patrick Lane, ScEYEnce Studios.
Historical timeline of platelets in relation to cancer. Professional illustration by Patrick Lane, ScEYEnce Studios.
A perspective on TEPs
Platelets are known for their function in coagulation of blood and wound healing, but their relationship with cancer has been studied extensively as well (reviewed in Franco et al,14 Lambert et al,15 Menter et al,16 Naderi-Meshkin and Ahmadiankia,17 Haemmerle et al,18 Olsson et al,19 Xu et al,20 Zhang et al,21 and Mancuso and Santagostino22 ). Two observations fundamental to the development of the concept of TEPs were made in the 19th century. First, in 1868, Trousseau shared his observation that spontaneous coagulation is common in cancerous patients,23 indicating that circulating platelets are affected by cancer.24 Second, in 1877, Billroth described “thrombi filled with specific tumor elements” as part of tumor metastasis,25 indicating a direct interaction between tumor cells and platelets.26-28 Evolving technology subsequently elucidated parts of the complex interactions among megakaryocytes, platelets, and cancer, leading to the concept of TEPs.5,8,11,29,30
In 1906, Wright reported that platelets are detached portions of the cytoplasm of megakaryocytes, which were identified in the bone marrow and spleen.31 However, circulating megakaryocytes were also observed in certain pathologic conditions,32 including cancer.33 In 2010, Zaslavsky et al showed that the number of megakaryocytes in the bone marrow increases in response to cancer.34 Kerr et al showed that communication between tumor and bone is mediated by platelets via tumor-derived proteins stored in granules.35 The increase in megakaryocytes and platelets was considered to be a systemic response to suppress tumor growth, established by elevated thrombospondin-1 levels in platelets.34 The relationship between platelet numbers and cancer was identified in the 1960s.36,37 A large-scale platelet screening study among 14 000 individuals revealed that thrombocytosis is associated with a variety of diseases, of which neoplasms in different organs resembled the largest group.36 In 1968, Gasic and colleagues showed a correlation between thrombocytopenia and cancer metastasis in mice. Injection with platelet-rich plasma reversed the antimetastatic effect observed for thrombocytopenic mice.38 In a follow-up study, they reported that in some instances, tumor cell–induced thrombocytopenia is accompanied by accumulation of platelets in the lung and that platelets with aggregating capacities usually correlate with lung metastases.37 It was recently demonstrated that the lung also is a site of platelet biogenesis,39 altogether suggesting a potential function of the lung (besides bone marrow) in the interaction between platelets and cancer metastases. The exact function of platelets from different origins has not been investigated yet. However, since ∼700 differentially expressed genes between lung and bone marrow megakaryocytes were identified,39 it is not unlikely that the RNA of platelets originating in bone marrow and lung differs as well. Hence, it may be of interest to study the spliced RNA profiles of the platelet populations derived from bone marrow and lung in response to various tumor-associated signals. It was shown that platelets also interact directly with intravasating and circulating tumor cells (CTCs), thereby altering platelet signaling.40-46 In 2017, Jiang et al were able to isolate platelet-covered CTCs from blood and observed that CTCs were extensively covered by platelets.45 Besides platelet numbers, the proteome and size of platelets and the platelet/immune cell ratio47-49 have also been reported to change in the presence of cancer.50-52 Moreover, meta-analyses showed that platelet inhibitors such as aspirin may be associated with a reduced risk of several cancer types.53 Experiments showed that inhibition of platelets by aspirin can affect their ability to induce cancer cell proliferation.54,55 Further research is warranted to determine how platelet inhibitors can be optimally employed as anticancer agents.18,20
For decades, it was believed that the platelet content was static, because platelets are cell fragments lacking a nucleus, and therefore no transcription and translation was expected. However, Warshaw et al performed experiments suggesting the ability of protein synthesis in platelets,56 a process extensively reviewed by Weyrich and colleagues.57 Booyse et al confirmed protein synthesis by platelets and suggested that the life span of the platelet mRNA determines the life span of the platelets.58 Subsequently, it was confirmed that platelet protein synthesis requires ribosomes.59 It took over 20 years until the involvement of mRNA in protein synthesis was confirmed by Newman et al using polymerase chain reaction.60
Power et al investigated the role of platelets in inflammation and used a complementary DNA library of human platelets and polymerase chain reaction to show the presence of mRNA coding for several chemokines in platelets.61 Weyrich et al demonstrated that protein synthesis is part of a signal-dependent activation response in human platelets,62 showing thrombin-induced 4E-BP1 phosphorylation and Bcl-3 expression in platelets. Also intra-platelet signaling by protein-tyrosine phosphorylation was revealed,63 and Nadal-Wollbold et al showed that thrombin-induced ERK2 activation is activated by conventional protein kinase Cs independently of Raf-1 and B-Raf activation.64 External stimuli, including lipopolysaccharide,65 P-selectin ligands,66 and thrombin,63,64 activate kinase pathway signaling, processes possibly resulting in “platelet education” that may lead to transformation of naive platelets into protumorigenic platelets.67 More explicit, Kuznetsov and colleagues found that platelets that absorb tumor-derived cytokines aid vessel formation at tumor sites.67
Various stimuli can alter platelet signaling and induce protein synthesis. The mechanism behind such observations was investigated by Denis et al in 2005. They showed that platelets possess a functional spliceosome including small nuclear RNAs, splicing proteins, and endogenous pre-mRNAs. The spliceosome can be induced to cause pre-mRNA splicing in response to stimuli.68 Schwertz et al reported that splicing of intron-rich tissue factor pre-mRNA into mature mRNA in healthy donors is controlled by Clk1.69 The involvement of RNA-binding proteins in the splicing mechanism of platelets was suggested as well.5,12 Platelets contain many different RNA species,70-73 including microRNAs72,74,75 and circular RNAs.76 It was shown that microRNA modulation induces proteome reorganization as well.77 A study combining proteomics and transcriptomics of platelets identified ∼8000 transcripts of which a set would undergo intron removal during platelet activation, concluding that maturation of specific pre-mRNAs contributes to the dynamic fine-tuning of the transcriptome.78 Thus, platelets arise as anucleate cytoplasmic fragments from megakaryocytes and retain megakaryocyte-derived cytoplasmic pre-mRNA, of which at least some are spliced into mature mRNAs and translated into proteins in response to external stimuli.79,80 Hence, tumor cells can directly stimulate platelet protein synthesis and platelet signaling. However, the protein content of platelets can consist of megakaryocyte-derived proteins, endocytosed proteins, and proteins translated in individual platelets. Therefore, it is important to determine which proteins are truly tumor derived and which proteins may fluctuate due to tumor-independent processes. Besides education via direct platelet–tumor cell interaction, tumor-associated biomolecules such as proteins35,81 and RNA9,10,82-84 can be sequestered by platelets. Examples of directly transferred transcripts include the cancer RNA biomarkers EGFRvIII,9,83 PCA3,9 EML4-ALK,82 KRAS, EGFR, and PIK3CA mutants,10 FOLH1, KLK2, KLK3, and NPY.84 Similar results have been reported for endothelial RNA transcripts taken up by platelets.85 The combination of specific splice events in response to external signals and the capacity of platelets to directly ingest (spliced) circulating mRNA provides TEPs with a highly dynamic transcriptome, with potential applicability as liquid biopsies for cancer diagnostics.9
Interestingly, platelets can respond to external stimuli by releasing RNA signaling complexes to other cells86,87 via microparticles, suggesting an intricate communicational crosstalk network. The role of platelet-derived microparticles (PMPs) in cancer development and progression has been previously reviewed.87 PMPs are the most abundant vesicle population in peripheral blood, accounting for ∼70% to 90% of all extracellular vesicles. It was found that PMPs are formed after platelet activation and are involved in angiogenesis, metastasis and multidrug resistance.87 Conversely, tumor-derived vesicles have the capability to sequester in platelets proteins and nucleic acids, possibly also including circulating free DNA, which seems to be inherent to platelets during their entire life span.5,9,88 Further research is needed to determine if platelets have a selection mechanism for specific vesicle sizes and types, and which internalization mechanisms are used.
Calverley et al and Nilsson et al demonstrated in 2010 and 2011, respectively, that the RNA profiles of platelets of cancer patients are altered as compared with healthy donors.9,89 In 2015, Best et al performed extensive RNA sequencing to determine differentially spliced RNA profiles in platelets from cancer patients (comprising 6 different types of cancer) and healthy individuals.10 This resulted in a predictive “pan-cancer” test with 96% accuracy.10 In addition, in a multiclass test across the 6 different tumor types (non-small cell lung carcinoma [NSCLC], colorectal cancer, glioblastoma, pancreatic cancer, hepatobiliary cancer, and breast cancer), the location of the primary tumor was correctly identified with 71% accuracy, indicating significant tumor-type-specific splicing within platelets. Detecting cancer using spliced TEP profiles was confirmed in a large follow-up study on platelets of NSCLC patients,12 and confirmed by others.90-92 Various individual spliced transcripts were detected in a validation study of NSCLC patients; the most significantly increased transcripts were CFL1, ACOT7, and ARPC1B, whereas DDX5, RPS5, and EEF1B2 were decreased, independent of age, smoking status, and blood storage, as well as various inflammatory conditions.12 Although no direct experiments were performed, it is likely that the spliced RNA detected in platelets from NSCLC patients is derived mostly from megakaryocytes and spliced in response to tumor-associated signals rather than tumor derived and ingested by the platelets. In addition, gene ontology analysis demonstrated that the spliced TEP transcripts correlated to translation, RNA-binding proteins, and intraplatelet signaling in low transcript splicing–levels NSCLC samples and interplatelet signaling and immune response in high transcript splicing–level samples. Additional clustering analyses revealed correlations between specific immune signaling pathways in TEPs of NSCLC patients and platelet homeostasis in platelets of individuals not diagnosed with cancer.12 Although further research is warranted, the mechanisms by which altered spliced RNA profiles may arise in TEPs include tumor-induced alteration of RNA transferred by megakaryocytes into platelets,34 signal-dependent platelet RNA splicing,68,69,78 exon skipping and alternative splicing, differential RNA-binding protein activity,12 and altered platelet aging/turnover.5,12
Platelet activation is associated with the initiation of coagulation cascades. During this process, platelets change shape and release the content of their granules, leading to platelet aggregation.93 A study comparing platelets from patients with ovarian cancer and those with benign ovarian tumors described that platelets are not hyperactive in patients with ovarian cancer.94 Cho and colleagues found that platelets increase the proliferation rate of ovarian cancer. Of note, they observed that direct contact between platelets and cancer cells or an intact structure of platelets was not required for the proliferative response. Moreover, blocking platelet adhesive surface proteins (GPIa, GPIIbIIIa, and P-selectin) did not diminish the proliferative effect of platelets. Aspirin only partially inhibited the proliferative effect of platelets on ovarian cancer cells.95 Although platelet activation can be induced by tumor cells, Best et al showed the absence of significant platelet activation during blood collection and storage of the analyzed platelet samples.12 Therefore, it is unlikely that the observed TEP RNA profiles are entirely driven by platelet activation. However, further exploration of the effects of both platelet activation and anticoagulation on TEP profiles is desired. Studies aiming at improving the power of TEP classifiers could include comparisons of changes in spliced RNA profiles in procoagulant states vs cancer-derived platelet activation, which would allow us to understand changes in platelet function induced by cancer vs other prothrombotic events. Clearly, further work is needed to validate these findings and evaluate potential events that generate noise or affect the diagnostic detection levels. Recently, a standardized protocol for thromboSeq, as well as TEP classifier development software, has been made available13 to further evaluate the diagnostic power of platelets. Studies comparing and combining different techniques are required to evaluate the (dis)advantages of individual biosources and the potential of combining them into a next-generation multianalyte liquid biopsy test.
Progress beyond the state of the art
The development and implementation of liquid biopsies in clinical settings requires a strong interdisciplinary effort, with a wide range of scientific competencies. Key will be to identify and select the best TEP biomarkers using the most sensitive techniques, including ultra-deep, massive parallel, and long-read sequencing of TEP transcripts (perhaps also including detection of epigenetic and epitranscriptomic features). Combining liquid biopsy sources for the detection and localization of cancer has proven to be effective.96 TEPs may offer certain advantages over other blood-based biosources, including their abundance and easy isolation, high-quality RNA, and capacity to process RNA in response to external signals.5,11,30 Combinatorial analysis of TEPs with complementary biosources such as extracellular vesicles, circulating tumor DNA, and CTCs, but possibly also imaging and protein markers, warrants consideration as next-generation biomarker troves, thereby seeking optimal diagnostic synergy.
Acknowledgments
Financial support was provided by the European Research Council (grants H2020-MSCA 765492, 713727, and 336540), the Netherlands Organisation for Scientific Research (grant 91711366), and the Dutch Cancer Society (grant VU2015-8080).
Authorship
Contribution: S.G.J.G.I.t.V. and T.W. wrote the manuscript.
Conflict-of-interest disclosure: T.W. received funding from Illumina Inc. and is a shareholder of GRAIL Inc. S.G.J.G.I.t.V. declares no competing financial interests.
Correspondence: Thomas Wurdinger, Amsterdam UMC, VU University Medical Center, De Boelelaan 1118, 1081 HV Amsterdam, The Netherlands; e-mail: t.wurdinger@vumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal