TO THE EDITOR:
Histiocytic neoplasms are rare hematological disorders that have diverse clinical manifestations and can pose significant management challenges for clinicians. Previously considered inflammatory, several of these disorders are now included in the World Health Organization classification of hematopoietic and lymphoid tumors.1 This was due, in part, to the discovery of recurrent MAP-ERK pathway alterations in Erdheim-Chester disease (ECD) and Langerhans cell histiocytosis (LCH).2,3 Recently, such mutations were also found to be present in one third of patients with Rosai-Dorfman disease (RDD).4 This suggests that at least a subset of these disorders is neoplastic in nature. The prognosis of histiocytic neoplasms is variable, and the natural course may be relatively benign and self-limiting in some cases, whereas in others it may be much more aggressive and life-threatening.5-7
Most adult patients with histiocytic neoplasms receive empiric systemic immunosuppressive or cytotoxic therapies because of the lack of approved treatments. Recently, vemurafenib was approved by the US Food and Drug Administration for the treatment of BRAF-V600–mutant ECD.8 However, the therapeutic options for non-BRAF-V600–mutant ECD and other histiocytic neoplasms are limited. Over the last decade, immune checkpoint inhibitors, such as programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1) inhibitors have shown significant improvement in outcomes among several hematological and solid organ malignancies.9-12 This has led to their accelerated approvals by the US Food and Drug Administration for these indications.
To identify appropriate treatment candidates for checkpoint inhibitor therapy, ongoing research has focused on identifying predictive biomarkers. PD-L1 immunohistochemistry (IHC) and microsatellite instability (MSI) status are used as complementary or companion diagnostics for immunotherapy prescription for some cancers. High levels of PD-1/PD-L1 expression and MSI are correlated with response to therapy.13,14 However, these biomarkers are not necessary to predict a benefit from immunotherapy. One promising new biomarker is tumor mutational burden (TMB), defined as the number of mutations per coding region within a tumor genome. Previous studies have demonstrated a strong and positive linear correlation between higher TMB and response to checkpoint inhibitors, as well as a lack of response to checkpoint inhibitors with a TMB < 5 mutations per megabase.15,16 The median TMB for tumors almost uniformly responsive to checkpoint inhibitor blockade, such as melanoma and microsatellite unstable colorectal cancer, is ∼15 and 45 mutations per megabase, respectively.16 To obtain additional insight into potential responsiveness of histiocytic neoplasms to immune checkpoint inhibitors, we performed genomic analyses on relevant tumor tissue samples. The objective of this study was to report the TMB, MSI status, and PD-L1 gene expression in histiocytic neoplasms.
All patients with histiocytic neoplasms diagnosed between 1 January 2017 and 30 June 2018 provided informed consent to participate in a prospective institutional protocol focused on tissue genotyping by next-generation sequencing (NGS). The Tempus xO Assay (performed at Tempus Labs) consists of a 1714–gene-targeted somatic and germline DNA sequencing panel and whole-transcriptome RNA sequencing to detect germline and somatic single-nucleotide polymorphisms, insertions/deletions, copy number alterations, and gene rearrangements causing chimeric messenger RNA transcript expression in a wide array of tumor types.17 The assay used formalin-fixed paraffin-embedded tumor samples and matched blood or saliva samples. Samples with low tumor percentage were macrodissected prior to RNA extraction. There was no cell separation or enrichment by antigen selection during sample preparation. TMB was calculated and reported as the number of nonsynonymous mutations per million base pairs or mutations per megabase. MSI was evaluated using a panel of >200 microsatellite loci covered on the Tempus xO assay and required a matched normal sample. PD-L1 gene expression was assessed using RNA sequencing. The percentile of the RNA expression value for each patient compared with a reference cohort of PD-L1+ IHC patients sequenced at Tempus Labs was determined using the log10-normalized count data generated by the bioinformatics RNA pipeline. The tumor cellularity threshold for clinical reporting of MSI status was 30%; however, for research purposes, these metrics were calculated for tumors with cellularity below the threshold.
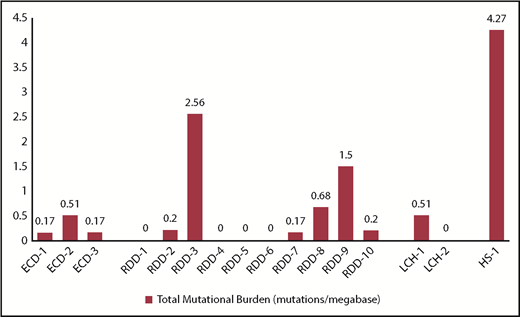
Sixteen patients with histiocytic neoplasms were included. The distribution of individual histiocytic neoplasms was as follows: RDD (n = 10), ECD (n = 3), LCH (n = 2), and histiocytic sarcoma (HS; n = 1). The median TMB for RDD and ECD patients was 0.17 and 0.19 mutations per megabase, respectively. The TMB for 2 LCH patients was 0.51 and 0 mutations per megabase. For the patient with HS, the TMB level was 4.27 mutations per megabase (Figure 1). Fifty percent (8/16) of patients expressed PD-L1 RNA at a level >20% of patients in the PD-L1+ IHC patient reference cohort. The RDD patients had variable RNA expression of PD-L1, but they also had the 4 highest expressing patients in the cohort. PD-L1 IHC results were available for 2 RDD patients (RDD-3 and RDD-9), which were 0% and 30%, respectively. The LCH patients had intermediate expression in this cohort, whereas the ECD and HS patients had the lowest expression (Table 1). For patients in whom evaluation of MSI status was feasible (n = 14), the MSI status was predicted as microsatellite stable.
Predictive immunotherapy biomarkers among patients with histiocytic neoplasms
| Patient ID . | Organ involvement . | Oncogenic mutations . | PD-L1 percentile (vs PD-L1+ cohort) . | MSI status (predicted) . | Treatment and outcomes* . |
|---|---|---|---|---|---|
| ECD-1 | Pituitary, bone, skin | BRAF p.V600E | 14 | MSS† | Pituitary hormone replacement (SD) |
| ECD-2 | Subcutis, bone | ND | 14 | MSS | Cladribine (PR), hydroxyurea (PR) |
| ECD-3 | CNS, orbit | BRAF p.V600E; PTCH1 copy loss | 15 | MSS† | Vemurafenib (CR) |
| RDD-1 | Lymph nodes | PTEN copy loss | 11 | MSS† | Prednisone (PR), cladribine (PR) |
| RDD-2 | Bone, lymph nodes, skin | ND | 41 | MSS† | Prednisone (PR), methotrexate (PR) |
| RDD-3 | Orbit, bone, skin | SMARCA4 p.K953 frameshift loss | 10 | UND‡ | Cladribine (PD), etoposide and vinblastine (CR), hydroxyurea (NR), radiation (PR), clofarabine (PR) |
| RDD-4 | Lymph nodes | ND | 43 | MSS | Observation (SD) |
| RDD-5 | Subcutis, bone, lymph node | ND | 43 | MSS | Observation (SD) |
| RDD-6 | Subcutis | ND | 24 | MSS | Surgical excision (PD) |
| RDD-7 | Subcutis, soft tissue | ND | 43 | MSS† | Observation (SD) |
| RDD-8 | Testis | MAP2K1 p.F53L | 26 | MSS | Observation (PD) |
| RDD-9 | Testis | ND | 13 | UND‡ | Prednisone (PR), cladribine (CR) |
| RDD-10 | Subcutis | KRAS p.K117N | 1 | MSS† | Surgical excision (SD) |
| LCH-1 | Bone, skin | BRAF p.V600E | 21 | MSS† | Surgical excision (CR), cryoablation (CR), surgical excision (CR) |
| LCH-2 | Lymph nodes | ND | 26 | MSS† | Cladribine (CR) |
| HS-1 | Nasal cavity | KRAS p.Q61R; PTCH1 p.Q118 stop gain | 5 | MSS† | Surgical excision, adjuvant radiation (CR) |
| Patient ID . | Organ involvement . | Oncogenic mutations . | PD-L1 percentile (vs PD-L1+ cohort) . | MSI status (predicted) . | Treatment and outcomes* . |
|---|---|---|---|---|---|
| ECD-1 | Pituitary, bone, skin | BRAF p.V600E | 14 | MSS† | Pituitary hormone replacement (SD) |
| ECD-2 | Subcutis, bone | ND | 14 | MSS | Cladribine (PR), hydroxyurea (PR) |
| ECD-3 | CNS, orbit | BRAF p.V600E; PTCH1 copy loss | 15 | MSS† | Vemurafenib (CR) |
| RDD-1 | Lymph nodes | PTEN copy loss | 11 | MSS† | Prednisone (PR), cladribine (PR) |
| RDD-2 | Bone, lymph nodes, skin | ND | 41 | MSS† | Prednisone (PR), methotrexate (PR) |
| RDD-3 | Orbit, bone, skin | SMARCA4 p.K953 frameshift loss | 10 | UND‡ | Cladribine (PD), etoposide and vinblastine (CR), hydroxyurea (NR), radiation (PR), clofarabine (PR) |
| RDD-4 | Lymph nodes | ND | 43 | MSS | Observation (SD) |
| RDD-5 | Subcutis, bone, lymph node | ND | 43 | MSS | Observation (SD) |
| RDD-6 | Subcutis | ND | 24 | MSS | Surgical excision (PD) |
| RDD-7 | Subcutis, soft tissue | ND | 43 | MSS† | Observation (SD) |
| RDD-8 | Testis | MAP2K1 p.F53L | 26 | MSS | Observation (PD) |
| RDD-9 | Testis | ND | 13 | UND‡ | Prednisone (PR), cladribine (CR) |
| RDD-10 | Subcutis | KRAS p.K117N | 1 | MSS† | Surgical excision (SD) |
| LCH-1 | Bone, skin | BRAF p.V600E | 21 | MSS† | Surgical excision (CR), cryoablation (CR), surgical excision (CR) |
| LCH-2 | Lymph nodes | ND | 26 | MSS† | Cladribine (CR) |
| HS-1 | Nasal cavity | KRAS p.Q61R; PTCH1 p.Q118 stop gain | 5 | MSS† | Surgical excision, adjuvant radiation (CR) |
CNS, central nervous system; CR, complete remission; HS, histiocytic sarcoma; MSS, microsatellite stable; ND, none detected; NR, no response; PD, progressive disease; PR, partial remission; SD, stable disease.
Based on clinical and fluorodeoxyglucose positron emission tomography–computed tomography criteria.
MSI status reported below clinical reporting threshold of 30% tumor content.
Undetermined (UND) because of the lack of a matched-normal sample.
In addition to the immunotherapy biomarkers, NGS on tumor DNA was able to identify a number of clinically actionable mutations, including the presence of BRAF-V600E in the tumor tissue of 2 of 3 ECD patients and 1 of 2 LCH patients. Pathogenic mutations were also detected in 4 of 10 RDD patients (PTEN, SMARCA4, KRAS, and MAP2K1) and in the HS patient (KRAS and PTCH1). Table 1 includes disease characteristics, molecular findings, treatments, and responses based on previously published criteria.18
In this limited cohort of histiocytic neoplasms, the TMB was low compared with cancers that have been shown to respond to immune checkpoint blockade. Among the cohort, the TMB was highest for the patient with HS, suggesting that this disease subgroup may benefit from further exploration of TMB and consideration of anti–PD-1/PD-L1 therapy. This is corroborated by a recent report of radiographic response to nivolumab, an anti–PD-1 antibody, in a patient with HS refractory to systemic chemotherapy.19 No patient was found to have MSI-high status or alterations in DNA mismatch-repair genes, suggesting that these neoplasms are typically microsatellite stable. It is notable that the majority of the patients in our study had RDD, and only a proportion of these have been found to demonstrate clonal neoplasia in previous studies, which may explain the low TMB and microsatellite stability. PD-L1 RNA expression was variable among the cohort and did not show a correlation with IHC assay in the 2 RDD specimens for which both results were available. These results should be confirmed by a clinical PD-L1 IHC test, because the RNA-based assay is unable to differentiate between PD-L1 RNA expression in tumor cells and adjacent cells.14 One study using PD-L1 IHC in histiocytic neoplasms found that 3 of 4 ECD and 7 of 8 LCH tumor specimens were positive for PD-L1 expression (≥5%).20
Our study is the first to explore TMB and MSI status in histiocytic neoplasms. In the current era of trials of checkpoint inhibitors in almost every malignancy, our data on TMB and MSI status suggest that these neoplasms may be less likely to respond to such agents. An exploration of all established immunotherapy biomarkers in a larger cohort of patients may be warranted prior to considering checkpoint inhibitor therapy in histiocytic neoplasms.
Presented in part at the 60th annual meeting of the American Society of Hematology, San Diego, CA, 1 December 2018, and at the Annual Erdheim Chester disease medical symposium, Orlando, FL, 15 November 2018.
The online version of this article contains a data supplement
Acknowledgments
This work was supported by the Mayo Clinic Cancer Center (grant P30 CA015083 from the National Institutes of Health, National Cancer Institute) and the Center for Individualized Medicine.
The contents are solely the responsibility of the authors and do not necessarily represent the official view of the National Institutes of Health.
Authorship
Contribution: G.G. collected clinical data and wrote the manuscript; D.L. and A.M.N. analyzed genomic and molecular data; R.V., K.L.R., J.H.R., C.J.D.-P., W.O.T., M.J.K., N.N.B., M.V.S., M.C.L., and R.S.G. critically revised the manuscript for important intellectual content; R.S.G. supervised the study; and all authors were involved in drafting the manuscript and approved the final version.
Conflict-of-interest disclosure: A.M.N. and D.L. are employees of Tempus Labs. R.V. has received research funding from Pfizer, Bristol-Myers-Squibb, and Sun Pharma. W.O.T. has received research funding from Mallinckrodt. The remaining authors declare no competing financial interests.
A complete list of the members of the Mayo Clinic Histiocytosis Working Group appears in the supplemental appendix, available on the Blood Web site.
Correspondence: Ronald S Go, Division of Hematology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: go.ronald@mayo.edu; and Minetta C. Liu, Department of Medical Oncology, Mayo Clinic, 200 1st St SW, Rochester, MN 55905; e-mail: liu.minetta@mayo.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal