Key Points
Type O platelets translocate farther over VWF at higher speeds compared with non-O platelets.
Binding kinetics between type O platelets and VWF are 40% lower than non-O platelets.
Abstract
Blood type O is associated with a lower risk of myocardial infarction. Platelets play a critical role in myocardial infarction. It is not known whether the expression of blood group antigens on platelet proteins alters platelet function; we hypothesized that platelet function would be different between donors with blood type O and those with non-O. To address this hypothesis, we perfused blood from healthy type O donors (n = 33) or non-O donors (n = 54) over pooled plasma derived von Willebrand factor (VWF) protein and purified blood type–specific VWF at arterial shear and measured platelet translocation dynamics. We demonstrate for the first time that type O platelets travel farther at greater speeds before forming stable bonds with VWF. To further characterize these findings, we used a novel analytical model of platelet interaction. Modeling revealed that the kinetics for GPIb/VWF binding rate are significantly lower for type O compared with non-O platelets. Our results demonstrate that platelets from type O donors interact less with VWF at arterial shear than non-O platelets. Our results suggest a potential mechanism for the reduced risk of myocardial infarction associated with blood type O.
Introduction
Platelet thrombus formation at a site of arterial vascular injury is a multistep process. When the vascular endothelium is damaged, exposed collagen captures von Willebrand factor (VWF) from flowing blood. VWF then undergoes a conformational change, enabling circulating platelets to tether to previously cryptic binding sites. In the arterial circulation, platelets bind to the A1 domain of anchored VWF through the platelet glycoprotein Ibα (GPIbα) receptor. This initial transient tethering of platelets to VWF slows platelets down as they translocate along bound VWF. The next step in this dynamic process is irreversible platelet adhesion mediated by the activated integrin GPIIbIIIa binding to the VWF C4 domain.
Models of platelet behavior have shown that adhesion rate is determined by the interplay between receptor kinetics and hydrodynamic effects of shear flow.1,2 GPIb is a mechanoreceptor; binding to uncoiled VWF is triggered in response to the mechanical stresses induced by shear flow. Binding kinetics are biphasic, with force deceleration inducing catch bonds and acceleration promoting dissociation or slipping apart of the bonds.3,4 Zhang et al5 recently identified a mechanosensitive domain (MSD) on GPIb that lies between the highly O-glycosylated macroglycopeptide region and the transmembrane domain. The unstable MSD is highly sensitive to tensile or pulling forces. The authors determined that the MSD unfolded at forces ranging from 5 to 20 pN, similar to those shown to induce catch bond formation when the VWF-A1 complexes with GPIb.
The macroglycopeptide region of GPIbα is located between the ligand-binding leucine-rich repeat region (AA1-282) and the recently documented MSD6,7 (AA417-483). It is an ∼90-kDa serine- and threonine-rich region containing ∼40 O-glycosylation sites.7 It is known that some of the glycosylated side chains of GPIbα carry the ABO(H) blood group antigens8 ; however, only 7 O-glycosites have been definitively identified thus far on GPIb,9 and the potential functional effects of variable glycosylation are not known. The core H antigen that determines blood type O is composed of a single chain. The addition of a single extra saccharide results in different side chains projecting from the core structure, with blood type A determined by the presence of N-acetylgalactoseamine and type B by the addition of a galactose to the terminus.10
Glycosylation is known to confer structural integrity to proteins.11 There is a strong association between ABO blood group and risk of thrombosis or bleeding.12 Blood group non-O (A, B, and AB) individuals have an increased incidence of arterial and venous thrombotic disease, compared with group O individuals.12 Two recent large prospective cohort studies and a meta-analysis of prospective studies have demonstrated that ABO blood group is associated with probability of coronary heart disease and myocardial infarction (MI) independent of other risk factors.13 Despite the association of blood group with cardiovascular disease,14,15 the mechanisms linking ABO blood type and disease have remained unclear.
Because platelets have a critical role in MI, we asked whether platelet function is different in donors with different blood groups. Thrombosis in the coronary circulation is mediated by platelets interacting with VWF under conditions of arterial shear; therefore, we used in vitro and in silico methods to measure platelet translocation and adhesion on VWF under arterial shear conditions. Because initiation of thrombosis in the arterial circulation is mediated primarily through the platelet GPIb and GPIIbIIIa receptors, we sought to characterize the dynamic interaction of platelets in flowing blood on VWF using a well-characterized assay of platelet function that mimics arterial shear conditions.16-18 This assay, which we have termed the dynamic platelet function assay (DPFA), measures the motional parameters of individually tracked platelets as they tether, translocate, and adhere to VWF.
Results of our in vitro experiments showed that platelets from donors with blood type O translocated more rapidly across immobilized VWF and traveled greater distances before arresting on the surface. Because it is not possible to selectively strip the glycans from GPIb while maintaining the integrity of the platelets, to study the effect of ABO(H) glycosylation on receptor function, we used a computational model to measure the kinetics of the platelet interaction with VWF. GPIb is a mechanoreceptor; therefore, we postulated that changes in the structure of the macroglycopeptide side chain relating to the presence of the different blood groups could change the kinetics of platelet GPIb binding to VWF. The results of our in silico assays confirm a role for blood group antigens in modulating platelet function.
Materials and methods
Ethical approval for the study was obtained from the Research Ethics Committee of the Royal College of Surgeons in Ireland. All participants were informed of the nature of the study, and written consent was obtained from all donors before recruitment. All blood samples were collected in accordance with the Declaration of Helsinki.
Sample preparation
Blood was collected from healthy volunteers who had not taken any medication known to affect platelet function within the previous 14 days. All donors gave informed consent. Blood was drawn from the antecubital vein through a 19-gauge Sarstedt Safety Needle into an S-Monovette 9NC blood collection tube (Sarstedt, Wexford, Ireland) containing the anticoagulant solution trisodium citrate dihydrate (final concentration by weight, 0.32%; citrate/blood ratio, 1:9). Donor blood cell counts (Sysmex KX21N; Sysmex, Kobe, Japan) were routinely recorded.
Blood typing
Blood typing was carried out using the clinically validated Ortho BioVue system19,20 (Ortho Clinical Diagnostics, Raritan, NJ). The procedure is based on the principle of agglutination. The Ortho BioVue System uses column agglutination technology, composed of glass beads and reagent contained in a column. Whole blood was centrifuged at 950g for 5 minutes, and the platelet-poor plasma (PPP) was removed to a fresh tube; 10 μL of packed red cells was mixed with 1 mL of phosphate-buffered saline (PBS), and 50 μL was added to each column of the BioVue cassette. The cassette was spun in the specially designed centrifuge for 5 minutes. Each cassette has 6 columns: anti-A, anti-B, and anti-A and -B combined columns, 2 anti-Rh D columns, and a single control column. For reverse typing, 40 μL of PPP was mixed with 10 μL of A or B Affirmagen and spun in a reverse-typing cassette. Upon addition of red blood cells and subsequent centrifugation of the cassette, agglutinated red blood cells are trapped by the glass beads, and nonagglutinated red blood cells travel to the bottom of the column.
Measuring VWF antigen levels
Plasma VWF levels were measured using a modification of an enzyme-linked immunosorbent assay from USBiological. Briefly, 96-well microtiter plates were coated overnight at 4°C with a 1:200 dilution of goat anti-human VWF capture antibody. Plates were blocked for 1 hour with 1% bovine serum albumin in PBS and then washed 3 times with PBS/0.1% Tween 20 (PBST). PPP was diluted 1:100 in HEPES/Tween/BSA buffer, and 100 μL was added to each well and incubated at room temperature for 1.5 hours. All samples were run in duplicate. Wells were washed 3 times with PBST, and 100 μL of goat anti-human horseradish peroxidase-labeled VWF detection antibody (1:200 dilution) was added to each well and incubated for 1.5 hours. Wells were washed with PBST 3 times, and 100 μL of freshly prepared O-phenylenediamine (Sigma, Wicklow, Ireland) was added to each well. The color was allowed to develop for 10 minutes, and the reaction was stopped by the addition of 50 μL of 2.5M H2SO4. Absorbance was read at 490 nm on a Wallac VictorII spectrophotometer (PerkinElmer, Dublin, Ireland). Plasma VWF levels were determined by extrapolating from the standard curve (100 μg/mL – 1.56 μg/mL).
DPFA
Whole-blood flow assays were performed using custom-designed parallel-plate flow chambers coated overnight at 4°C with 100 μg/mL of VWF (Haemate-P; CSL-Behring, Marburg, Germany). Parallel-plate flow chambers were mounted on an inverted microscope (Zeiss Axiovert-200 epifluorescence). Whole blood was fluorescently labeled with 1 μM of DiOC6, drawn through biocompatible platinum-cured silicone tubing (1/16 in internal diameter; Nalgene; Thermo Fisher Scientific, Rochester, New York), and perfused through the VWF-coated flow chamber using a NEMESYS syringe pump (Cetoni GmbH, Korbussen, Germany). A flow rate (Q) of 75 μL/min was used corresponding to an arterial shear rate (γ) of 1500 s−1 and a shear stress (τ) of 6 Pa (6 N/m2) at the wall of the surface where the platelets were interacting. Images were captured using an cooled (−80°C) digital EM-CCD camera (iXON EM+; Andor Technology, Belfast, Ireland) connected to MetaMorph software (version 7.7; Molecular Devices Ltd, Wokingham, United Kingdom) and illuminated with an Osram 103-W mercury light source and a fluorescein isothiocyanate (FITC) filter set providing excitation and emission at 480 to 500 nm and 508 to 548 nm, respectively (Chroma Technology Corp, Bellows Falls, VT). Images were acquired at 30 frames per second for 500 frames using a 63× objective (512 × 512 pixels; 128.8 × 128.8 μm) field of view for visualization of platelet interactions with VWF in real time.
Type-specific VWF was purified from fresh frozen plasma obtained from known blood type O (VWF_O) and type A (VWF_A) donors as previously described.21 VWF_A and VWF_O were then used to coat the parallel-plate flow chambers. Whole blood from paired type A and type O donors was perfused across VWF_A- and VWF_O-coated chambers.
A number of modifications were made to our previously described algorithm18 to enhance the detection of platelets; images were analyzed as follows: a combination of wavelet filtering and template matching was used to identify single platelets in each frame, and their position was refined by their intensity-weighted centers of mass. Platelets were tracked from frame to frame using the single-particle tracking software uTrack.22 The performance of our modified detection and tracking software was benchmarked against simulated ground-truth platelet motion video sequences, generating a Jaccard index (number of correct tracks divided by all detected and missed tracks), typically >85%. Thus, this assay accurately measures platelet motional behavior. From the resultant position vs time tracks, the following parameters were generated18 : the number of platelets that interact with VWF (platelet tracks); the number of platelets that adhere stably to the surface (static platelets, defined as platelets that move <3 µm); the number of platelets that translocate on the surface (platelet translocation); the distance traveled by each platelet in micrometers (mean translocation distance); and the velocity of platelet movement across VWF as micrometers per second (mean translocation velocity).
Receptor counts
Surface expression of platelet glycoprotein receptors was measured by flow cytometry using the Biocytex Gp Screen kit (Stago, Marsailles, France) according to the manufacturer’s instructions.
Statistical analysis
All statistics were produced using Graphpad Prism for Mac (version 6). Mann-Whitney t tests were used for analysis of nonparametric data produced by the DPFA.
Results
Blood group alters platelet interaction with VWF under conditions of arterial shear: modeling binding and translocation kinetics
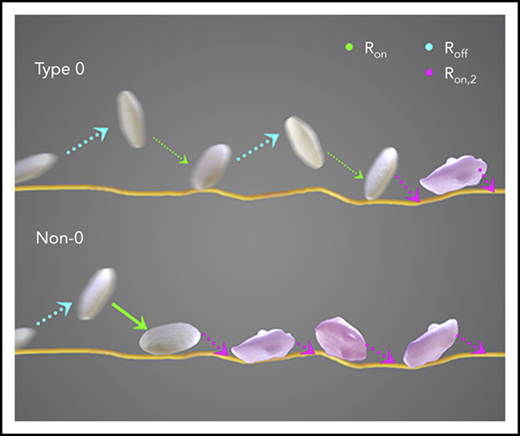
To determine the effect of blood group on platelet function under conditions of arterial shear, we used our well-characterized DPFA that mimics conditions of arterial blood flow ex vivo and accurately measures platelet behavior.18,23-25 Blood from a total of 87 healthy volunteers was assayed (33 with type O and 54 with a non-O blood group). Video microscopy was used to determine the sequential stages of platelet interaction with VWF (ie, the initial GPIb-determined tethering and slowing translocation of the platelets before firm adhesion through the engagement of GPIIbIIIa).
The results of this study demonstrate that the translocation velocity of type O platelets was significantly greater than that of non-O platelets (Table 1). The fraction of platelets that adhered stably to the surface was lower for the type O cohort (Table 1).
Blood group modulates platelet translocating behavior on VWF
| . | Mean ± SD . | P . | |
|---|---|---|---|
| Type O (n = 33) . | Non-O (n = 54) . | ||
| Platelet tracks | 273 ± 130 | 272 ± 95 | .62 |
| Proportion of all platelets stably adhered to VWF surface | 0.55 ± 0.1 | 0.63 ± 0.1 | .009 |
| Mean translocation distance, µm | 5.77 ± 1.8 | 4.74 ± 1.4 | .037 |
| Mean translocating velocity, µm/s | 4.6 ± 2.1 | 3.4 ± 1.7 | .002 |
| Rate of platelet adhesion, 1/s (Ron) | 0.11 ± 0.06 | 0.16 ± 0.05 | .011 |
| Rate of platelet detachment, 1/s (Roff) | 0.06 ± 0.03 | 0.08 ± 0.04 | .39 |
| Platelet count, ×109/L | 217 ± 57 | 215 ± 55 | .72 |
| Haematocrit, % | 39.9 ± 4.2 | 38.5 ± 3.9 | .19 |
| Male sex, % | 55 | 39 | .16 |
| CD41 (n = 20 non-O; n = 20 type O) | 62 600 ± 13 600 | 61 600 ± 11 900 | .81 |
| CD42b (n = 20 non-O; n = 20 type O) | 46 800 ± 6 800 | 44 300 ± 8 300 | .33 |
| VWF, IU/dL | 51.0 ± 32.3 | 62.1 ± 34.7 | .14 |
| . | Mean ± SD . | P . | |
|---|---|---|---|
| Type O (n = 33) . | Non-O (n = 54) . | ||
| Platelet tracks | 273 ± 130 | 272 ± 95 | .62 |
| Proportion of all platelets stably adhered to VWF surface | 0.55 ± 0.1 | 0.63 ± 0.1 | .009 |
| Mean translocation distance, µm | 5.77 ± 1.8 | 4.74 ± 1.4 | .037 |
| Mean translocating velocity, µm/s | 4.6 ± 2.1 | 3.4 ± 1.7 | .002 |
| Rate of platelet adhesion, 1/s (Ron) | 0.11 ± 0.06 | 0.16 ± 0.05 | .011 |
| Rate of platelet detachment, 1/s (Roff) | 0.06 ± 0.03 | 0.08 ± 0.04 | .39 |
| Platelet count, ×109/L | 217 ± 57 | 215 ± 55 | .72 |
| Haematocrit, % | 39.9 ± 4.2 | 38.5 ± 3.9 | .19 |
| Male sex, % | 55 | 39 | .16 |
| CD41 (n = 20 non-O; n = 20 type O) | 62 600 ± 13 600 | 61 600 ± 11 900 | .81 |
| CD42b (n = 20 non-O; n = 20 type O) | 46 800 ± 6 800 | 44 300 ± 8 300 | .33 |
| VWF, IU/dL | 51.0 ± 32.3 | 62.1 ± 34.7 | .14 |
Data were analyzed using Mann-Whitney U tests to account for their nonparametric distribution.
SD, standard deviation.
It was not clear initially whether these differences were due to the platelet or VWF associated with a given blood type. In matched experiments, whole blood from type A and type O donors was perfused across flow chamber surfaces coated with VWF_A or VWF_O (n = 7). Thus, type O platelets were perfused separately over purified A and O VWF, respectively. Similarly, A platelets were perfused separately over A and O VWF, respectively. There was no difference in the motional parameters of A platelets when perfused over VWF_A or O (Table 2). In contrast, platelets from type O donors showed clear differences in motional behavior on both A and O type VWF, in that they translocated for greater distances at higher velocity before arresting stably on the surface of VWF_O than they did on VWF_A (Table 3). We saw no significant differences in the translocation of A and O platelets over VWF_O (Table 4); this would indicate that the glycosylation pattern of both VWF and the platelet is important in modulating their interaction.
Translocation characteristics of platelets from type A donors are independent of VWF type
| Donor type VWF source . | Mean ± SD . | P . | |
|---|---|---|---|
| Type A VWF_O . | Type A VWF_A . | ||
| Platelet tracks | 98 ± 33 | 108 ± 12 | .57 |
| Proportion of all platelets stably adhered to VWF surface | 0.66 ± 0.1 | 0.63 ± 0.1 | .72 |
| Mean translocation distance, µm | 5.3 ± 0.9 | 5.1 ± 1.4 | .86 |
| Mean translocation velocity, µm | 4.6 ± 0.6 | 3.8 ± 1.5 | .49 |
| Donor type VWF source . | Mean ± SD . | P . | |
|---|---|---|---|
| Type A VWF_O . | Type A VWF_A . | ||
| Platelet tracks | 98 ± 33 | 108 ± 12 | .57 |
| Proportion of all platelets stably adhered to VWF surface | 0.66 ± 0.1 | 0.63 ± 0.1 | .72 |
| Mean translocation distance, µm | 5.3 ± 0.9 | 5.1 ± 1.4 | .86 |
| Mean translocation velocity, µm | 4.6 ± 0.6 | 3.8 ± 1.5 | .49 |
SD, standard deviation.
Type O platelets travel farther and stick less on type O VWF
| Donor type VWF source . | Mean ± SD . | P . | |
|---|---|---|---|
| Type O VWF_O . | Type O VWF_A . | ||
| Platelet tracks | 157 ± 106 | 198 ± 84 | .26 |
| Proportion of all platelets stably adhered to VWF surface | 0.67 ± 0.1 | 0.78 ± 0.1 | .05 |
| Mean translocation distance, µm | 5.6 ± 0.7 | 4.4 ± 0.5 | .007 |
| Mean translocation velocity, µm | 5.0 ± 1.6 | 2.8 ± 1.1 | .03 |
| Donor type VWF source . | Mean ± SD . | P . | |
|---|---|---|---|
| Type O VWF_O . | Type O VWF_A . | ||
| Platelet tracks | 157 ± 106 | 198 ± 84 | .26 |
| Proportion of all platelets stably adhered to VWF surface | 0.67 ± 0.1 | 0.78 ± 0.1 | .05 |
| Mean translocation distance, µm | 5.6 ± 0.7 | 4.4 ± 0.5 | .007 |
| Mean translocation velocity, µm | 5.0 ± 1.6 | 2.8 ± 1.1 | .03 |
SD, standard deviation.
VWF type does not influence platelet translocation behavior
| Donor type VWF source . | Mean ± SD . | P . | |
|---|---|---|---|
| Type O VWF_O . | Type A VWF_O . | ||
| Platelet tracks | 157 ± 106 | 98 ± 33 | .22 |
| Proportion of all platelets stably adhered to VWF surface | 0.67 ± 0.1 | 0.66 ± 0.1 | .91 |
| Mean translocation distance, µm | 5.6 ± 0.7 | 5.3 ± 0.9 | .59 |
| Mean translocating velocity, µm | 5.0 ± 1.6 | 4.6 ± 0.6 | .60 |
| Donor type VWF source . | Mean ± SD . | P . | |
|---|---|---|---|
| Type O VWF_O . | Type A VWF_O . | ||
| Platelet tracks | 157 ± 106 | 98 ± 33 | .22 |
| Proportion of all platelets stably adhered to VWF surface | 0.67 ± 0.1 | 0.66 ± 0.1 | .91 |
| Mean translocation distance, µm | 5.6 ± 0.7 | 5.3 ± 0.9 | .59 |
| Mean translocating velocity, µm | 5.0 ± 1.6 | 4.6 ± 0.6 | .60 |
SD, standard deviation.
These results demonstrated a blood group–dependent difference in platelet receptor function. To further investigate why these changes occurred, in silico modeling was used to determine the rate of platelet adhesion and/or detachment from the surface-bound VWF. The modeling process focused on the 2 critical receptor-ligand bonds that determine platelet adhesion to VWF. GPIb binding through catch/slip bonds is responsible for initial reversible platelet translocating behavior, followed by persistent, irreversible adhesion mediated by the binding of GPIIbIIIa to VWF.
Rate equations for reversible and irreversible reactions associated with platelet adhesion were derived in previous studies.2,26 On the basis of these studies, the following equations were are used to describe the instantaneous numbers of transiently adhered platelets (Nplatelet,1) and stably adhered platelets (Nplatelet,2) and the cumulative number of adhesion events (ie, number of tracks of adhered platelets [Ntrack]):
and
Our tracking algorithm measured Ntrack and Nplatelet. Total platelet count N0 was measured using a Sysmex KX21N Cell Counter. The equations shown above can therefore be used to fit Ntrack vs time (t) and Nplatelet vs t plots to calculate the rate of adhesion (Ron), the rate of detachment (Roff), and the rate of stable adhesion (Ron,2). Ntrack vs t plots show linear growth up to 500 frames, and thus, the process of platelets from within the flowing blood stream tethering and adhering to immobilized VWF can be approximated as a Poisson process with a constant rate Ron. In this model, the fast and transient nature of the GPIb-VWF interaction does not support stable adhesion and thus does not influence the GPIIbIIIa-determined Ron,2. We have noted that a small number of platelets can bypass transient adhesion, becoming stably adhered upon initial contact with the VWF surface. This is most likely due to platelets binding to VWF through integrin GPIIbIIIa that is already in a conformationally active state. These events are infrequent, ≤3% of the total number of platelet tracks when platelet motional parameters are assayed after initiating video recording.
Transient adhesion of platelets involves multiple GPIb-VWF bonds breaking and forming, resulting in platelet translocation. The rate of adhesion Ron is influenced by factors such as the number of GPIb receptors on the platelet surface that are available to bind to immobilized VWF. Therefore, the rate of adhesion depends not only on GPIb-VWF(A1) binding rate kon27 but also on GPIb receptor density on the platelet surface. Data generated by the DPFA demonstrate that the rate of platelet adhesion was significantly lower for type O blood compared with non-O (P = .01; Table 1). However, no correlation was observed between receptor count and blood type (Table 1), demonstrating that the number of GPIb receptors on the surface did not influence Ron in our cohorts (Pearson correlation r = −0.077; P = .719 for GPIb receptor count and blood group).
The rate of adhesion is also a function of near-wall platelet concentration, which differs from the overall platelet concentration (N0) because platelets are not uniformly distributed throughout the flowing blood stream. When N0 is fixed, near-wall platelet concentration depends on hematocrit.28 Hematocrit was in the normal range in our donors, with no significant difference between the 2 cohorts (P = .19; Table 1).
The results of this model therefore suggest that the observed differences in platelet translocating behavior between type O and non-O platelets is due to a fundamental change in GPIb-VWF binding kinetics (ie, kon). With all other factors simply amplifying the frequency of binding events, kon is linearly related to Ron as described in previous publications.2,28 Our data determined that kon is >40% lower for type O compared with non-O platelets.
The immobilized pooled VWF used to characterize the interaction of platelets with VWF was from a single lot number of Haemate P. Therefore, changes in the observed adhesion rates are due to differences in the donor’s platelet GPIb receptor and not due to variations in the VWF preparation.
Once tethered, platelets translocate on the VWF-coated surface while they are subject to flow shear. The inverse of Roff is the translocation lifetime (ie, the characteristic duration of translocation). Platelet translocation is recorded as a saltatory or stop-go motion where platelets move along the flow direction for brief periods of time. We therefore also calculated the translocation distance based on our image tracking algorithm. When flow conditions are fixed, changes in translocating behaviors are indicative of blood group–determined differences in GPIb receptors that influence the interaction with VWF. Type O platelets traveled significantly longer distances than non-O platelets (P = .037; Table 1), with a higher translocation velocity (P = .002; Table 1), before adhering stably to the immobilized surface.
Representative MP4 video files of type O and non-O platelets translocating across the VWF surface can be accessed in supplemental Data, available on the Blood Web site. Supplemental Video 1 is representative of platelets from a type O donor; supplemental Video 2 is a recording of platelets from a non-O donor translocating over immobilized VWF.
Autologous plasma VWF concentration does not influence platelet interactions with the pooled VWF surface
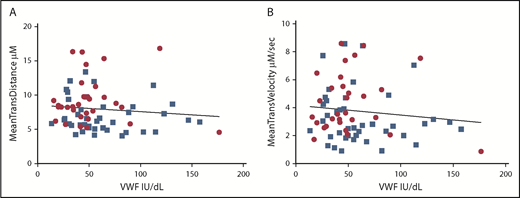
It is known that soluble and surface-bound VWF can self-associate, promoting platelet tethering and adhesion.29 It is also known that type O donors have an average of 25% less circulating plasma VWF than non-O donors.30,31 To exclude any confounding effect of donor plasma VWF levels affecting our results, VWF levels were determined by enzyme-linked immunosorbent assay for each donor (Figure 1A). The average plasma VWF concentration was 57.5 IU/dL, with a range of 13.5 to 176.5 IU/dL. Although VWF concentration was lower in the type O donors (mean, 51.0 IU/dL; standard deviation, 32 IU/dL) compared with the non-O cohort (mean, 62.1 IU/dL; standard deviation, 34.7 IU/dL), this difference was not statistically significant. Moreover, linear regression confirmed the absence of any link between plasma VWF levels and platelet translocation distance (r2 = 0.01) or translocation velocity (r2 = 0.01; Figure 1A-B, respectively). Our laboratory has also shown that spiking whole blood with a twofold excess of exogenous VWF has no effect on platelet translocation speeds or distances as measured by our assay, indicating that plasma VWF levels have no effect on this assay (data not shown).
Platelet interaction with the VWF coated–surface is independent of donor plasma VWF levels. There was no correlation between donor plasma VWF level and mean translocation distance (Y = −0.0009395*X + 8.529; linear regression model) (A) or mean translocation velocity (Y = −0.000699*X + 4.174; linear regression model) (B). Blue squares represent non-O donors; red circles represent type O donors.
Platelet interaction with the VWF coated–surface is independent of donor plasma VWF levels. There was no correlation between donor plasma VWF level and mean translocation distance (Y = −0.0009395*X + 8.529; linear regression model) (A) or mean translocation velocity (Y = −0.000699*X + 4.174; linear regression model) (B). Blue squares represent non-O donors; red circles represent type O donors.
Discussion
The results of our experiments demonstrate for the first time that ABO(H) blood type modulates platelet function. Platelets from donors with blood type O translocated across VWF for greater distances at higher velocities than non-O donor platelets in our DPFA. The proportion of platelets from type O donors that were able to form stable bonds with immobilized VWF over the duration of the assay was significantly reduced. When blood from donors was perfused over type-specific VWF, only differences in O blood on different types of VWF was noted. Thus, the A and O platelets must differ in some details of their interactions with the 2 types of VWF; our results suggest that this is most likely due to altered glycosylation patterns on the platelets’ GPIb receptors. It is not possible to selectively deglycosylate specific receptors on platelets in whole blood and maintain normal platelet function. Also, cells transfected with platelet surface receptors do not adequately mimic the physiological behavior of platelets in native whole blood under conditions of shear. Therefore, to further characterize our results, we developed a computational model to study the kinetics of platelet receptor binding to VWF.
Our modeling demonstrated that differences observed between type O and non-O donor platelets under physiological flow conditions arise from changes in the binding kinetics between GPIb and VWF. kon is >40% lower for O platelets. The dissociation kinetics of GPIIbIIIa/VWF interactions, measured as Roff, showed no difference between the groups. These results suggest that ABO(H) modification specifically alters platelet GPIb receptor function.
The computer model highlights how the increased distance and translocation velocity for type O platelets are driven by alterations in the binding kinetics with VWF. Our experiments on homogenous, type-specific VWF further refine this and point to blood group–dependent differences in platelet function. Differences in type O platelet translocation over VWF_A and VWF_O point toward the importance of the glycosylation pattern on VWF. However, because we did not observe similar differences when type A platelets were perfused over type-specific VWF, and we have noted differences in the dynamics of how non-O and type O platelets interact with pooled VWF, this indicates that there are also important differences in the glycosylation of the platelet VWF receptors. Taken together with the in silico results, our hypothesis is that the glycosylation of GPIb on type O platelets renders them sensitive to the different glycosylation patterns of VWF. GPIb on type A platelets is not sensitive to this difference. In the context of MI, the platelets of the patient will only be exposed to his or her blood type–specific VWF; therefore, it is the modification of GPIb that is key to the reduced risk of MI in type O individuals.
Previous work by Moshfegh et al32 showed that the deglycosylation of GPIbα gives rise to a 50% reduced response to ristocetin- and botrocetin-induced agglutination but has no effect on VWF binding in a static adhesion assay. The removal of the glycans had a significant structural effect on GPIb, reducing the length of the protein from 50 to 20 nm after O-deglycosylation. This is a clear indication that the glycosylated residues confer structural stability on the protein. They postulate that the reduction in length prevents the protein from binding to its VWF ligand. Deng et al6 recently proposed a trigger model to describe the activation of GPIb: ligand binding transmits a pulling force through the macroglycopeptide region, inducing unfolding of the MSD and culminating in GPIb signaling. Quach et al33 showed that it is not the site of ligand binding that is important for the unfolding of the GPIb MSD; rather, it is the pulling force exerted on the protein and the bound ligand under shear conditions. Our work highlights further the importance of the macroglycopeptide region of GPIb. Our results suggest that blood group–determined differences in the carbohydrate residues along the peptide chain alter platelet function and their interaction with VWF under conditions of arterial flow.
There have been many studies linking blood group with susceptibility to a variety of human diseases.34,35 The severity of Plasmodium falciparum malarial infection is reduced in type O individuals compared with non-O individuals,36 whereas the reverse is true for norovirus infections.37 In metastatic cancers, it has been noted that downregulated expression of A and B antigens is inversely proportional to tumor metastatic potential.38 Additional studies are required to determine if blood group–associated glycosylation of the platelet is implicated in these or other disease types.
There is a well-established link between blood group and risk of MI13 ; the mechanism, however, has yet to be determined. We have shown that type O platelets interact less with VWF under conditions of arterial shear, traveling farther and faster than non-O platelets before forming stable bonds. Our model-derived results provide evidence that differential glycosylation patterns on GPIb influence platelet binding kinetics during the initial tethering stages of thrombus development. Thus, platelets in donors with blood group O react less with VWF. Our results suggest a potential mechanism that may explain why individuals with blood group O have a reduced risk of MI in that O type platelets are less sticky on VWF, in a manner analogous to the effect of antiplatelet agents.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the Health Research Board of Ireland (HRA_POR/2013/233). Q.M.Q. is supported by Stanford Graduate Fellows in Science and Engineering.
Authorship
Contribution: E.D., J.S.O., and D.K. conceived and designed the experiments; E.D. and J.M.O. performed the experiments and analyzed the data; I.S. modified the platelet tracking algorithm; Q.M.Q., E.S.S., and A.J.R. devised the modeling; Q.M.Q. analyzed data and performed the modeling; and E.D., Q.M.Q., and D.K. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dermot Kenny, Royal College of Surgeons in Ireland, 123 St Stephen’s Green, Dublin D02 YN77, Ireland; e-mail: dkenny@rcsi.ie.

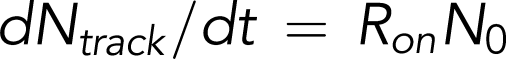
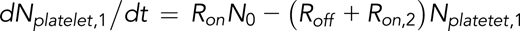
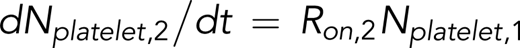

Comments
platelet-vWF interactions
The increased mobility of type O platelets can be explained by the lower circulating levels (25%) of vWF antigen found in group O individuals. 2,3 Alao, homozygotes for blood group O show reduced biological activities related to glycoprotein Ib, botrocetin cofactor, and ristocetin cofactor,4 suggesting that type O platelets have fewer vWF-mediated binding sites for attachment to the subendothelial matrix. This explains the slower binding kinetics of GPIb/vWF interactions in type O platelets, especially during adhesion to subendothelial surface, and the reduced interaction under arterial shear stress.
These findings may be linked to the ABO, where the lack of functional glycosyltransferase enzymes in individuals with blood group O leads to the expression of an unmodified H glycan structure. 5
This structural distinction likely contributes to the differences in platelet binding kinetics and behaviors. This underscores the importance of glycosylation in regulating coagulation proteins, highlighting the need for further research into post-translational modifications and their impact on hemostatic responses across blood groups.