Abstract
Introduction
Tyrosine kinase inhibitors (TKI) have improved survival in patients with chronic myeloid leukemia in chronic phase (CML-CP). There is a growing interest in treatment discontinuation after achieving sustained MR4.5. Over the course of therapy patients dose reduction of their TKI or switch to another TKI for various reasons. Multivariate joint models for dynamic personalized assessment incorporate survival and longitudinal data of multiple repeated measurements. Such models can handle longitudinal data of BCR-ABL levels over time as well as the dose of each TKI in every clinic encounter. The aim of this study is to evaluate the impact of dose adjustments and TKI switch on the achievement of sustained MR4.5.
Methods
From July 2000 to January 2017, 646 patients who enrolled in frontline TKI trials (imatinib 400 mg/day, 73 patients; imatinib 800 mg/day, 208 patients; nilotinib 800 mg/day, 148 patients; dasatinib 100 mg/day, 150 patients; dasatinib 50 mg/day, 16 patients; ponatinib 45 mg/day, 43 patients; ponatinib 30 mg, 8 patients) including 10,623 clinical visits to verify the dose of TKI, and 8,319 measurements of BCR-ABL by reverse transcriptase polymerase chain reaction. Sustained MR4.5 was defined as at least 2-year duration of MR4.5. Multivariate joint modeling with multiple longitudinal measurements was performed for dynamic personalized assessment with the combination of Cox proportional hazard model with generalized linear mixed models. For the estimation of parameters of the joint model, a Bayesian approach was used with Markov Chain Monte Carlo methods. The dose of each TKI, and the BCR-ABL/ABL ratio were considered as time-dependent covariates in the generalized linear mixed model. The dose of each TKI was handled as numeric values which enabled to accommodate treatment effect of dose-reduced TKI and alternative TKI after front-line TKI failure throughout different clinical trials.
Results
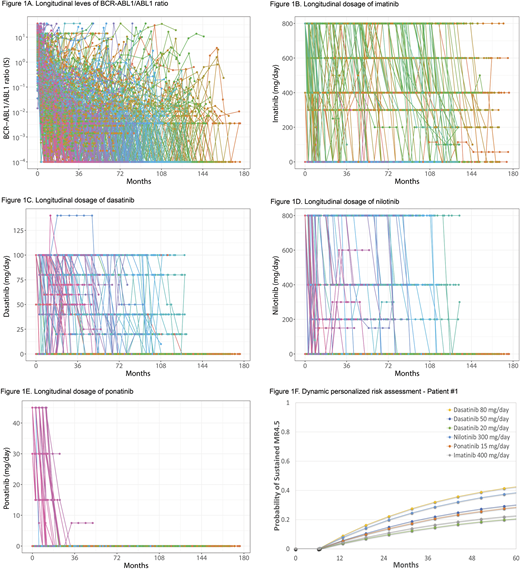
Overall median follow-up was 105 months (range, 0.3-213.4); imatinib 400 mg/day, 193 months; imatinib 800 mg/day, 166 months; nilotinib 800 mg/day, 81 months; dasatinib 100 mg/day, 79 months; dasatinib 50 mg/day, 10 months; ponatinib 45 mg/day, 48 months; ponatinib 30 mg/day, 40 months. A total of 334 patients (52%) achieved sustained MR4.5; median time to sustained MR4.5 was 44 months (95% confidence interval, 30.3-56.1). The trajectories of BCR-ABL levels (Figure 1A) and the dose of TKI are shown (imatinib, Figure 1B; dasatinib, Figure 1C; nilotinib, Figure 1D; ponatinib, Figure 1E). Multivariate joint model identified the daily dose of imatinib (p <0.001; hazard ratio [HR], 1.0017; 95% credible interval [CI], 1.0006-1.0028), dasatinib (p <0.001; HR, 1.0153; 95% CI, 1.0078-1.0237), nilotinib (p <0.001; HR, 1.0017; 95% CI, 1.0006-1.0028), ponatinib (p <0.001; HR, 1.0243; 95% CI, 1.0036-1.0471) as prognostic factors for the achievement of sustained MR4.5. An example of dynamic personalized assessment for the guidance of dose reduction and alternative TKI was shown in Figure 1F. Hypothetical Patient #1 was treated with front-line dasatinib 100 mg/day. At 6.24 months of dasatinib 100 mg/day, BCR-ABL1/ABL1 ratio was 0.18 % on the international scale. Assuming Patient #1 developed refractory pleural effusion not controlled with steroid and diuretics, expected cumulative rate of sustained MR4.5 at dasatinib 80 mg/day, dasatinib 50 mg/day, dasatinib 20 mg/day, nilotinib 300 mg/day, ponatinib 15 mg/day, imatinib 400 mg/day were calculated in Figure 1F. Dynamic personalized assessment by multivariate joint model estimated sustained MR4.5 at 5 years of TKI therapy after the suggested treatment options as follows; dasatinib 80 mg/day, 41% (95% CI, 31.0-55.8); dasatinib 50 mg/day, 29% (95% CI, 18.9-41.7); dasatinib 20 mg/day, 20%, (10.8-32.5); nilotinib 300 mg/day, 37% (95% CI, 28.8-45.6); imatinib 400 mg/day, 28% (19.0-37.1); ponatinib 15 mg/day, 22% (95% CI, 13.0-32.5).
Conclusion
Dynamic personalized assessment of TKI dose reduction and alternative TKI selection optimizes treatment decision at the time of TKI failure in patients with CML-CP.
Sasaki:Otsuka Pharmaceutical: Honoraria. Ravandi:Bristol-Myers Squibb: Research Funding; Amgen: Honoraria, Research Funding, Speakers Bureau; Astellas Pharmaceuticals: Consultancy, Honoraria; Xencor: Research Funding; Abbvie: Research Funding; Orsenix: Honoraria; Astellas Pharmaceuticals: Consultancy, Honoraria; Jazz: Honoraria; Macrogenix: Honoraria, Research Funding; Xencor: Research Funding; Jazz: Honoraria; Sunesis: Honoraria; Orsenix: Honoraria; Amgen: Honoraria, Research Funding, Speakers Bureau; Sunesis: Honoraria; Seattle Genetics: Research Funding; Bristol-Myers Squibb: Research Funding; Macrogenix: Honoraria, Research Funding; Seattle Genetics: Research Funding; Abbvie: Research Funding. Konopleva:Stemline Therapeutics: Research Funding. Wierda:AbbVie, Inc: Research Funding; Genentech: Research Funding. Daver:Otsuka: Consultancy; Kiromic: Research Funding; Sunesis: Consultancy; ImmunoGen: Consultancy; Karyopharm: Consultancy; Novartis: Consultancy; Incyte: Research Funding; Pfizer: Consultancy; Incyte: Consultancy; BMS: Research Funding; ARIAD: Research Funding; Novartis: Research Funding; Alexion: Consultancy; Sunesis: Research Funding; Daiichi-Sankyo: Research Funding; Karyopharm: Research Funding; Pfizer: Research Funding. Jabbour:Bristol-Myers Squibb: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Novartis: Research Funding; Abbvie: Research Funding; Takeda: Consultancy, Research Funding. Cortes:Novartis: Consultancy, Research Funding; Astellas Pharma: Consultancy, Research Funding; Daiichi Sankyo: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Arog: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal