Abstract
Approximately 45% of adult AML pts do not have cytogenetic abnormalities visible by microscopic karyotype analysis and are classified as CN-AML. However, these pts can still have one or more UPD (the loss of a chromosome or its segment and duplication of the remaining chromosome or segment), since UPDs are not detectable by typical karyotyping methods. Because UPDs have been shown to contribute to malignant transformation and outgrowth in various cancers and leukemias, we sought to identify recurrent UPDs in CN-AML pts and explore their associations with disease presentation, gene mutations and outcome. We used Infinium OmniExpress SNP arrays to detect UPDs in a cohort of 425 pts, who were similarly treated with intensive chemotherapy on Alliance for Clinical Trials in Oncology therapeutic trials. Pts having early death (<30 days) were excluded. The mutation status of 80 genes were previously assessed by targeted sequencing (Leukemia, 2017;31(10):2211-8).
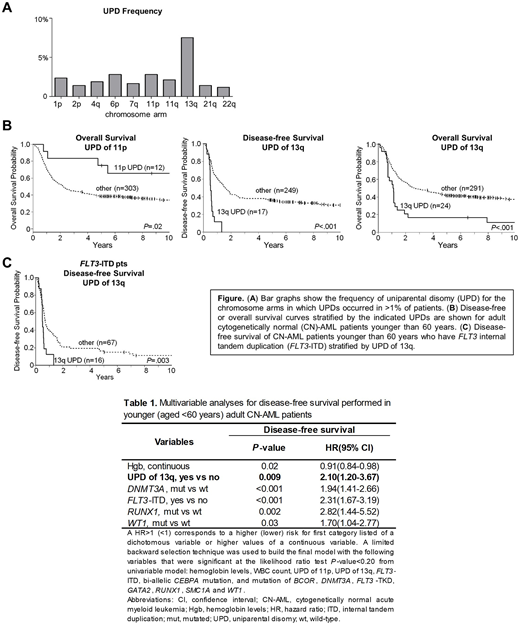
UPDs of 10 different chromosome arms were detected in >1% of pts (Figure A). The most commonly occurring UPDs were UPD of 13q (present in 7.5% of pts), UPD of 6p (in 2.4% of pts) and UPD of 11p (in 2.4% of pts). We examined associations between UPDs of 13q, 6p and 11p with demographic and clinical diagnostic characteristics. UPD of 6p was associated with higher peripheral blood (PB) blast percentage (median 86% vs 70%, P=.02); UPD of 11p was associated with lower platelet counts (41 vs. 60 x 109/L, P=.03) and higher PB blast percentage (85% vs 60%, P=.002); and UPD of 13q was associated with higher white blood cell count (53 vs 29 x 109/L, P=.02), higher PB blast percentage (77% vs 58%, P=.004) and higher bone marrow blast percentage (80% vs 69%, P=.005). We also examined the associations between UPDs and gene mutations present in >2% of CN-AML pts in our sample series. 13q UPD significantly co-occurred with FLT3-internal tandem duplication (ITD) (P<.001), and UPD of 11p significantly co-occurred with bi-allelic CEBPA mutations (P<.001). The co-occurrence of FLT3-ITD with 13q UPD likely has biologic relevance since FLT3 is located on 13q, whereas the association between 11p UPD with CEBPA mutation is less clear, as CEBPA is on 19q.
Assessment of the association between UPDs and outcome was performed. Because of differences in induction therapies between pts younger and older than 60 years (y), outcome analyses must be performed separately in these two age groups. Based on sample size limitations we were only able to assess the impact of 11p UPD and 13q UPD on achievement of complete remission (CR), disease-free survival (DFS) and overall survival (OS) in the 315 pts younger than 60 y. UPD of 11p was associated with longer OS (3 y rates, 83% vs 45%, P=.02), and UPD of 13q was associated with shorter DFS and OS (3 y rates, 0% vs 38%, P<.001; 17% vs 49%, P<.001) (Figure B). To examine the combined effect of mutations and UPDs on outcome we performed multivariable analyses (MVAs). We found that UPD of 13q was significantly associated with shorter DFS (P=.009) after adjusting for hemoglobin levels (P=.02), FLT3-ITD status (P <.001), and mutation of DNMT3A (P<.001), RUNX1 (P=.002) and WT1 (P=.03) (Table). UPD of 13q did not impact on CR or OS when considered in a MVA, and UPD of 11p did not remain significant in MVA.
Finally, since FLT3-ITD and UPD of 13q often co-occurred and were both found to be associated with shorter DFS in the MVA of adult younger pts, we examined younger pts who harbored FLT3-ITD for differences in DFS associated with UPD of 13q. We found those pts with 13q UPD had significantly shorter DFS (P=.003) compared to those without (Figure C), which further demonstrates 13q UPD status is useful for prognostic stratification independent from its association with the known prognostic marker FLT3-ITD.
These data show the incorporation of 13q UPD might improve current genetic risk stratification models. Follow-up studies investigating the mechanistic roles of these recurrent UPDs, especially the relationship between UPD of 11p and bi-allelic CEBPA mutation, could shed light on novel pathways underlying leukemic transformation and proliferation in CN-AML.
Powell:Rafael Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees. Kolitz:Magellan Health: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal