Abstract
Background: Allogeneic hematopoietic stem cell transplantation (alloSCT) is the only curative option for patients with high risk acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). Whereas, relapse is the main event in therapeutic failure for these patients. We previously reported the utility of residual circulating tumor DNA (ctDNA) status for identifying patients with AML and MDS at high risk for relapse post myeloablative alloSCT (Nakamura et al, ASH. 2017). However, it remains to be elucidated whether persistent mutation status in serum and bone marrow (BM) have comparable ability to identify patients at high risk for relapse. Additionally, recent reports indicated mutation persistence (MP) in BM based on three genes regarding clonal hematopoiesis, DNMT3A, TET2, and ASXL1 (DTA), was not informative for relapse prediction of patients with AML in the setting of chemotherapy (Jongen-Lavrencic et al, N Engl J Med. 2018). Therefore, the prognostic impact of residual ctDNA status based on DTA genes should also be tested.
Methods: To address these questions, we retrospectively collected tumor and matched serum samples at diagnosis and 1 and 3 months post-alloSCT from 53 patients with AML and MDS. Cell-free DNA was extracted from serum samples. We subjected tumor DNA, extracted from BM or peripheral blood, and buccal swab DNA, to next-generation sequencing (NGS), identifying candidate driver mutations. After identifying driver mutations, we designed droplet digital PCR (ddPCR) assay. The primary endpoint was the cumulative incidence of relapse (CIR) rate, and the secondary endpoint was the overall survival (OS) rate. We used DeLong's test to compare the performance between two assays based on the area under the curve (AUC) of receiver operating characteristics (ROC) curves.
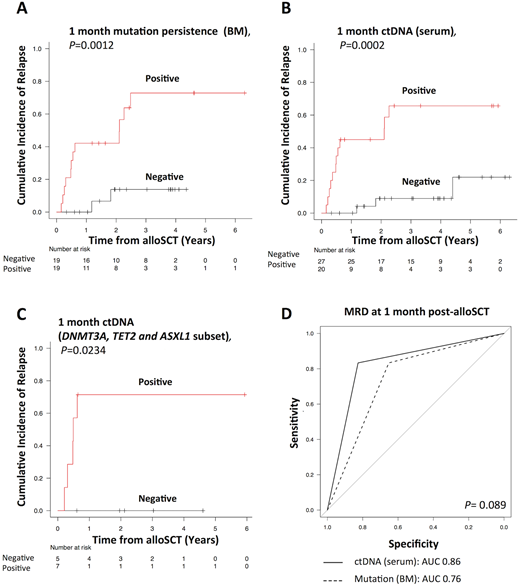
Results: Driver mutations were identified in 51 of 53 patients by NGS, and our cohort consisted of 37 patients with AML and 14 patients with MDS. The median age of the patients was 53 years. The conventional cytogenetic risk category was an adverse or high risk in 39.2% of patients, and 49.0% were in relapse or refractory disease status at alloSCT, and all patients received myeloablative conditioning; in most cases, the stem cell source was cord blood. The most frequent mutations found involved epigenetic regulators (DNMT3A/TET2/ASXL1, mutated in 32.1%), followed by signal transduction proteins (NRAS/FLT3, 31.4%). We could design at least one representative ddPCR assay for 51 patients. There was a clear correlation of variant allele frequency measurement between diagnostic ctDNA and matched tumor DNA (r2 = 0.67; P < 0.0001). Sixteen patients relapsed after a median of 7 months post-alloSCT. Both MP in BM at 1 and 3 months post-alloSCT and corresponding ctDNA persistence (CP) in serum (MP1 and MP3; CP1 and CP3, respectively) were comparably associated with higher 3-year CIR rates and inferior OS rates [3-year CIR (3-year OS): MP1 vs. non-MP1: 72.9 (50.0)% vs. 13.8 (88.0)%; P = 0.0012 (.0304) (Figure 1A); CP1 vs. non-CP1: 65.6 (45.8)% vs. 9.0 (91.7)%; P = 0.0002 (.0014) (Figure 1B); MP3 vs. non-MP3; 80.0 (30.0)% vs. 11.6 (94.1)%; P = 0.0002 (.0007); CP3 vs. non-CP3: 71.4 (53.4)% vs. 8.4 (92.5)%; P < 0.0001 (.0021)]. We next tested whether CP based on DTA could also be helpful in relapse prediction, we performed a subset analysis of patients with DTA based ddPCR assays (n=12). As a result, CP based on DTA genes also had the prognostic impact on CIR (Figure 1C). Finally, we compared the discriminatory ability of CP with those of MP. There was no significant difference between either CP and MP (Figure 1D). Additionally, when CP1 was compared with CP3, CP3 was found to be a better indicator of CIR and OS.
Conclusions: In summary, we, for the first time, demonstrated that non-invasive serum ctDNA-testing, regardless of DTA genes, had comparable utility to molecular MRD testing of BM with regard to identifying patients at high risk for relapse in AML and MDS undergoing myeloablative alloSCT. Although prospective large-scale analyses are needed to confirm our findings, such non-invasive ctDNA-testing might allow for rapid clinical decision-making and, ultimately, subsequent risk-adapted therapeutic interventions post-alloSCT in AML and MDS.
Figure 1. CIR based on the 1 month (A) MP and (B) CP status. (C) CIR based on the 1month CP status according to DTA subset. (D) Comparison of ROC curves for relapse prediction between 1 month CP and MP.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal