Abstract
ADAMTS13 (a disintegrin and metalloproteinase with thrombospondin type 1 motif, 13) is a metalloprotease responsible for cleavage of ultra-large von Willebrand factor (VWF) multimers. Severely deficient activity of the protease can trigger an acute episode of thrombotic thrombocytopenic purpura (TTP). Our understanding of the pathophysiology of TTP has allowed us to grasp the important role of ADAMTS13 in other thrombotic microangiopathies (TMAs) and thrombotic disorders, such as ischemic stroke and coronary artery disease. Through its action on VWF, ADAMTS13 can have prothrombotic and proinflammatory properties, not only when its activity is severely deficient, but also when it is only moderately low. Here, we will discuss the biology of ADAMTS13 and the different assays developed to evaluate its function in the context of TTP, in the acute setting and during follow-up. We will also discuss the latest evidence regarding the role of ADAMTS13 in other TMAs, stroke, and cardiovascular disease. This information will be useful for clinicians not only when evaluating patients who present with microangiopathic hemolytic anemia and thrombocytopenia, but also when making clinical decisions regarding the follow-up of patients with TTP.
Introduction
The term microangiopathic hemolytic anemia (MAHA) is used to describe the mechanical destruction of red blood cells that may occur in the setting of hemodynamic turbulence or in thrombotic microangiopathies (TMAs), as the red blood cells are sheared by microthrombi in the arterioles. It is the hallmark of diseases such as thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS), but it can also be seen in many other acute illnesses, such as sepsis, malignant hypertension, and preeclampsia. These diseases may have similar clinical presentations yet very different treatments and prognoses. Whereas a majority of patients with immune-mediated TTP (iTTP) will respond to plasma exchange (PEX) and steroids, only 30% of patients with complement-mediated HUS (cm-HUS) will respond to PEX, and most will require complement-inhibition therapy with eculizumab. The treatment of other diseases presenting with TMA findings is directed toward treating the underlying illness.1
TTP can be distinguished from other causes of MAHA by the finding of severely deficient ADAMTS13, typically <10% of normal.2 ADAMTS13 is a plasma protease responsible for the cleavage of von Willebrand factor (VWF), preventing the accumulation of ultra-large VWF (ULVWF) multimers that can spontaneously interact with platelets, leading to microvascular thrombosis.
The role of ADAMTS13 goes beyond TTP. Endothelial activation resulting from inflammation can induce the release of VWF into the circulation with subsequent ADAMTS13 consumption, leading to moderately decreased ADAMTS13 activity. This can be seen in other forms of TMA and thrombotic disorders, such as myocardial infarction and ischemic stroke. The ADAMTS13 protease has also been the focus of recent research toward the development of novel agents,3-8 with exciting new potential therapeutic options for patients with TTP. In the same way, a better characterization of the function of ADAMTS13 in maintaining normal vascular homeostasis has opened the door for potential new treatments in patients with inflammatory and thrombotic disorders but only moderately decreased ADAMTS13.
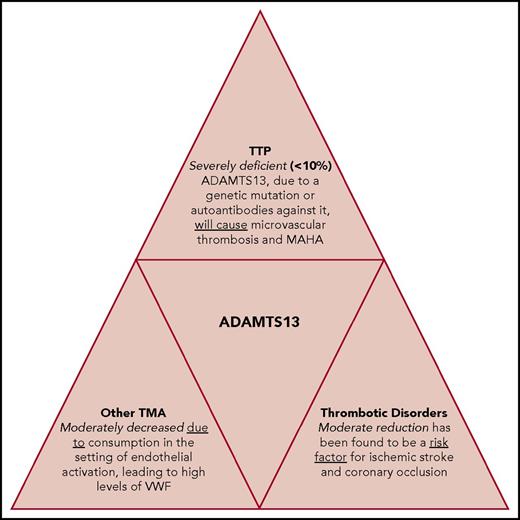
This article will discuss the structure and function of ADAMTS13, its role in the pathophysiology of TMAs, and potential future directions and clinical utilities of ADAMTS13 activity testing in the diagnosis and treatment of TMAs and other thrombotic conditions (Figure 1).
ADAMTS13 deficiency. ADAMTS13 deficiency is the cause of TTP but also has a significant role in the pathophysiology, diagnosis, and prognosis of other TMAs and thrombotic disorders, such as stroke and myocardial infarction.
ADAMTS13 deficiency. ADAMTS13 deficiency is the cause of TTP but also has a significant role in the pathophysiology, diagnosis, and prognosis of other TMAs and thrombotic disorders, such as stroke and myocardial infarction.
Biology of ADAMTS13
ADAMTS13 has a structure similar to that of other members of the ADAMTS family, composed of 14 different domains: a metalloprotease, a disintegrin, a first thrombospondin type 1 repeat (TSP1), cys-rich, and spacer domains. The distal part of the protease contains 7 additional TSP1 repeats and 2 CUB domains.9,10 Each domain has a different role in the function of ADAMTS13 activity, with the CUB and spacer domains being particularly important in TTP. Even though ADAMTS13 is released as an active enzyme,11,12 it circulates in a closed conformation through the interaction between CUB and spacer domains.13,14 When VWF binds to ADAMTS13, the protease changes its configuration and becomes activated. Roose et al15 used antibodies that recognized cryptic epitopes in the spacer domain to further elucidate the conformational changes in the protease. Although ADAMTS13 was found to circulate in a folded conformation in all healthy donors and in 78% of patients in TTP remission, it was found to have an open conformation (defined as a conformation index of >0.5) in 92% of patients with acute TTP. Additional studies are needed to determine the role of these conformational changes of ADAMTS13 in the diagnosis and prognosis of TTP.
ADAMTS13 is synthesized primarily in the interstitial area of hepatic stellate cells,10,16,17 but it is also produced in trace amounts in the endothelial cells,11,18 megakaryocytes, platelets,19,20 renal podocytes, and tubular epithelial cells.21,22 Studies in rats and humans have shown that ADAMTS13 activity can drop 30% to 40% after a partial hepatectomy, suggesting that ADAMTS13 produced in other tissues has a small but important contribution to normal plasma protease levels. Endothelial-derived ADAMTS13 may cleave newly formed ULVWF multimers, contributing to the maintenance of a cell surface that is free of hyperadhesive VWF.12,23 Although the amount of ADAMTS13 produced outside the liver is small, it may still be biologically important because of its role in thrombus formation, although more studies are needed to better understand this relationship.
ADAMTS13 has a plasma half-life of 2 to 3 days,24 with 3% to 5% of ADAMTS13 circulating bound to VWF.25 There are no data regarding the clearance of the protease, nor any known inhibitor of its function. Therefore, the regulation of ADAMTS13 is likely to be at the substrate level (VWF), with 3 factors known to regulate its activity: (1) fluid shear stress, found in the microcirculation, which allows VWF to unfold and expose its A2 domain for ADAMTS13 binding; (2) factor VIII, which enhances the ADAMTS13 proteolytic activity by affecting the A1A2A3 domain-domain interaction when ADAMTS13 binds VWF under shear forces26,27 ; and (3) platelet glycoprotein 1bα (GP1bα), which increases ADAMTS13 function under static or shear conditions by exposing the VWF A2 domain when it binds to the A1 domain.28 The main function of the ADAMTS13 protease is the binding and cleavage of cell-bound ULVWF strings at the Tyr1605-Met1606 bond.29,30 Under shear stress, as seen in the microcirculation or after thrombus formation, VWF undergoes conformational changes that unfold its A2 domain, making the ADAMTS13 cleavage site accessible.31-34
Assays of ADAMTS13 activity
There are several ADAMTS13 biomarker assays that are either available for routine clinical use or under study for their potential clinical applications. The most common types of these ADAMTS13 assays measure in vitro functional ADAMTS13 activity.
ADAMTS13 activity assays
Measuring ADAMTS13 activity has become increasingly important in the evaluation and treatment of patients with TTP. One of the primary limitations of ADAMTS13 activity assays remains the need for nonphysiologic assay conditions (guanidine hydrochloride to expose the VWF cleavage site, low potential of hydrogen and sodium chloride concentrations) to simulate the high shear forces important for in vivo ADAMTS13 function. This limitation has been overcome using a VWF substrate with the VWF cleavage site instead of the full-length VWF molecule.
There have been multiple assays developed in the past 20 years, with the FRETS-WWF73–based assay35 being the most commonly used in clinical settings given the relative ease to perform it, although it is still primarily done in reference laboratories.
ADAMTS13 antigen assay
The levels of the ADAMTS13 antigen can be measured by an enzyme-linked immunosorbent assay (ELISA). Although this test is not yet part of routine testing for patients with TTP, Awan et al36 recently showed that patients with the ADAMTS13 antigen at presentation in the lowest quartile (<1.5%) had a higher TTP-related mortality rate, compared with those with higher levels of the antigen, suggesting a prognostic value of the test.
ADAMTS13 autoantibody and inhibitor assays
Patients with iTTP will have an autoantibody that targets the spacer domain of the protease.37 The detection of an acquired inhibitor is essential to differentiate iTTP from congenital TTP (cTTP). Most of the autoantibodies against ADAMTS13 are of the immunoglobulin G (IgG) isotype (with IgG4 being the most common, followed by IgG1), although IgM and IgA have also been reported.38,39 However, quantifying the amount of anti-ADAMTS13 antibodies through an ELISA does not confirm the functional activity of the antibody. Although most of the autoantibodies will have an inhibitory function (which can be measured by a Bethesda-like assay), ∼10% to 25% of patients with iTTP have autoantibodies that do not neutralize the protease, but rather are thought to be involved in its in vivo clearance.40,41 In the evaluation of patients with TTP, the use of the Bethesda-like assay to demonstrate inhibitory function is important to confirm the diagnosis of iTTP and exclude cTTP.
ADAMTS13-specific CICs
Anti-ADAMTS13 autoantibodies can circulate freely in plasma or bind to ADAMTS13, forming a complex42-44 that can be detected by an ELISA. Circulating immune complexes (CICs) are found in patients with TTP, during acute episodes and in remission. Although the role of CICs in the pathophysiology of TTP is not entirely clear, Mancini et al45 noted a higher risk of recurrence in those patients who had CICs detected during acute episodes of TTP.
Assays of ADAMTS13 in TTP
When a patient presents with thrombocytopenia and MAHA without an alternative clinical explanation, ADAMTS13 activity of <10% confirms the diagnosis of TTP. Additional studies to detect the presence of an inhibitor of the protease with a Bethesda-like assay, as described in “ADAMTS13 autoantibody and inhibitor assays,” will help to differentiate between iTTP or cTTP. If no inhibitor is detected, and the clinical picture is consistent with cTTP (first manifestation in childhood, more rapid initial recovery, persistently deficient ADAMTS13 activity over time with a full recovery of activity with plasma infusion), then an ADAMTS13 mutational analysis should be ordered to confirm the diagnosis of cTTP.
Once a patient with iTTP has achieved clinical response (platelet count >150 × 109/L and normal lactate dehydrogenase, with stabilization or improvement of symptoms), 3 scenarios can occur2 : (1) clinical remission (continuous clinical response after cessation of PEX, maintained for >30 days); (2) exacerbation, defined as a recurrent thrombocytopenia and the need to restart PEX within 30 days of the last PEX procedure, which can occur in 40% to 50% of cases46 ; and (3) relapse, defined as a recurrence of TTP >30 days after the last PEX procedure (incidence of ∼30%-50%).46-48 Interestingly, neither the normalization of ADAMTS13 activity nor the variability during remission has been incorporated into the clinical definitions of TTP.
Although there is no question regarding the diagnostic role of ADAMTS13 testing at the time of clinical presentation,47,49-52 there are uncertainties regarding the role of these assays at different points of the disease (Table 1).
Prediction of relapse using different ADAMTS13-related biomarkers at different points of disease activity
| Biomarker . | Measured at beginning of acute episode . | Measured at clinical response . | Measured in remission . |
|---|---|---|---|
| ADAMTS13 activity <10% | Diagnostic of TTP | Predictor of relapse | Predictor of relapse38,57,58 |
| ADAMTS13 Ag | No association81 | Higher levels associated with sustained remission82 | Associated with relapse vs no association58 |
| ADAMTS13 inhibitor | No association81 | No association | Associated with relapse |
| Anti-ADAMTS13 Ab | IgG associated with relapse | IgG39 associated with relapse (IgG4) | |
| VWF Ag | No association | No association | No association |
| Biomarker . | Measured at beginning of acute episode . | Measured at clinical response . | Measured in remission . |
|---|---|---|---|
| ADAMTS13 activity <10% | Diagnostic of TTP | Predictor of relapse | Predictor of relapse38,57,58 |
| ADAMTS13 Ag | No association81 | Higher levels associated with sustained remission82 | Associated with relapse vs no association58 |
| ADAMTS13 inhibitor | No association81 | No association | Associated with relapse |
| Anti-ADAMTS13 Ab | IgG associated with relapse | IgG39 associated with relapse (IgG4) | |
| VWF Ag | No association | No association | No association |
Ab, antibody; Ag, antigen.
ADAMTS13 activity during the acute episode
Wu et al53 reported the prognostic value of ADAMTS13 activity when measured daily during PEX in 19 patients with iTTP who were severely deficient (<10%) at presentation. The first PEX did not significantly change the ADAMTS13 activity levels. Even after 3 PEX procedures, 14 of the 19 patients still had ADAMTS13 activity <10% (measured immediately before the next PEX procedure). After a week of PEX procedures, 12 of the 19 patients had not achieved ADAMTS13 activity >10%.
Compared with those who recovered their activity before day 7, these patients took a longer time to achieve a clinical response (P = .001). There was also a trend toward more exacerbations in the group with more delayed recovery of ADAMTS13 activity in the first week, but the difference was not statistically significant. These data confirm that even in situations in which ADAMTS13 activity is not measured before initiating PEX, there is still a diagnostic role for the test, with a sensitivity of 89%, 83%, and 78% after days 1, 2, and 3 of PEX, respectively.
ADAMTS13 at clinical response of TTP
A retrospective study at out institution evaluated the role of ADAMTS13 activity measured at clinical response to predict exacerbations in 44 acute episodes from 26 patients with iTTP.54 In this study, African American race and lower pretreatment ADAMTS13 activity were both associated with an increased risk for exacerbation.
ADAMTS13 activity measured in the first week after stopping PEX was significantly lower in those patients who experienced an exacerbation (1.2% vs 22.5%) compared with those who did not, but the difference was no longer significant after accounting for race as a covariable.
Two recent studies evaluating caplacizumab, a nanobody that binds to the A1 of VWF, preventing the interaction between platelets and VWF, also provide important information regarding the prognostic value of ADAMTS13 to predict exacerbation. In the TITAN study,55 75 patients with a clinical diagnosis of iTTP were randomly assigned to receive caplacizumab or placebo daily with PEX and to continue 30 days after the last PEX. In this study, 11 patients (28%) experienced an exacerbation in the placebo arm, compared with 3 (8%) in the caplacizumab group, supporting the hypothesis that caplacizumab prevents exacerbations of iTTP. Importantly, ADAMTS13 activity was <10% at the time of clinical response in 13 of the 14 patients who experienced an exacerbation. In the follow-up phase 3 Hercules study, a similar decrease in exacerbations was seen in the caplacizumab arm compared with placebo (3% vs 28%), with the presumption that persistently severely deficient ADAMTS13 activity was a significant factor in the development of exacerbations.56 In this study, physicians were encouraged (but not required) to continue caplacizumab until there was clear improvement and correction of the severe ADAMTS13 deficiency to avoid recurrences of TTP after stopping caplacizumab.
These data provide the rationale for the use of ADAMTS13 at the time of PEX discontinuation to identify those patients who may be more prone to exacerbations and would benefit from additional immune suppressive therapy.
The French Clinical and Biological Network on Adult TMA published its experience regarding the prognostic value of ADAMTS13 activity and anti-ADAMTS13 antibody characteristics (Ig isotype, titer, and inhibitory effect) in a cohort of 35 patients.38 Samples were obtained within 7 days of achieving clinical response. Thirteen patients (41%) had undetectable activity at the time of clinical response. These patients had a higher relapse rate (38.5%) when compared with those who recovered their activity at clinical response (5%). A high IgG autoantibody level at clinical presentation was also associated with persistently deficient ADAMTS13 activity at clinical response.
Although presently the treatment of acute TTP is directed only at the correction of the thrombocytopenia and any end-organ injury present, these results suggest that weekly monitoring of ADAMTS13 activity after PEX is discontinued may be an equally important biomarker of treatment response, with the goal of correcting the severely deficient ADAMTS13 activity with immune suppressive therapy to prevent exacerbations and relapse of iTTP.
ADAMTS13 monitoring during remission
There are uncertainties surrounding the measurement of ADAMTS13 activity during remission, including how often should it be monitored, the level of ADAMTS13 activity that would prompt the use of preemptive rituximab or another immune suppressive agent, and the correlation of the known variability of ADAMTS13 activity during remission with relapse risk. Jin et al57 found an association between lower ADAMTS13 activity measured in remission and an increased risk of relapse in 24 patients with iTTP followed with serial ADAMTS13 monitoring during remission for an average of 23 months. There was no association found between the risk of relapse and the ADAMTS13 IgG levels. Another study involving 109 patients with iTTP with samples obtained during remission (most commonly 1 sample per patient, at variable times after achieving clinical response; range, 1-96 months) found that ADAMTS13 activity was severely deficient in 32% of patients at remission. Of these 109 patients, 46 had a recurrence. The prevalence of severe ADAMTS13 deficiency was higher in patients with recurrent TTP (46% vs 22%; P = .01). The likelihood of recurrence associated with severe ADAMTS13 deficiency when adjusted in the multivariate analysis was statistically significant (odds ratio, 2.9; 95% confidence interval [CI], 1.3-6.8). Despite lower ADAMTS13 antigen levels in the relapse group (median, 36% vs 58%; P = .003), the odds ratio did not predict relapse. Finally, regarding the anti-ADAMTS13 antibody analysis, autoantibodies were present in 64% of patients in the relapse group, compared with 36% in the nonrelapse group.58
These studies and others (Table 1) form the basis to consider prophylactic rituximab to prevent relapses in patients who are found to have severely deficient ADAMTS13 activity during remission.59-61 However, there are also data from patients with iTTP and persistently deficient ADAMTS13 activity in remission who do not uniformly experience relapse, with some patients having an isolated ADAMTS13 activity <10% that improves spontaneously.62 We recently calculated the sensitivity and specificity of ADAMTS13 activity measured during remission to predict relapses in the following 30 and 90 days.63 The assays were obtained every 3 months in 39 patients (total of 557 samples). There was a significant variability in ADAMTS13 activity, with 145 (26%) of the samples showing severely deficient activity of <10%; however, only 11 of these were followed by a relapse in the subsequent 90 days. We estimated that the sensitivity and specificity of ADAMTS13 activity <10% to predict a relapse in the next 30 and 90 days were 40.74% and 74.72%, respectively (likelihood ratio, 1.61; CI, 1-2.6). When we used 20% as a threshold, the sensitivity and specificity were both 66% (likelihood ratio, 1.97; CI, 1.47-2.64).
In summary, although severely deficient ADAMTS13 activity in remission may be a risk factor for relapse, it does not mean a relapse will absolutely occur. For decisions regarding prophylactic treatment with rituximab, ADAMTS13 activity <20% may be a more useful threshold than ADAMTS13 <10%. Better models for a more accurate prediction of relapse, incorporating additional biomarkers, are needed. The most appropriate use of these ADAMTS13 activity data would be to have an informed discussion with patients to balance the risk of relapse with the risks of prophylactic rituximab therapy. Our approach to the use of ADAMTS13 testing and prophylactic rituximab therapy is shown in Figure 2. We follow patients after their first acute TTP episode indefinitely, not only to try to prevent relapses, but also to monitor the long-term complications described in these patients.64-66
Approach to patient follow-up after initial diagnosis of iTTP. CBC, complete blood count; LDH, lactate dehydrogenase.
Approach to patient follow-up after initial diagnosis of iTTP. CBC, complete blood count; LDH, lactate dehydrogenase.
ADAMTS13 activity in other TMAs
Although severely deficient ADAMTS13 activity (<10%) is unique to TTP, there is ample evidence showing that ADAMTS13 is moderately decreased in other TMAs. Martin et al67 reported moderately decreased ADAMTS13 activity in 30 patients with severe sepsis (43.2%; interquartile range, 32.7-67.0) when compared with 29 patients with organ failure resulting from other causes (67.8%; interquartile range, 57.4-87.9; P < .05) and 30 healthy participants (105.6%; interquartile range, 87.2-125.6; P < .001). A study in 53 patients with TMAs associated with different systemic illnesses, including sepsis, solid organ transplantation, malignancy, and autoimmune diseases, showed decreased ADAMTS13 activity (median activity, 33.5%; range, 16%-47%) when compared with control patients and healthy participants.68 Similar findings have been reported in patients with malignant hypertension, in which the median ADAMTS13 activity was 64%.69 Increased release of VWF in the setting of endothelial stimulation seen in these disorders is thought to be the cause of the lower ADAMTS13.70 These studies and many others highlight the delicate balance between the ADAMTS13 protease and VWF to permit physiologic hemostasis while at the same time preventing pathological thrombosis. Although low ADAMTS13 activity is not the cause of TMAs other than TTP, it seems to have an important prognostic role.
ADAMTS13 in thrombotic disorders
Via its cleavage of VWF, ADAMTS13 is thought to have antithrombotic properties. It is no surprise then that the protease may also have a role in the pathophysiology of cardiovascular disease (CVD). In animal models, ADAMTS13−/− mice showed significantly larger infarctions after middle cerebral artery occlusion compared with wild-type (WT) mice.71 Fujioka et al72 found similar results, noting progressively decreased regional cerebral blood flow in ADAMTS13−/− mice compared with WT mice after reperfusion after an induced middle cerebral artery occlusion. VWF is released from Weibel-Palade bodies in endothelial cells during episodes of ischemia, making the function of ADAMTS13 even more important, not only before the event but also after reperfusion. Another animal model showed a significant reduction in microvascular length and area, as well as decreased perfusion, in ADAMTS13−/− mice 14 days after stroke. ADAMTS13−/− mice had significantly reduced endothelial proliferation 2 weeks after stroke, with decreasing neovascularization. They also had an 82% increase in blood-brain barrier permeability compared with WT mice. The mechanism involved in this process may be related to downregulation of angiopoietin-1 and galectin-3, as well as a decrease in phosphorylation of VEGFR-2 in ADAMTS13-deficient mice.73 These studies noted abnormal findings in ADAMTS13−/− mice, but mice that were also deficient in VWF behaved similarly to WT mice, implying that the role of ADAMTS13 in stroke is dependent on its action on VWF.
Although severely deficient ADAMTS13 activity in TTP may lead to microthrombi that can be clinically evident as seizures, focal neurologic deficits, and coma, a moderate reduction in ADAMTS13 activity has been found to be a risk factor for ischemic stroke and coronary occlusion. The most complete information in humans comes from the Rotterdam study, a population-based cohort study in which almost 6000 individuals had their ADAMTS13 activity measured at enrollment to evaluate the association between ADAMTS13 activity, VWF/Ag level, and stroke. Over a median follow-up of 10.7 years, 461 participants had a stroke, 306 of which were ischemic, and 315 individuals had a transient ischemic attack. Patients were divided into 4 quartiles based on their ADAMTS13 activity. Comparing the group in the lowest quartile (ADAMTS13 activity <80%) with those with the highest activity (>102.27%), the former had higher risk of ischemic stroke (absolute risk, 7.3% vs 5.3%), transient ischemic attack (absolute risk, 6.8% vs 4%), and all cerebrovascular events (absolute risk, 17.8% vs 9.3%). Moreover, adding ADAMTS13 to the traditional risk factors used to predict a stroke improved the performance of the model. The investigators also observed a 5.68% decrease in ADAMTS13 activity per 10-year increase in age and an 8.6% higher activity in women compared with men. Diabetes and smoking were also associated with decreased ADAMTS13 activity. These findings were all statistically significant in the population studied.74 A case-control study measuring ADAMTS13 antigen and plasma VWF levels in 85 healthy volunteers, 104 patients with an acute ischemic stroke, and 112 patients with chronic cerebrovascular disease found that patients with an acute ischemic stroke had significantly lower levels of ADAMTS13 antigen than healthy volunteers (82.6% ± 21.0% vs 110.6% ± 26.9%; P < .0001). Patients with chronic cerebrovascular disease also had lower ADAMTS13 activity than healthy volunteers (99.6% ± 24.5%; P < .03), but not as low as those with an acute stroke (P < .0001). Furthermore, a higher VWF/ADAMTS13 ratio was associated with stroke severity.75
Moderately decreased ADAMTS13 is seen not only in patients with neurovascular disorders but also in those with CVD. A study of 27 patients undergoing emergency cardiac catheterization for ST-elevation myocardial infarction showed moderately decreased ADAMTS13 activity (median, 75%). The study also showed significantly reduced ADAMTS13 activity in coronary blood compared with peripheral blood.76 Another subset of the Rotterdam study evaluated the risk for coronary artery disease (CAD) based on ADAMTS13 activity. Of almost 6000 individuals followed for a median of 9.7 years, 456 had CAD; 156 of these cases occurred in the group with ADAMTS13 activity in the lowest quartile (mean, 70.5%), compared with only 85 cases in the group in the highest quartile of ADAMTS13 activity (mean,114.3%),77,78 suggesting a 42% higher risk of developing CAD with moderately deficient ADAMTS13 activity. A follow-up study also reported an association with low ADAMTS13 activity and higher cardiovascular mortality (hazard ratio, 1.46; 95% CI, 1.09-1.96).77 Taken together, these findings should be the basis for clinical trials that may lead to new prognostic tools and treatment options for patients with CAD and CVD.
Conclusions and future directions
In the evaluation of a patient with suspected TTP, severely deficient ADAMTS13 <10% will confirm the diagnosis of TTP and differentiate it from other forms of TMA. A better understanding of the role of ADAMTS13 TTP has led to insights regarding its role in CVD, as a regulator of thrombi formation and neovascularization through its action on VWF.
Recombinant ADAMTS13 has shown promising results in a phase 1 study of patients with cTTP in whom the drug was well tolerated, without ADAMTS13 antibody detection, and a good clinical response was seen.3 Moreover, the drug has shown preclinical activity in iTTP via the neutralization of autoantibodies in vitro8 and ADAMTS13 immune complex formation in animal models,6,79 with reconstitution of ADAMTS13 activity. In thrombotic disorders, infusion of rADAMTS13 in animal models of ischemic stroke has been shown to reduce stroke volume,71 increase endothelial cell proliferation and perfusion, and decrease the permeability of the blood-brain barrier.73 A model using occlusive VWF-rich thrombi in the middle cerebral arteries of mice showed dissolution of the thrombi with rhADAMTS13 but not with tissue plasminogen activator.80 These results suggest a role for rADAMTS13 beyond cTTP and comprise the rationale for future studies.
Authorship
Contribution: C.M. and S.R.C. both wrote, edited, and reviewed the manuscript.
Conflict-of-interest disclosure: S.R.C. serves as a consultant to Ablynx and Shire Pharmaceuticals. The remaining author declares no competing financial interests.
Correspondence: Spero R. Cataland, Department of Hematology, Ohio State University, A361 Starling Loving Hall, 320 W. 10th Ave Columbus, OH 43210; e-mail: spero.cataland@osumc.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal