Key Points
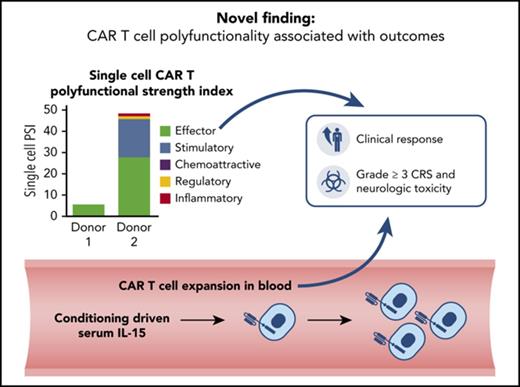
The PSI of manufactured CAR T cells was associated with clinical response and toxicities.
Monitoring CAR T-cell polyfunctionality as a key product attribute may complement other characteristics including T-cell proliferation.
Abstract
After treatment with chimeric antigen receptor (CAR) T cells, interleukin-15 (IL-15) elevation and CAR T-cell expansion are associated with non-Hodgkin lymphoma (NHL) outcomes. However, the association of preinfusion CAR product T-cell functionality with clinical outcomes has not been reported. A single-cell analysis of the preinfusion CD19 CAR product from patients with NHL demonstrated that CAR products contain polyfunctional T-cell subsets capable of deploying multiple immune programs represented by cytokines and chemokines, including interferon-γ, IL-17A, IL-8, and macrophage inflammatory protein 1α. A prespecified T-cell polyfunctionality strength index (PSI) applied to preinfusion CAR product was significantly associated with clinical response, and PSI combined with CAR T-cell expansion or pretreatment serum IL-15 levels conferred additional significance. Within the total product cell population, associations with clinical outcomes were greater with polyfunctional CD4+ T cells compared with CD8+ cells. Grade ≥3 cytokine release syndrome was associated with polyfunctional T cells, and both grade ≥3 neurologic toxicity and antitumor efficacy were associated with polyfunctional IL-17A–producing T cells. The findings in this exploratory study show that a preinfusion CAR product T-cell subset with a definable polyfunctional profile has a major association with clinical outcomes of CAR T-cell therapy. This trial was registered at www.clinicaltrials.gov as #NCT00924326.
Introduction
Genetic reprograming of T cells to express a chimeric antigen receptor (CAR) offers a novel approach for treating hematologic malignancies.1-3 T cells transduced with an anti-CD19 CAR composed of CD28 and CD3ζ signaling domains produce interferon-γ (IFN-γ) in a CD19-specific manner, kill primary leukemia cells, and undergo CD19 target–dependent proliferation.4 Treatment of B-cell malignancies with anti-CD19 CAR T cells results in durable remission in a significant number of patients. This treatment is associated with B-cell aplasia because of endogenous CD19 expression on B cells. Additionally, treatment with anti-CD19 CAR T-cell therapy can cause cytokine release syndrome (CRS) and neurologic toxicity (NT). Anti-CD19 CAR T-cell therapy has not yet been fully characterized mechanistically.5,6 An optimized, low-dose conditioning chemotherapy regimen of cyclophosphamide and fludarabine has been shown to enhance engraftment of CAR T cells through lymphodepletion and by increasing serum cytokine levels, most notably interleukin-15 (IL-15).6 Levels of IL-15 are correlated with CAR T-cell expansion, which in turn correlates with clinical response and toxicities.6 This study also showed that greater expansion of CAR T cells in blood and higher serum levels of IL-15, granzyme B, and IL-10 were associated with grade ≥3 NT.6
T cells deploy a broad spectrum of immune programs,7 and it is unclear which specifically influence in vivo expansion and activity of CAR T cells. We hypothesized that CAR T cells orchestrate clinical activity by deploying multiple immune programs that functionally complement one another. To address this hypothesis, we evaluated CAR T-cell product functionality using a high-content single-cell multiplex cytokine analysis8,9 that allowed for identification of a subset of polyfunctional T cells in CAR T-cell products that produce ≥2 cytokines upon stimulation with CD19 antigen in vitro. We also examined associations of a prespecified polyfunctionality strength index (PSI) applied to CAR T-cell products, CAR T-cell expansion in vivo, objective response (OR), and toxicities. We show that highly polyfunctional T cells within CAR T-cell products are significantly associated with clinical response and that a subset of polyfunctional CD4+ T cells producing IL-17A is associated with grade ≥3 NT.
Methods
Patient demographics and treatment protocol
The study cohort comprised 22 patients with recently described clinical outcomes.6 Of 22 treated patients, 19 had diffuse large B-cell lymphoma (DLBCL), 2 had follicular lymphoma, and 1 had mantle cell lymphoma (Table 1). Of 19 patients with DLBCL, 11 had chemotherapy-refractory lymphoma. Five patients with DLBCL had lymphoma that relapsed ≤10 months after autologous stem-cell transplantation as their last treatment before protocol enrollment. Eleven patients with DLBCL were high risk, according to the second-line, age-adjusted International Prognostic Index.10 The median number of unique lymphoma therapies received before protocol enrollment was 4 (range, 1-7). Products from 20 patients were evaluable by single-cell multiplex cytokine profiling and used in this exploratory study (Table 1). OR is defined as partial or complete response according to Cheson et al11 2014 criteria. Stable and progressive disease correspond to lack of OR (nonresponders). CRS and NT were graded as previously reported.6
Patient demographics, clinical response, and adverse events
| Patient ID . | Age, y . | Sex . | Lymphoma . | Lymphoma category . | N of prior lines of therapy . | BRESP . | OR subgroup . | Grade ≥3 CRS . | Grade ≥3 NT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66.5 | M | DLBCL | Aggressive | 3 | PR | OR | CRS | — |
| 2 | 63.5 | M | FL | Indolent | 6 | CR | OR | CRS | NT |
| 3 | 65.3 | M | DLBCL | Aggressive | 4 | PR | OR | CRS | — |
| 4 | 47.1 | M | DLBCL | Aggressive | 2 | PR | OR | — | — |
| 5 | 28.8 | M | DLBCL | Aggressive | 7 | PD | — | — | — |
| 6 | 62.7 | M | DLBCL | Aggressive | 7 | CR | OR | — | NT |
| 7 | 54.7 | M | DLBCL | Aggressive | 3 | PD | — | CRS | — |
| 8 | 28.6 | M | DLBCL | Aggressive | 2 | SD | — | — | — |
| 9 | 29.5 | M | PMBCL | Aggressive | 3 | SD | — | — | — |
| 10 | 40.4 | M | PMBCL | Aggressive | 2 | PD | — | — | NT |
| 11 | 67.8 | M | DLBCL | Aggressive | 3 | CR | OR | — | NT |
| 12 | 50.4 | M | MCL | Indolent | 1 | CR | OR | CRS | NT |
| 13 | 53.2 | M | DLBCL | Aggressive | 4 | CR | OR | CRS | NT |
| 14 | 67.0 | F | FL | Indolent | 3 | CR | OR | CRS | NT |
| 15 | 51.9 | M | DLBCL | Aggressive | 3 | CR | OR | CRS | NT |
| 16 | 52.0 | F | DLBCL | Aggressive | 5 | CR | OR | CRS | NT |
| 17 | 39.0 | M | DLBCL | Aggressive | 4 | PR | OR | CRS | NT |
| 18 | 67.1 | F | DLBCL | Aggressive | 4 | CR | OR | CRS | NT |
| 19 | 64.4 | M | DLBCL | Aggressive | 4 | CR | OR | CRS | NT |
| 20 | 52.0 | M | DLBCL | Aggressive | 5 | PD | — | CRS | — |
| Patient ID . | Age, y . | Sex . | Lymphoma . | Lymphoma category . | N of prior lines of therapy . | BRESP . | OR subgroup . | Grade ≥3 CRS . | Grade ≥3 NT . |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 66.5 | M | DLBCL | Aggressive | 3 | PR | OR | CRS | — |
| 2 | 63.5 | M | FL | Indolent | 6 | CR | OR | CRS | NT |
| 3 | 65.3 | M | DLBCL | Aggressive | 4 | PR | OR | CRS | — |
| 4 | 47.1 | M | DLBCL | Aggressive | 2 | PR | OR | — | — |
| 5 | 28.8 | M | DLBCL | Aggressive | 7 | PD | — | — | — |
| 6 | 62.7 | M | DLBCL | Aggressive | 7 | CR | OR | — | NT |
| 7 | 54.7 | M | DLBCL | Aggressive | 3 | PD | — | CRS | — |
| 8 | 28.6 | M | DLBCL | Aggressive | 2 | SD | — | — | — |
| 9 | 29.5 | M | PMBCL | Aggressive | 3 | SD | — | — | — |
| 10 | 40.4 | M | PMBCL | Aggressive | 2 | PD | — | — | NT |
| 11 | 67.8 | M | DLBCL | Aggressive | 3 | CR | OR | — | NT |
| 12 | 50.4 | M | MCL | Indolent | 1 | CR | OR | CRS | NT |
| 13 | 53.2 | M | DLBCL | Aggressive | 4 | CR | OR | CRS | NT |
| 14 | 67.0 | F | FL | Indolent | 3 | CR | OR | CRS | NT |
| 15 | 51.9 | M | DLBCL | Aggressive | 3 | CR | OR | CRS | NT |
| 16 | 52.0 | F | DLBCL | Aggressive | 5 | CR | OR | CRS | NT |
| 17 | 39.0 | M | DLBCL | Aggressive | 4 | PR | OR | CRS | NT |
| 18 | 67.1 | F | DLBCL | Aggressive | 4 | CR | OR | CRS | NT |
| 19 | 64.4 | M | DLBCL | Aggressive | 4 | CR | OR | CRS | NT |
| 20 | 52.0 | M | DLBCL | Aggressive | 5 | PD | — | CRS | — |
The table depicts major demographic characteristics of the evaluable patients.
BRESP, best response by Cheson et al11 criteria; CR, complete response; FL, follicular lymphoma; MCL, mantle cell lymphoma; PD, progressive disease; PMBCL, primary mediastinal B-cell lymphoma; PR, partial response; SD, stable disease.
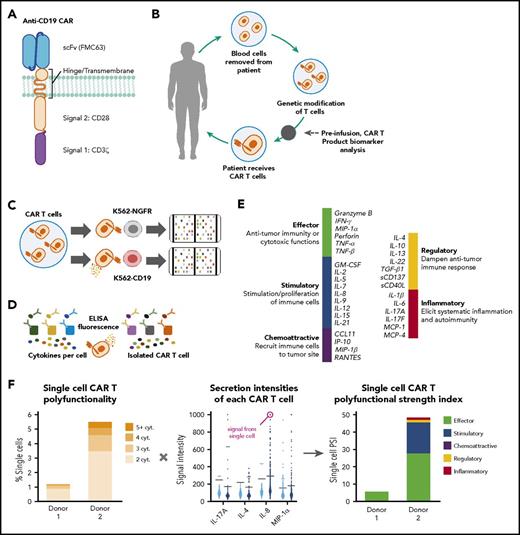
T-cell polyfunctionality evaluation by single-cell cytokine profiling and calculation of PSI
Cryopreserved CAR T-cell products comprising ∼40% to 60% CAR+ T cells, measured by surface expression of the single-chain variable fragment by flow cytometry, were thawed and cultured in complete Cell Therapy Systems (Thermo Fisher Cell Therapy Systems, Waltham, MA) medium with IL-2 (10 ng/mL; Biolegend, San Diego, CA) at a density of 1 × 106/mL in a 37°C, 5% CO2 incubator. After overnight recovery, viable T cells were enriched using Ficoll-Paque Plus medium (Fisher Scientific, Pittsburgh, PA). CD4+/CD8+ T-cell subsets were separated using anti-CD4 or anti-CD8 microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany) and stimulated with K562 cells transduced with either CD19 or nerve growth factor receptor (NGFR) at a ratio of 1:2 for 20 hours at 37°C, 5% CO2. The cocultured CD4+ or CD8+ T cells were further enriched using anti-CD19– or anti-NGFR–conjugated magnetic beads to deplete CD19-K562 or NGFR-K562 cells. Presence of CD4+ or CD8+ CAR T cells was confirmed by staining with anti-CD4 or anti-CD8 antibodies conjugated to Alexa Fluor 647 (Thermo Fisher Cell Therapy Systems) at room temperature for 10 minutes, rinsing once with phosphate-buffered saline, and resuspending in complete Cell Therapy Systems medium at a density of 1 × 106/mL. Approximately 30 µL of the cell suspension was loaded onto the single-cell barcode chip (SCBC) microchip9 for single-cell secretomic evaluation.
For each sample, a 32-plex assay measured secreted proteins from ∼2000 T cells (Figure 1). Raw microscopy and microarray scans of the cell samples (loaded onto the SCBC) and protein secretion data were analyzed using proprietary image processing software to determine the locations of chambers containing single cells and subsequent extraction of their secretion readouts. Data from empty cell chambers were used to measure background intensity levels for each analyzed protein. Single-cell readouts were then normalized using the background readouts to determine significant secretions and compare profiles across assays. Proprietary software and the R statistical package (R Foundation for Statistical Computing, Vienna, Austria) were used for statistical data analysis and visualizations.
Schematic representation of the method used to evaluate T-cell polyfunctionality. (A-B) Schematic representations of CAR construct configuration and treatment protocol. (C-D) Product T-cell polyfunctionality was assessed by using enzyme-linked immunosorbent assay (ELISA) detection of proteins from each single-cell chamber after T-cell stimulation. (E-F) Polyfunctionality was measured through a PSI, spanning a prespecified panel of 32 key immunologically relevant molecules across major categories: homeostatic/proliferative, inflammatory, chemotactic, regulatory, and immune effector. cyt, cytokine; MIP-1α, macrophage inflammatory protein 1α; scFv, single-chain variable fragment.
Schematic representation of the method used to evaluate T-cell polyfunctionality. (A-B) Schematic representations of CAR construct configuration and treatment protocol. (C-D) Product T-cell polyfunctionality was assessed by using enzyme-linked immunosorbent assay (ELISA) detection of proteins from each single-cell chamber after T-cell stimulation. (E-F) Polyfunctionality was measured through a PSI, spanning a prespecified panel of 32 key immunologically relevant molecules across major categories: homeostatic/proliferative, inflammatory, chemotactic, regulatory, and immune effector. cyt, cytokine; MIP-1α, macrophage inflammatory protein 1α; scFv, single-chain variable fragment.
A single-cell functional profile was determined for each evaluable product. Profiles were categorized into effector (granzyme B, IFN-γ, macrophage inflammatory protein [MIP]-1α, perforin, tumor necrosis factor [TNF]-α, TNF-β), stimulatory (granulocyte-macrophage colony-stimulating factor, IL-2, IL-5, IL-7, IL-8, IL-9, IL-12, IL-15, IL-21), regulatory (IL-4, IL-10, IL-13, IL-22, TGF-β1, sCD137, sCD40L), chemoattractive (CCL-11, IP-10, MIP-1β, RANTES), and inflammatory (IL-1B, IL-6, IL-17A, IL-17F, MCP-1, MCP-4) groups. Polyfunctional product CAR T cells were defined as cells cosecreting at least 2 proteins from the prespecified panel per cell coupled with the amount of each protein produced (ie, combination of the number of proteins secreted and degree of intensity). Cutoffs for any given cytokine were computed based on background levels from wells not containing cells plus 3 standard deviations. Knowing the functional profile of each cell enables the calculation of other metrics, including a breakdown of each sample according to cell polyfunctionality (ie, percentage of cells secreting multiple cytokines vs nonsecreting or monofunctional cells) and a breakdown of the sample by functional groups (ie, mono- and polyfunctional groups secreted by cells in the sample and level of frequency).
Furthermore, the PSI of each sample was computed using a prespecified formula,8 defined as the percentage of polyfunctional cells, multiplied by mean fluorescence intensity (MFI) of the proteins secreted by those cells: PSIsample = (% polyfunctional cells in sample)  MFI of secreted protein i of the polyfunctional cells.
MFI of secreted protein i of the polyfunctional cells.
The CD4+ and CD8+ PSIs were computed for corresponding CD19-K562 or NGFR-K562 stimulated samples from each donor. An overall PSI, (the average of CD4+ and CD8+ PSIs), was also computed.
The CD4+ PSI calculation was performed by analyzing all polyfunctional CD4+ cell readouts of the sample. Each readout was an n dimensional vector (n = number of analyzed cytokines) of signal intensities (MFI, random fluorescence intensity). The average signal intensity of each cytokine was found across the set of polyfunctional cells. Multiplying this number by the fraction of polyfunctional cells provided the individual cytokine PSI values (eg, IL-17A CD4+ PSI). The sum of all the individual cytokine PSI values provided the overall CD4+ PSI for the sample. The same calculation was performed to find the CD8+ PSI and the individual CD8+ PSI of each cytokine. Averaging CD4+ and CD8+ PSIs provided the overall PSI for each sample. We also calculated a monofunctional strength index, defined as the percentage of monofunctional cells multiplied by the secretion intensity of that cytokine.
Any notable statistically significant associations between preinfusion single-cell CAR T data and clinical outcome were determined, cognizant of the clinical outcome of these patients (OR, CRS, and NT). More specifically, associations between overall PSI, CD4+ PSI, CD8+ PSI, IL-17A CD4+ PSI, and clinical outcomes were determined. Mann-Whitney U tests were used to determine P values of these associations. Any associations between single-cell metrics (eg, PSI) and other pre- and postinfusion metrics described throughout this report (eg, in vivo CAR T-cell expansion, IL-15 day 0 patient levels, coculture and serum cytokine levels, T-cell phenotype frequencies) were determined by measuring the Spearman correlation between these metrics.
In some cases, 2 metrics were combined into a joint metric to test their association with patient outcome. The metrics were added to each other after each was first standardized to have unit variance. This standardization was achieved by dividing the metrics by their respective standard deviation to bring them to a common magnitude/scale. Therefore, when added to each other, the metrics carry a similar weight, and neither overpowers the other.
Measurement of in vivo CAR T-cell levels
CAR T-cell presence and expansion in blood were measured by quantitative polymerase chain reaction (PCR) as previously described.5
Measurement of cytokines in coculture and serum by multiplex analysis
Coculture experiments were performed using K562 cells engineered to express CD19 or NGFR (negative control) mixed 1:1 with CAR T-cell product. Cell culture medium was harvested 24 hours postincubation for subsequent analysis. Thirty-one analytes were evaluated by Meso Scale Discovery (Rockville, MD), MULTI-SPOT, and EMD Millipore (Burlington, MA) Luminex xMAP multiplex assays. Serum IL-15 was measured using EMD Millipore Luminex xMAP multiplex assays. Data acquisition and analysis were performed using a Luminex 200 instrument and xPONENT 3.1 data analysis software.
Evaluation of percentage of T17 and T regulatory cells by epigenetic analysis
Epigenetic analysis was performed based on previously characterized methods,12 at Epiontis GmbH (Berlin, Germany). Genomic DNA was isolated using the DNeasy tissue kit (Qiagen) following the protocol for cultured animal cells. Bisulfite treatment of genomic DNA was performed. PCR was performed in a final volume of 25 µL containing 1× PCR buffer, 1 U of Taq DNA polymerase (Qiagen), 200 µmol/L of 2′-deoxynucleoside 5′-triphosphate, 12.5 pmol each of forward and reverse primers, and 7 ng of bisulfite-treated genomic DNA at 95°C for 15 minutes, and 40 cycles of 95°C for 1 minute, 55°C for 45 seconds, and 72°C for 1 minute, and a final extension step of 10 minutes at 72°C. The PCR products were purified using ExoSAP-IT (USB Corp, Cleveland, OH) and sequenced by applying the PCR primers and the ABI Big Dye Terminator version 1.1 chemistry (Applied Biosystems) followed by capillary electrophoresis on an ABI 3100 genetic analyzer; epigenetic sequencing methylation analysis software was used to interpret AB1 files.
Determination of product T-cell phenotypes by flow cytometry
For CD4, CD8, and central memory phenotype determination, we used a method similar to that described previously.5 CAR+ CD3+ events were gated, and the percentage of cells expressing memory markers CCR7 and CD45RA was determined. Appropriate isotype control antibodies were used in all experiments. The memory antibodies used were anti-CD45RA (eBioscience, San Diego, CA; clone HI100) and anti-CCR7 (R&D Systems; clone 150503).
Gene expression analysis
Gene expression analysis was performed at NanoString Technologies (Seattle, WA). Cryopreserved cells were thawed and enumerated using a Vi-CELL automated cell counter (Beckman Coulter, Brea, CA). Live cell suspensions were used as input into the NanoString Vantage 3D RNA:Protein Immune Cell Profiling Panel (NanoString Technologies), a 770-plex gene and 30-plex protein expression panel that profiles the human immune response. For CD19-K562 or NGFR-K562 samples, 50 000 cells were used for RNA and 100 000 cells for protein. For K562 plus CAR T-cell cocultures, 150 000 cells were used for RNA and 200 000 cells for protein. For CAR T cells, 300 000 cells were used for RNA and 200 000 cells for protein. A custom gene expression panel of 191 additional immune- and metabolic-related markers, including probes specific for the anti-CD19 CAR, was run on cell lysates made for the RNA:Protein assay. Raw data were imported from the MAX digital analyzer into nSolver software v3.0 (NanoString Technologies). Standard quality control checks assessing imaging, binding density, positive control linearity, and limit of detection were performed. By utilizing internal positive controls in the panels, raw gene and protein expression data were first normalized. The messenger RNA (mRNA) data were further normalized by the geometric mean of a set of stably expressed reference genes and the protein data were normalized by the geometric mean of a set of stably expressed proteins. The mRNA and proteins expressed below background were filtered from the analysis using cutoffs of mean plus 2 standard deviations of negative controls (for mRNA) and 3 times the geometric mean of immunoglobulin G-negative controls (for protein).
Results
Preinfusion CAR T-cell product polyfunctional profiles are associated with clinical response and toxicities
The polyfunctional profiles of CAR product T cells stimulated with CD19+ cells were dominated by effector molecules, stimulatory/proliferative cytokines, and chemokines. Detailed evaluation including principal component analysis showed a complex and heterogeneous functional profile of product T cells, with both polyfunctional CD4+ and CD8+ T-cell subsets secreting predominantly IFN-γ, IL-8, IL-5, granzyme B, and/or MIP-1α (supplemental Figure 1, available on the Blood Web site). Notably, the CD4+ but not CD8+ T-cell population also contained IL-17A+ polyfunctional T cells.
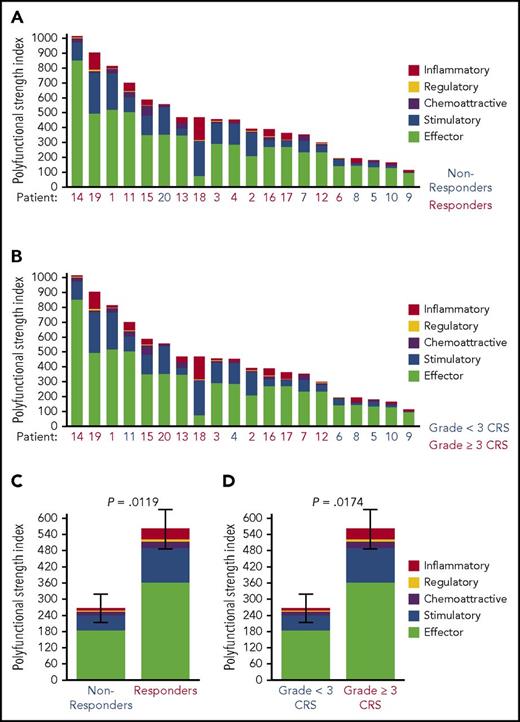
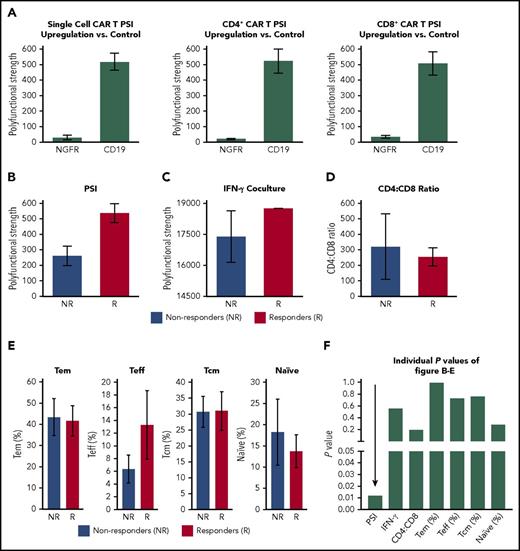
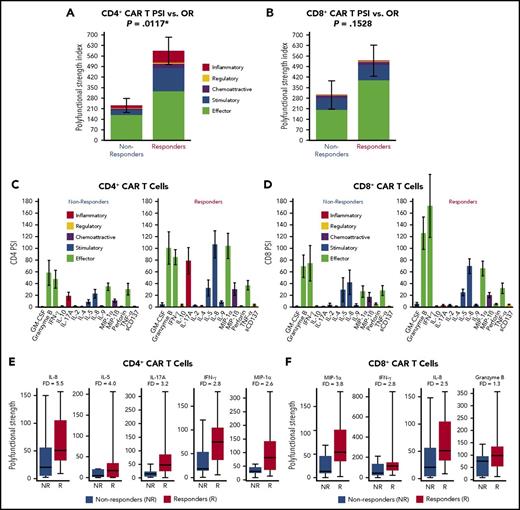
Overall, products displayed a wide range of PSI values; the polyfunctional cells comprised ∼20% to 25% of all cytokine-producing product T cells. Global product PSI was significantly associated with OR (P = .0119; Figure 2). The median PSI was twice as high for responders vs nonresponders. Higher product PSI was statistically associated with grade ≥3 CRS (P = .0174; Figure 2). In contrast to PSI findings, a similarly computed index corresponding to monofunctional T cells in the product did not have associations with OR, grade ≥3 NT, or grade ≥3 CRS (data not shown). In addition, in this study of limited sample size, major product phenotypes defined by flow cytometry and IFN-γ in coculture were not associated with clinical response (Figure 3). The major cytokines and chemokines produced by product polyfunctional T cells in the responding patients comprised IFN-γ, MIP-1α, IL-8 in both CD4 and CD8 T cells, granzyme B in CD8 T cells, and IL-17A and IL-5 in CD4 T cells (Figure 4). These results suggest that the combination of frequency and cytokine production levels of polyfunctional T cells in the product are associated with clinical response to and toxicity resulting from treatment with CAR T cells.
Association of PSI with OR and treatment-related adverse events. Single-cell product PSI was determined for 20 patient donors by using SCBC proteomic analysis of a panel of 32 secreted cytokines, chemokines, and cytotoxic molecules. Of the 20 patients, 14 had an OR to the CAR T-cell therapy. The products were ranked according to CAR T-cell PSI levels, and the PSI was associated with the OR (A,C) or grade ≥3 CRS (B,D) as indicated. The results are shown as patient-level PSI (A-B) and mean ± standard error PSI (C-D). All statistical values were computed using the Mann Whitney U test.
Association of PSI with OR and treatment-related adverse events. Single-cell product PSI was determined for 20 patient donors by using SCBC proteomic analysis of a panel of 32 secreted cytokines, chemokines, and cytotoxic molecules. Of the 20 patients, 14 had an OR to the CAR T-cell therapy. The products were ranked according to CAR T-cell PSI levels, and the PSI was associated with the OR (A,C) or grade ≥3 CRS (B,D) as indicated. The results are shown as patient-level PSI (A-B) and mean ± standard error PSI (C-D). All statistical values were computed using the Mann Whitney U test.
Association of CAR product polyfunctionality with CD19 recognition and clinical outcome. (A) PSI of CAR T cells and CD4+ or CD8+ subset, ex vivo stimulated with CD19+ as compared with CD19− cells (NGFR transfected). (B-E) Association between OR and product PSI, IFN-γ measured in product coculture with CD19+ cells, or major product T-cell subsets defined by flow cytometry. T naïve, central memory (cm), effector memory (em), and effector (eff) cells were defined by staining for CD45RA and CCR7. (F) Individual P values (Mann Whitney U test) corresponding to the strength of association of major product attributes with clinical response.
Association of CAR product polyfunctionality with CD19 recognition and clinical outcome. (A) PSI of CAR T cells and CD4+ or CD8+ subset, ex vivo stimulated with CD19+ as compared with CD19− cells (NGFR transfected). (B-E) Association between OR and product PSI, IFN-γ measured in product coculture with CD19+ cells, or major product T-cell subsets defined by flow cytometry. T naïve, central memory (cm), effector memory (em), and effector (eff) cells were defined by staining for CD45RA and CCR7. (F) Individual P values (Mann Whitney U test) corresponding to the strength of association of major product attributes with clinical response.
Major cytokines driving polyfunctional product CD4+and CD8+T cells by CD19 stimulation that distinguish responders to the therapy from nonresponders. (A-B) Single-cell proteomic analysis of a panel of 32 secreted cytokines, chemokines, and cytotoxic molecules was performed on product T cells from 20 patients treated with CAR T cells. The analysis was performed on all product cells or select CD4+ and CD8+ T cells. The product T cells were first stimulated with CD19-expressing target cells or control NGFR cells before the analysis. The graphs show PSI (mean ± standard error) with or without CD19 stimulation for all cells and for CD4+ and CD8+ subsets separately. The main cytokine drivers for each product T-cell subpopulation are also shown. (C-D) Product CD4+ and CD8+ T-cell PSI profiles were broken down per cytokine, between patient groups with no response and OR to CAR T-cell therapy. Only CD4 and CD8 cytokines that were upregulated relative to mock stimulation are shown. Each cytokine PSI level reflects its average secretion intensity in polyfunctional single cells. The diagram shows the cytokines that contribute to the polyfunctionality index in the CD8+ and CD4+ T-cell populations. (E-F) Polyfunctional strength of major cytokines and chemokines in CD4+ and CD8+ CAR T cells from nonresponders and responders. FD, fold difference; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Major cytokines driving polyfunctional product CD4+and CD8+T cells by CD19 stimulation that distinguish responders to the therapy from nonresponders. (A-B) Single-cell proteomic analysis of a panel of 32 secreted cytokines, chemokines, and cytotoxic molecules was performed on product T cells from 20 patients treated with CAR T cells. The analysis was performed on all product cells or select CD4+ and CD8+ T cells. The product T cells were first stimulated with CD19-expressing target cells or control NGFR cells before the analysis. The graphs show PSI (mean ± standard error) with or without CD19 stimulation for all cells and for CD4+ and CD8+ subsets separately. The main cytokine drivers for each product T-cell subpopulation are also shown. (C-D) Product CD4+ and CD8+ T-cell PSI profiles were broken down per cytokine, between patient groups with no response and OR to CAR T-cell therapy. Only CD4 and CD8 cytokines that were upregulated relative to mock stimulation are shown. Each cytokine PSI level reflects its average secretion intensity in polyfunctional single cells. The diagram shows the cytokines that contribute to the polyfunctionality index in the CD8+ and CD4+ T-cell populations. (E-F) Polyfunctional strength of major cytokines and chemokines in CD4+ and CD8+ CAR T cells from nonresponders and responders. FD, fold difference; GM-CSF, granulocyte-macrophage colony-stimulating factor.
Combined indices of product PSI and CAR T-cell expansion or pre–CAR T-cell infusion levels of IL-15 correlated strongly with clinical response and toxicity
After infusion, CAR T cells rapidly expand, with peak levels occurring within the first 7 to 14 days. CAR T-cell expansion, measured as peak and cumulative levels during the first month after infusion, is associated with OR and grade ≥3 NT, but not grade ≥3 CRS.6
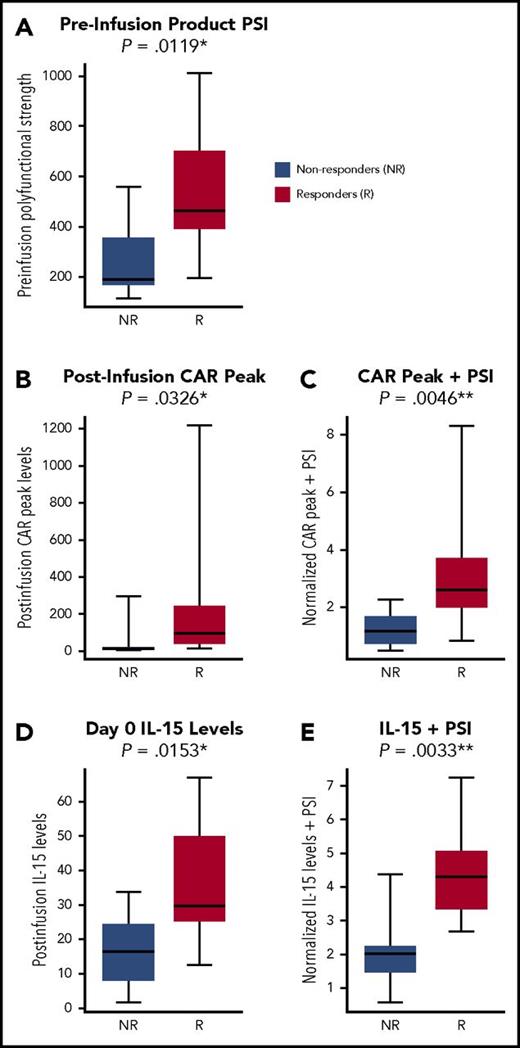
Because both CAR T-cell expansion in vivo and product PSI individually correlated with OR, we tested the impact of a composite index integrating PSI and CAR T-cell expansion on the correlation with OR. This index was computed by adding the 2 metrics to each other, after first standardizing them to have comparable variances (described in “Methods”). This composite index combining PSI and CAR T-cell expansion improved the association with OR (P = .0046; not adjusted for multiplicity) compared with each covariate alone (Figure 5). Furthermore, OR was associated with CD4+ PSI plus CAR peak levels (P = .0023), but only marginally with CD8+ PSI plus CAR peak levels (P = .0507; supplemental Figure 2). There was no apparent association between CAR peak levels and PSI (supplemental Figure 3). These results suggest that PSI in conjunction with CAR T-cell expansion may be associated with clinical outcome of CAR T-cell therapy.
PSI in conjunction with CAR T-cell expansion in vivo or in conjunction with conditioning-driven IL-15 pre–CAR T-cell infusion correlates with OR. CAR T-cell levels in blood measured by quantitative PCR were correlated with clinical outcome. A composite index integrating PSI and CAR T-cell expansion in vivo was developed as detailed in the supplemental Methods and was associated with OR. The metrics were added to each other after each was first standardized to have unit variance. This standardization was achieved by dividing the metrics by their respective standard deviation to bring them to a common magnitude/scale. Whole product PSI alone (A), peak CAR levels in blood alone (B), and product PSI in conjunction with peak CAR levels (C) are shown in association with OR. Pre–CAR T-cell infusion (day 0) IL-15 serum levels alone (D) or in conjunction with product PSI (E) are shown in association with OR. Statistical values were computed using the Mann Whitney U test (P values were not adjusted for multiplicity).
PSI in conjunction with CAR T-cell expansion in vivo or in conjunction with conditioning-driven IL-15 pre–CAR T-cell infusion correlates with OR. CAR T-cell levels in blood measured by quantitative PCR were correlated with clinical outcome. A composite index integrating PSI and CAR T-cell expansion in vivo was developed as detailed in the supplemental Methods and was associated with OR. The metrics were added to each other after each was first standardized to have unit variance. This standardization was achieved by dividing the metrics by their respective standard deviation to bring them to a common magnitude/scale. Whole product PSI alone (A), peak CAR levels in blood alone (B), and product PSI in conjunction with peak CAR levels (C) are shown in association with OR. Pre–CAR T-cell infusion (day 0) IL-15 serum levels alone (D) or in conjunction with product PSI (E) are shown in association with OR. Statistical values were computed using the Mann Whitney U test (P values were not adjusted for multiplicity).
Because PSI was not significantly associated with commonly assessed product phenotypes based on CCR7 or CD45RA expression (Figure 3), we sought to identify other potential product characteristics associated with PSI. We hypothesized that the anti-CD19 CAR construct enabled the polyfunctionality of product T cells. Therefore, CAR gene expression levels were measured by NanoString (Seattle, WA) in bulk product together with T-cell housekeeping molecules. Global PSI was associated with the ratio between CAR mRNA copies and T-cell–specific markers (supplemental Table 1). This finding suggests that product CAR expression level influences product T-cell polyfunctionality.
In contrast to PSI alone, combining product PSI or CD4+ PSI with CAR peak levels was significantly associated with grade ≥3 NT (supplemental Figure 4A). As specified in “Methods,” CD4+ and CD8+ PSIs were calculated for each product by applying the same prespecified formula to the 2-product T-cell subsets separately. On the basis of these findings and the putative pathogenic role of T helper 17 (Th17) cells,13,14 we asked whether subsets of polyfunctional T cells producing cytokines including IL-17A were associated with grade ≥3 NT. Cytokine-specific PSI was computed by multiplying the percentage of polyfunctional T cells secreting a given cytokine with the average signal intensity for that cytokine. These cytokine PSI values were analyzed in relation to outcome. Interestingly, IL-17A PSI, defined post hoc, plus CAR peak cell levels had a significant association with grade ≥3 NT (P = .0007; supplemental Figure 4A). Individually, CAR peak cell levels and IL-17A PSI were associated with grade ≥3 NT (P = .0015 and P = .0574, respectively). To strengthen these findings, we explored other product attributes that may relate to IL-17A PSI. As expected, IL-17A PSI correlated strongly with the percentage of Th17 cells measured by epigenetic marking and IL-17A production in product coculture (P < .0001 for both comparisons) and was associated with the CD4/CD8 ratio, percentage of Th cells, and IL-6 levels in product coculture (supplemental Table 2). In turn, grade ≥3 CRS was associated with the global product PSI plus CAR peak levels (supplemental Figure 4B).
Because IL-15 levels initially elevated by conditioning chemotherapy were associated with CAR T-cell expansion and OR,6 we investigated whether pre–CAR T-cell infusion levels of IL-15, in combination with PSI, were associated with clinical outcome. Indeed, product PSI combined with IL-15 levels at day 0 had a statistically significant association with OR (P = .0033). OR also was associated with PSI (P = .0119) and IL-15 (P = .0153) individually (Figure 5). However, PSI and IL-15 on day 0 did not have a statistically significant association with each other. IL-15 on day 0 in conjunction with CD4+ PSI, or with IL-17A PSI, was also strongly associated with OR (P < .0004; supplemental Figure 2). Finally, day 0 levels of IL-15 and IL-17A PSIs were associated with grade ≥3 NT and CRS (supplemental Figure 5).
These results show that product PSI, together with CAR T-cell expansion or pre–CAR T- cell infusion levels of IL-15, contributes jointly to clinical outcomes after CAR T-cell therapy. These findings also highlight the prominent role of IL-17A polyfunctional T cells in clinical outcomes, particularly NT, associated with CAR T-cell therapy.
Discussion
We describe a novel product attribute for CAR T cells associated with T-cell polyfunctionality and correlated with clinical outcome. This polyfunctionality index, combined with conditioning-driven IL-15, a cytokine with potent T-cell proliferative capabilities,15,16 or CAR T-cell expansion in vivo,6,17-19 is associated with clinical outcomes post–CAR T-cell therapy.
The study comprised a cohort of patients with aggressive refractory non-Hodgkin lymphoma treated with CAR T cells.6 In response to ex vivo coculture with CD19+ target cells, product T cells rapidly secrete a broad array of cytokines, chemokines, and immune effector molecules. These cytokines and chemokines, as well as T-cell characteristics defined by commonly used phenotypic markers in a heterogeneous population of T cells, were not significantly associated with clinical outcomes (data not shown). To understand the parameters that are associated with clinical outcome in anti-CD19 CAR T-cell therapy, we characterized product T cells at the single-cell level using PSI8 based on the frequency and production levels of homeostatic/proliferative, inflammatory, chemotactic, regulatory, and immune effector molecules. To perform this analysis, an SCBC platform and a prespecified formula were used. In brief, product PSI was defined as the percentage of polyfunctional cells multiplied by MFI of the cytokines secreted by those cells (Figure 1; details in “Methods”).
This analysis showed that polyfunctional profiles for both CD4+ and CD8+ T cells stimulated with CD19+ target cells were composed of select effector molecules (granzyme B), stimulatory/immune-modulating cytokines (IFN-γ, IL-5), and chemokines (IL-8, MIP-1α). In contrast to CD8+ T cells, the CD4+ T-cell subset also comprised IL-17A–secreting polyfunctional cells. The diversity and coparticipation of CD4+ and CD8+ T cells with regard to the polyfunctional T-cell population are consistent with a CAR product containing a CD28 costimulatory domain, which is known to play a signaling role in both subsets.4 Strikingly, only 20% to 25% of all product cells were polyfunctional upon stimulation with CD19-expressing target cells.
The major finding of this study is that clinical response and toxicities resulting from CAR T-cell treatment were associated with the PSI of whole product, or defined T-cell subpopulations, computed using a prespecified algorithm. Patient 20 is a notable outlier, because this patient had a high product PSI but did not achieve an OR (Figure 2A). In this patient, CAR T cells did not expand well. We hypothesize multiple possible reasons, including limited T-cell proliferative capability and/or an immune-exclusionary tumor environment.
Because both CAR T-cell expansion in vivo and product PSI individually correlated with OR, we explored the impact of a composite index integrating PSI and CAR T-cell expansion and association with OR. Similarly, we combined day 0 IL-15 serum levels with product PSI. Combined indices of PSI with either peak CAR T-cell levels in blood or with day 0 IL-15 serum levels had more significant associations with clinical outcomes vs either parameter alone. Day 0 IL-15 serum levels, elevated by conditioning chemotherapy,6 were measured just before CAR T-cell infusion. Although the number of evaluable patients in this cohort was limited, OR was associated with both CD4+ PSI plus day 0 IL-15 and IL-17A PSI plus day 0 IL-15. IL-17A PSI was determined using a formula-specified post hoc analysis to address the hypothesis that an IL-17A–producing T-cell subset may be preferentially associated with outcomes. CD4+ T cells producing IL-17A and IFN-γ can be potent Th cells, with the capability of reprogramming the tumor microenvironment and enabling robust, effective immune-mediated responses.20 In addition, IL-8 and MIP-1α, coproduced by these polyfunctional Th cells, are well-known chemokines for a wide range of immune cells, including lymphocytes and myeloid cells.20-22 Although the results point to specialized polyfunctional CD4+ T cells correlating with clinical response to a greater degree than CD8+ T cells, they do not exclude a role for effector CD8+ T cells, consistent with reports of CD8+ T-cell effectiveness in metastatic melanoma.23
Grade ≥3 NT had the most statistically significant association with IL-17A PSI plus peak CAR levels in blood, suggesting the participation of specialized T cells. Grade ≥3 CRS correlated with whole product PSI plus either CAR peak levels or day 0 serum IL-15 levels. These findings point to mechanisms underlying serious adverse events. The best correlates of grade ≥3 NT were peak CAR levels in blood and an index combining peak CAR levels in blood plus IL-17A PSI. These results raise the possibility that high numbers of IL-17A–producing polyfunctional CAR T (Th17) cells in vivo could be implicated in the pathogenesis of serious NT, while also contributing to OR. Previous evidence implicated IL-17A in the pathogenesis of neuroinflammation13,14 and participation in antitumor activity.24 Although global product PSI was not associated with major T-cell phenotypic markers, it was associated with the level of CAR gene expression in product T cells.
Polyfunctional T cells have not been extensively evaluated in the context of engineered T-cell therapies, but they were previously analyzed in vaccines and infectious diseases, where their presence was associated with increased protective immunity.7,25 Additionally, presence and characterization of polyfunctional T cells were reported recently in CAR T cells generated from healthy individuals.26
Concurrent studies conducted with larger patient cohorts in conjunction with platform optimization and comprehensive validation of this methodology are anticipated to enable a better understanding of single-cell metrics over other technologies.
In summary, this exploratory study underscores the potential usefulness of monitoring CAR T-cell polyfunctionality as a key product attribute, complementing other characteristics including T-cell proliferative capability. Nevertheless, other key questions remain, such as whether polyfunctionality is partially predetermined by T-cell status before manufacturing, and whether polyfunctionality and clinical outcome could be manipulated through optimizing T-cell genetic programming, the manufacturing process, or dosing strategy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Allen Xue of Kite for performing a formal review of the statistical analysis and methods used in this report.
This study was funded by Kite, a Gilead Company.
Authorship
Contribution: J.R. helped design the study and performed experiments, analysis, and data interpretation; P.P. helped to design the study, performed statistical analysis and visualizations of the presented data, developed the software suite for single-cell data acquisition and analysis, and assisted with writing the manuscript; K.M., B.F., and A.K. performed all single-cell experiments, data acquisition, and validation experiments; Y.-W.S. contributed to product analysis; C.N. and T.C. managed and assisted with execution of single-cell analysis processes, validation processes, and additional related biological procedures; K.G. performed single-cell data acquisition and assisted with statistical analysis; R.F and J.R.H. contributed to the discussion and analysis of the project results and assisted with writing the manuscript; S.M. supervised the project and contributed to the overall analysis and interpretation and the writing of the manuscript; S.A.R. and J.N.K. were the investigators of the clinical study, made intellectual contributions, and shared materials and biospecimens; J.Z. developed the single-cell cytokine profiling experimental protocol, managed the execution of the experiments, and contributed to the data analysis and writing of the manuscript; A.B. oversaw the study and the drafting of the manuscript; and all authors reviewed and provided final approval of the manuscript.
Conflict-of-interest disclosure: J.R., Y.-W.S., and A.B. are employed by Kite, a Gilead Company, and have equity ownership in Gilead Sciences, Inc.; P.P. is employed by, has equity ownership in, and is a patent holder with IsoPlexis; K.M., B.F., A.K., T.C., and J.Z. are employed by and have equity ownership in IsoPlexis; C.N. and K.G. are employed by IsoPlexis; R.F. is cofounder and scientific advisor of IsoPlexis; S.M. is employed by, has equity ownership in, and is a patent holder with IsoPlexis and received honoraria from Kite, a Gilead Company; J.R.H. is a founder and board member of IsoPlexis; S.A.R. received research funding from Kite, a Gilead Company, and is a patent holder with National Cancer Institute Cooperative Research and Development Agreement/Kite; and J.N.K. received research funding from Kite, a Gilead Company and BlueBird Bio, is a patent holder with Kite, a Gilead Company BlueBird Bio, and Novartis, and has received travel expenses from Kite, a Gilead Company, and Celgene Corporation.
Correspondence: Adrian Bot, Kite, a Gilead Company, 2225 Colorado Ave, Santa Monica, CA 90404; e-mail: abot@kitepharma.com.
References
Author notes
J.R. and P.P. are joint lead authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal