Key Points
The incidence of venous thromboembolism is high in patients with a solid tumor and implanted port in the real-life practice setting.
The risk factors for catheter-related thrombosis differ from those for venous thromboembolism unrelated to the catheter.
Abstract
The need to accurately identify cancer outpatients at high risk of thrombotic complications is still unmet. In a prospective, multicenter cohort study (ONCOlogie et Chambres ImPlantables [ONCOCIP]), consecutive adult patients with a solid tumor and implanted port underwent 12-month follow-up. Our primary objective was to identify risk factors for (1) catheter-related thrombosis, defined as ipsilateral symptomatic upper-limb deep-vein thrombosis with or without pulmonary embolism, and (2) venous thromboembolism other than catheter-related, defined as any symptomatic superficial- or deep-vein thrombosis (other than catheter-related) or pulmonary embolism, and incidental pulmonary embolism. All events were objectively confirmed and centrally adjudicated. Rate assessments integrated competing risk of death. Overall, 3032 patients were included (median age: 63 years; women: 58%). The most frequent cancer locations were breast (33.7%), lung (18.5%), and colorectal (15.6%), cancer being metastatic in 43.2% of patients. Most patients (97.1%) received chemotherapy. By 12 months, 48 (1.6%) patients had been lost to follow-up and 656 (24.6%) had died; 3.8% (n = 111) of patients had experienced catheter-related thrombosis, and 9.6% (n = 276) venous thromboembolism other than catheter-related. By multivariate analysis, use of cephalic vein for catheter insertion predicted catheter-related thrombosis, whereas ongoing antiplatelet therapy was protective; risk factors for venous thromboembolism other than catheter-related were advanced age, previous venous thromboembolism, cancer site, and low hemoglobin level or increased leukocyte count before chemotherapy. In conclusion, this large prospective cohort study showed a high rate of venous thromboembolism in patients with a solid tumor and implanted port. Risk factors for catheter-related thrombosis differed from those for venous thromboembolism not catheter-related. This trial was registered at www.clinicaltrials.gov as #NCT02025894.
Introduction
Cancer-associated thrombosis in ambulatory patients with solid tumors represents a considerable burden in terms of mortality, morbidity, and cost.1-6 Cancer is associated with a hypercoagulable state, including specific procoagulant activities of cancer cells, decreased levels of coagulation inhibitors, impaired fibrinolysis, increased antiphospholipid antibodies, activated protein C-resistance, enhanced platelet aggregation, and interactions between different factors of coagulation and inflammation.7 Besides cancer-related variables (eg, site and stage) and patient-related factors, specific conditions associated with cancer such as stasis because of immobilization, surgery, infections, and use of prothrombotic chemotherapeutic agents may contribute to this hypercoagulable state.8-10 Long-term central venous catheters, including those involving port implantation, are increasingly used with the expansion of the population of cancer patients who require chemotherapy and intravenous administration of supportive care treatments, but their placement may be complicated by thrombotic events, in particular upper extremity deep-vein thrombosis owing to direct vascular damage.11-13 Specific antineoplastic agents infused through implanted ports may additionally influence the risk of catheter-related thrombosis.
Yet current guidelines all advise against routine primary thromboprophylaxis in such patients,1-6 including those with central venous catheters.2,5,11,12,14,15 Reasons for this include the wide variation in thrombotic risk among cancer patients, the annual incidence of events ranging from 0.5% to 20%,16 and results of thromboprophylactic trials showing only a small absolute benefit17,18 or inconclusive results.19,20
Because some cancer outpatients might benefit from primary thromboprophylaxis, guidelines emphasize the need to identify patients at high risk of thrombotic events.1-6,11,12,14,15 However, data regarding risk factors for catheter-related thrombosis are scarce.21 Moreover, although various risk assessment models have been developed to identify patients at high risk for venous thromboembolism,22-24 one being widely used,22 their positive predictive value remains limited.25,26 More accurate risk stratification, based on further cohort and intervention studies, is still considered a research priority.25-27
We therefore conducted a prospective, multicenter, observational cohort study of consecutive ambulatory adult patients with a solid tumor requiring port implantation who were followed for 12 months. Our primary objective was to identify, in a real-life practice setting, risk factors for catheter-related thrombosis, as well as risk factors for venous thromboembolism unrelated to the catheter.
Methods
Study design
The ONCOCIP (ONCOlogie et Chambres ImPlantables) study was a prospective, multicenter, observational, French cohort study conducted in accordance with the ethical principles stated in the Declaration of Helsinki, Good Clinical Practice, and relevant French regulations regarding ethics and data protection. The protocol and amendments were approved by a central independent Ethics Committee, and written informed consent was obtained from all patients before inclusion. The trial was registered at www.clinicaltrials.gov number as #NCT02025894.
Patients
Consecutive ambulatory patients aged 18 years or over were potentially eligible for the study if they presented a solid tumor and had undergone port implantation, whether at inclusion or within 1 month prior to inclusion. The main exclusion criteria were the following: hematologic malignancies (including lymphoma), insertion of a central venous catheter via the femoral vein, temporary port implantation (removal planned within the subsequent 2 weeks), indication for curative anticoagulant treatment, and inability to attend follow-up visits.
Study procedures and data collection
Before inclusion, all participating patients were informed of the study aims and procedures. Patients were followed through face-to-face visits every month for 6 months, and every 3 months thereafter, up to 12 months. They were instructed to report to the study center immediately in the event of any symptoms or signs, particularly those suggestive of a thromboembolic event, infection or local port-related complication. The port could be replaced at any time as deemed necessary by the investigator. For each patient, the study ended (1) when the port was definitively removed, (2) at the planned 12-month visit, or (3) in the event of death.
All medical and surgical procedures were performed according to each center’s usual practice, except that the study protocol recommended that the catheter tip position be verified radiologically just after insertion. No routine examinations to screen for thromboembolic complications were required during follow-up.
Each patient’s data were recorded in a dedicated case report form, including (1) at baseline: demographic and cancer characteristics, medical history, risk factors for venous thromboembolism (including well-known risk factors in general and those already documented in cancer patients), ongoing treatments, and details regarding port implantation; and (2) at each visit: changes in treatments received, port local tolerability and patency, and clinical events (thromboembolic events, infections, or local complications). All centers were regularly monitored by a clinical research assistant, who reviewed the data entry in the case report form and collected source documents.
Study objectives and thrombotic event definitions
The primary objective of the study was to identify risk factors for catheter-related thrombosis, as well as risk factors for venous thromboembolism unrelated to the catheter. Secondary objectives included determination of the incidence and nature of thrombotic events observed in the study population.
Catheter-related thrombosis was defined as the composite of symptomatic upper-extremity deep-vein thrombosis occurring in the clinical and anatomic setting of the central venous catheter14 or symptomatic pulmonary embolism associated with upper-extremity deep-vein thrombosis. Upper-extremity deep-vein thrombosis was confirmed by ultrasonography, venography, or tomodensitometry. Symptomatic pulmonary embolism was confirmed by computed tomography pulmonary angiography, ventilation–perfusion scanning, autopsy in the event of death, or, in the absence of autopsy, if no other cause could be identified to explain sudden death. If symptomatic pulmonary embolism was objectively confirmed, an ultrasonographic examination of the upper limb ipsilateral to the catheter was to be performed, and pulmonary embolism was considered as catheter-related if an associated upper-limb venous thrombosis was confirmed ipsilateral to the catheter. Isolated catheter occlusions were not included in the primary outcome.
Venous thromboembolism unrelated to the catheter was defined as the composite of any symptomatic upper- (other than catheter-related) or lower-extremity deep- or superficial-vein thrombosis (confirmed by ultrasonography, venography, or tomodensitometry), symptomatic pulmonary embolism (confirmed as described previously), any other symptomatic venous thrombotic event confirmed by an objective test (eg, renal, splanchnic, or cerebral venous thrombosis), and incidental pulmonary embolism. Incidental pulmonary embolisms were those discovered on scans performed for reasons other than suspected pulmonary embolism and were included in the composite outcome, because guidelines acknowledge a similarly poor prognosis as that associated with symptomatic events and thus recommend similar treatment.28
Symptomatic arterial thrombotic events were also recorded and included acute myocardial infarction (confirmed by a combination of ischemic symptoms, electrocardiogram changes, and biomarker samples), stroke or transient ischemic attacks (confirmed by tomodensitometry or magnetic resonance imaging), and acute lower-limb ischemia (confirmed by arteriography).
All thromboembolic events and deaths were adjudicated by an independent central critical event committee.
Statistical analysis
The sample size was calculated to allow determination of independent risk factors for venous thromboembolic events in a real-life patient cohort. At the time the study was designed, the most recently published rate of such events was 3.4% without thromboprophylaxis.18 Our aim was to detect any risk factor with a prevalence of at least 20% and associated with a relative risk of 2.5, corresponding approximately to an expected incidence of venous thromboembolic complications of 2.5% in unexposed patients and 6.0% in exposed patients. Based on these considerations, it was calculated using EpiTools epidemiological calculators (available at http://epitools.ausvet.com.au/) that a sample size of 3000 patients would have a power of 90% to detect such risk factors with a 5% type-I error.
Analyses were performed on all included patients with available data. Quantitative variables were presented as the number of cases, mean (± standard deviation) and median (interquartile range) where appropriate. Qualitative variables were presented as the number of cases and percentages, with 95% confidence intervals (CIs) where appropriate. Time to death was estimated by the Kaplan-Meier method, and time-to-event thrombotic outcomes were estimated by competitive risk analysis using the Kalbfleisch and Prentice method,29 accounting for death as a competing risk with thromboembolic events.30-32 Patients were censored at their last available follow-up. Cumulative incidences and corresponding 95% CIs were calculated.
Univariate analysis was performed to screen for potential variables associated with catheter-related thrombosis and venous thromboembolism other than catheter-related at 12 months. We included well-known risk factors for thromboembolic complications on the basis of previous epidemiological studies and expert clinical opinion (see supplemental Data; available on the Blood Web site). Variables with a prevalence of >3% and a P value of at least .20 were considered for inclusion in the final multiple analyses. A bivariate correlation matrix was used to detect relationships between all variables that could potentially be introduced in the multivariate model. If 2 variables were found to be significantly (P > .6) correlated, the choice of the variable to be introduced in the multivariate model was based on clinical judgement. A multivariate stepwise proportional hazards model was built using the Fine and Gray method, taking into account competing risks.33 Hazard ratios and corresponding 95% CIs were calculated. Factors with a P value <.05 in the multivariate analysis were considered as independent risk factors.
Data were processed and analyzed using SAS-WINDOWS software version 9.4 (SAS Institute Inc., Cary, NC).
Role of the funding source
ONCOCIP was supported by unrestricted grants from the Ligue Nationale Contre le Cancer, Paris, France, and from Bayer Healthcare, Loos, France, as well as by regional public funding from the Plateforme Régionale d’Aide à la Recherche Clinique, Auvergne et Rhône-Alpes, supported by the Ligue Nationale Contre le Cancer, Lyon, France, and from the Région Rhône-Alpes (CIBLE 2009), Saint-Etienne, France. The sponsor was a public cancer hospital, the Institut de Cancérologie Lucien Neuwirth, Saint-Priest en Jarez, France. The funding sources were not involved in designing or conducting the study, collecting, managing, analyzing, or interpreting the data; preparing, reviewing, or approving the manuscript; or deciding to submit this for publication. An academic steering committee assumed overall responsibility for all these steps. Data were collected and analyzed by the Centre d’Investigation Clinique CIC 1408, Saint-Etienne, France. The database (including adjudicated outcomes) was managed by the Centre d’Investigation Clinique CIC 1408, Saint-Etienne, France. All authors contributed to designing the study, had full access to the data and analyses, contributed to interpreting the data and developing the report, agreed on the final version of the manuscript, and had final responsibility for the report content and the decision to publish the findings.
Results
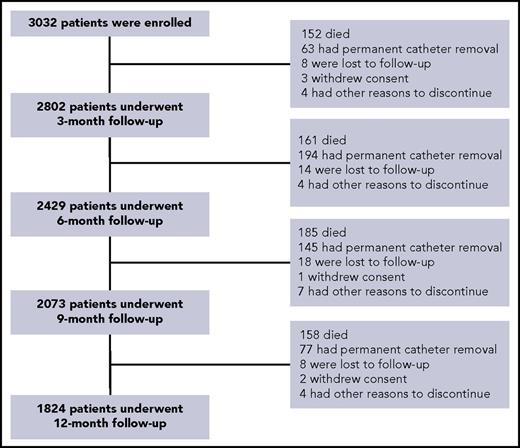
Between June 2010 and February 2014, 3032 patients were enrolled in 29 French centers. A total of 1208 (39.8%) discontinued the study prematurely, the main reasons being death (n = 656; Kaplan-Meier estimate: 24.6%) and definitive port removal (n = 479); by 12 months, 48 (1.6%) patients had been lost to follow-up (Figure 1; supplemental Tables 1-3).
The median age of the study population was 63 years, 58% of patients being women, and the Eastern Cooperative Oncology Group performance status at inclusion was rated as 0 or 1 in 91.0% of patients (Table 1). The most frequent cancer locations were breast (33.7%), lung (18.5%), and colorectal (15.6%), cancer being an adenocarcinoma in 77.9% of patients and metastatic in 43.2% (Table 2). Most patients (97.1%) received chemotherapy, surgical tumor resection being performed in 44.5% (Table 2; supplemental Table 4).
Baseline characteristics of the study patients
| . | Total (N = 3032) . |
|---|---|
| Age, y* | 61.8 ± 12.2 |
| >70 y, no. (%) | 795 (26.2) |
| Female, no. (%) | 1751 (57.8) |
| Body-mass index, kg/m2* | 24.7 ± 5.0 |
| ≤18 kg/m2, no. (%) | 128 (4.2) |
| ≥30 kg/m2, no. (%) | 405 (13.4) |
| Eastern Cooperative Oncology Group performance status, no. (%)† | |
| 0 | 1642 (58.1) |
| 1 | 930 (32.9) |
| 2 | 227 (8.0) |
| 3 | 28 (1.0) |
| 4 or 5 | 0 (0.0) |
| Medical history, no. (%)‡ | |
| Previous thromboembolism | 448 (14.8) |
| Pulmonary embolism | 42 (1.4) |
| Deep-vein thrombosis | 129 (4.3) |
| Superficial-vein thrombosis | 54 (1.8) |
| Myocardial infarction | 104 (3.4) |
| Acute lower-limb ischemia | 114 (3.8) |
| Stroke or transient ischemic attack | 81 (2.7) |
| Previous implanted port | 231 (7.6) |
| Diabetes | 346 (11.4) |
| Ongoing medications at the time of inclusion | |
| Immunosuppressive therapy | 19 (0.6) |
| Corticosteroids | 161 (5.3) |
| Statins | 515 (17.0) |
| Erythropoietin | 13 (0.4) |
| Hormone therapy§ | 57 (1.9) |
| Anticoagulant agents at prophylactic dose|| | 247 (8.1) |
| Antiplatelet agents | 458 (15.1) |
| . | Total (N = 3032) . |
|---|---|
| Age, y* | 61.8 ± 12.2 |
| >70 y, no. (%) | 795 (26.2) |
| Female, no. (%) | 1751 (57.8) |
| Body-mass index, kg/m2* | 24.7 ± 5.0 |
| ≤18 kg/m2, no. (%) | 128 (4.2) |
| ≥30 kg/m2, no. (%) | 405 (13.4) |
| Eastern Cooperative Oncology Group performance status, no. (%)† | |
| 0 | 1642 (58.1) |
| 1 | 930 (32.9) |
| 2 | 227 (8.0) |
| 3 | 28 (1.0) |
| 4 or 5 | 0 (0.0) |
| Medical history, no. (%)‡ | |
| Previous thromboembolism | 448 (14.8) |
| Pulmonary embolism | 42 (1.4) |
| Deep-vein thrombosis | 129 (4.3) |
| Superficial-vein thrombosis | 54 (1.8) |
| Myocardial infarction | 104 (3.4) |
| Acute lower-limb ischemia | 114 (3.8) |
| Stroke or transient ischemic attack | 81 (2.7) |
| Previous implanted port | 231 (7.6) |
| Diabetes | 346 (11.4) |
| Ongoing medications at the time of inclusion | |
| Immunosuppressive therapy | 19 (0.6) |
| Corticosteroids | 161 (5.3) |
| Statins | 515 (17.0) |
| Erythropoietin | 13 (0.4) |
| Hormone therapy§ | 57 (1.9) |
| Anticoagulant agents at prophylactic dose|| | 247 (8.1) |
| Antiplatelet agents | 458 (15.1) |
Plus-minus values are means ± standard deviation.
Data were missing for 205 patients.
Some patients had experienced >1 event.
Estrogen-containing hormone replacement therapy/oral contraception.
Mainly low-molecular-weight heparin (in 207 patients).
Cancer characteristics and treatments initiated at inclusion
| . | N = 3032, no. (%) . |
|---|---|
| Site of the primary cancer | |
| Breast | 1023 (33.7) |
| Lung | 560 (18.5) |
| Colorectal | 472 (15.6) |
| Upper aerodigestive tract | 168 (5.5) |
| Pancreas | 162 (5.3) |
| Prostate | 108 (3.6) |
| Gastric | 103 (3.4) |
| Ovarian or peritoneal | 98 (3.2) |
| Bladder or urinary tract | 92 (3.0) |
| Liver or biliary tract | 65 (2.1) |
| Uterus | 64 (2.1) |
| Testis | 25 (0.8) |
| Kidney | 24 (0.8) |
| Brain | 9 (0.3) |
| Bone | 7 (0.2) |
| Other | 21 (0.7) |
| Missing | 31 (1.0) |
| Cancer histological subtype | |
| Adenocarcinoma | 2362 (77.9) |
| Squamous cell carcinoma | 305 (10.1) |
| Sarcoma | 32 (1.1) |
| Other | 333 (11.0) |
| Metastasis | 1310 (43.2) |
| Mediastinal, thoracic, or cervical lymph adenopathies | 792 (26.1) |
| Treatments initiated at inclusion* | |
| Surgical tumor resection | 1350 (44.5) |
| Chemotherapy (conventional or targeted) | 2921 (97.1) |
| Growth factors | 910 (30.1) |
| Radiotherapy | 239 (7.9) |
| Antiangiogenic agents | 231 (7.6) |
| Hormone therapy | 43 (1.4) |
| Parenteral nutrition | 89 (3.0) |
| . | N = 3032, no. (%) . |
|---|---|
| Site of the primary cancer | |
| Breast | 1023 (33.7) |
| Lung | 560 (18.5) |
| Colorectal | 472 (15.6) |
| Upper aerodigestive tract | 168 (5.5) |
| Pancreas | 162 (5.3) |
| Prostate | 108 (3.6) |
| Gastric | 103 (3.4) |
| Ovarian or peritoneal | 98 (3.2) |
| Bladder or urinary tract | 92 (3.0) |
| Liver or biliary tract | 65 (2.1) |
| Uterus | 64 (2.1) |
| Testis | 25 (0.8) |
| Kidney | 24 (0.8) |
| Brain | 9 (0.3) |
| Bone | 7 (0.2) |
| Other | 21 (0.7) |
| Missing | 31 (1.0) |
| Cancer histological subtype | |
| Adenocarcinoma | 2362 (77.9) |
| Squamous cell carcinoma | 305 (10.1) |
| Sarcoma | 32 (1.1) |
| Other | 333 (11.0) |
| Metastasis | 1310 (43.2) |
| Mediastinal, thoracic, or cervical lymph adenopathies | 792 (26.1) |
| Treatments initiated at inclusion* | |
| Surgical tumor resection | 1350 (44.5) |
| Chemotherapy (conventional or targeted) | 2921 (97.1) |
| Growth factors | 910 (30.1) |
| Radiotherapy | 239 (7.9) |
| Antiangiogenic agents | 231 (7.6) |
| Hormone therapy | 43 (1.4) |
| Parenteral nutrition | 89 (3.0) |
Missing data were distributed as follows: surgical tumor resection (n = 1), chemotherapy (n = 23), growth factors (n = 6), radiotherapy (n = 12), antiangiogenic agents (n = 5), hormone therapy (n = 7), parenteral nutrition (n = 36); details regarding treatments within each category are provided in supplemental Table 4.
The initial procedure for port implantation was successful in 3030 patients (after 2 attempts during the same procedure in 247 patients), the rate of immediate operative complications being 1.6% (Table 3). Two patients required a second procedure for successful port placement, and 96 patients and 1 patient required insertion of a second and third port, respectively, after removal of the first port. The proportions of patients who received prophylactic doses of anticoagulant, therapeutic doses of anticoagulant, and antiplatelet agents at any time during the study were 22.3% (n = 664), 15.1% (n = 451), and 17.3% (n = 514), respectively (supplemental Table 5).
Characteristics of port implantation (initial procedure)
| . | N = 3032 . |
|---|---|
| Interval between cancer diagnosis and first port implantation, months* | 1.4 (0.8-3.1) |
| Duration of the procedure, minutes*,† | 25 (20-32) |
| Successful implantation, no. (%)‡ | 3030 (99.9) |
| First attempt | 2783 (91.8) |
| Second attempt | 247 (8.1) |
| Catheter tip position verified radiologically just after insertion, n/N (%) | 3013/3030 (99.4) |
| At the junction of the superior vena cava and the right atrium | 2977/3013 (98.8) |
| Medical specialty of the operator, no. (%) | N = 3028 |
| Surgeon | 2845 (94.0) |
| Radiologist | 159 (5.3) |
| Anesthesiologist | 22 (0.7) |
| Other | 2 (0.1) |
| Type of anesthesia, no. (%) | N = 3015 |
| Local | 1679 (55.7) |
| General | 1336 (44.3) |
| Side of implantation, no. (%)§ | N = 3031 |
| Right | 2260 (74.6) |
| Left | 771 (25.4) |
| Vein used, no. (%) | N = 3019 |
| Cephalic | 1551 (51.4) |
| Subclavian | 705 (23.4) |
| Internal jugular | 711 (23.6) |
| External jugular | 52 (1.7) |
| Vein approach, no. (%) | N = 3016 |
| Denudation | 1770 (58.7) |
| Percutaneous | 1246 (41.3) |
| Port chamber size, no. (%) | N = 2924 |
| Small | 1110 (38.0) |
| Large | 1814 (62.0) |
| Chemical composition of the catheter, no. (%) | N = 2906 |
| Silicone | 2029 (69.8) |
| Polyurethane | 877 (30.2) |
| Operative complications (all attempts combined), no. (%) | N = 3016 |
| Overall | 47 (1.6) |
| Hematoma | 25 (0.8) |
| Pneumothorax | 11 (0.4) |
| Other | 12 (0.4) |
| Prophylactic treatment initiated after port implantation, n/N (%) | |
| Antibiotic prophylaxis | 753/3012 (25.0) |
| Thromboprophylaxis | 143/3008 (4.8) |
| . | N = 3032 . |
|---|---|
| Interval between cancer diagnosis and first port implantation, months* | 1.4 (0.8-3.1) |
| Duration of the procedure, minutes*,† | 25 (20-32) |
| Successful implantation, no. (%)‡ | 3030 (99.9) |
| First attempt | 2783 (91.8) |
| Second attempt | 247 (8.1) |
| Catheter tip position verified radiologically just after insertion, n/N (%) | 3013/3030 (99.4) |
| At the junction of the superior vena cava and the right atrium | 2977/3013 (98.8) |
| Medical specialty of the operator, no. (%) | N = 3028 |
| Surgeon | 2845 (94.0) |
| Radiologist | 159 (5.3) |
| Anesthesiologist | 22 (0.7) |
| Other | 2 (0.1) |
| Type of anesthesia, no. (%) | N = 3015 |
| Local | 1679 (55.7) |
| General | 1336 (44.3) |
| Side of implantation, no. (%)§ | N = 3031 |
| Right | 2260 (74.6) |
| Left | 771 (25.4) |
| Vein used, no. (%) | N = 3019 |
| Cephalic | 1551 (51.4) |
| Subclavian | 705 (23.4) |
| Internal jugular | 711 (23.6) |
| External jugular | 52 (1.7) |
| Vein approach, no. (%) | N = 3016 |
| Denudation | 1770 (58.7) |
| Percutaneous | 1246 (41.3) |
| Port chamber size, no. (%) | N = 2924 |
| Small | 1110 (38.0) |
| Large | 1814 (62.0) |
| Chemical composition of the catheter, no. (%) | N = 2906 |
| Silicone | 2029 (69.8) |
| Polyurethane | 877 (30.2) |
| Operative complications (all attempts combined), no. (%) | N = 3016 |
| Overall | 47 (1.6) |
| Hematoma | 25 (0.8) |
| Pneumothorax | 11 (0.4) |
| Other | 12 (0.4) |
| Prophylactic treatment initiated after port implantation, n/N (%) | |
| Antibiotic prophylaxis | 753/3012 (25.0) |
| Thromboprophylaxis | 143/3008 (4.8) |
Median (interquartile range).
Data were missing in 195 patients.
In 2 patients, the port was successfully implanted during a second procedure a few days later.
The majority of patients with left-side implantation (540/771) were those with breast (426/1023) and lung (114/560) cancers.
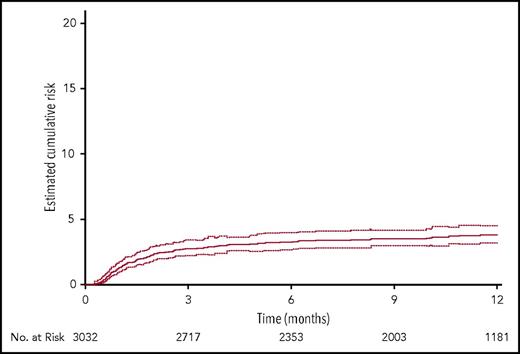
By 12 months, thromboembolic complications (any symptomatic event or incidental pulmonary embolism) had occurred in 397 of 3032 patients (13.8%; 95% CI, 12.6-15.0) (Table 4). The median time (interquartile range) to any thromboembolic event was 67.0 (30.0-137.0) days (Figure 2). Venous thromboembolism had occurred in 378 of 3032 patients (13.1%; 95% CI, 12.0-14.3); 142 (5.0%) patients had experienced pulmonary embolism, fatal in 37 (1.3%) and incidental in 58 (2.0%). Symptomatic catheter-related thrombosis had occurred in 111 of 3032 patients (3.8%; 95% CI, 3.2-4.5), being pulmonary embolism associated with upper-extremity deep-vein thrombosis ipsilateral to the catheter in 5 (0.2%) and isolated (without pulmonary embolism) upper-extremity deep-vein thrombosis ipsilateral to the catheter in 106 (3.8%) (Table 4). The median time (interquartile range) to any symptomatic catheter-related thrombosis was 45.0 (23.0-99.0) days (Figure 3). Of the 378 patients who experienced venous thromboembolism, the event was not catheter-related in 276 (9.6%). The incidence of arterial thromboembolism was 0.9%. Among the 397 who experienced a thromboembolic event, 329 (82.9%) received anticoagulation at therapeutic dose.
Thromboembolic outcomes and death at 12 months
| Patients presenting at least 1 event . | N = 3032 . | |
|---|---|---|
| No. (%) . | 95% CI . | |
| Any thromboembolic event* | 397 (13.8) | 12.6; 15.0 |
| Venous thromboembolism | 378 (13.1) | 12.0; 14.3 |
| Any pulmonary embolism | 142 (5.0) | 4.3; 5.9 |
| Symptomatic | 87 (3.1) | 2.5; 3.9 |
| Fatal | 37 (1.3) | — |
| Incidental (asymptomatic) | 58 (2.0) | 1.6; 2.5 |
| Lower-extremity deep-vein thrombosis | 106 (3.7) | 3.1; 4.4 |
| Proximal | 72 | |
| Distal | 34 | |
| Upper-extremity deep-vein thrombosis | 119 (4.1) | 3.4; 4.8 |
| Superficial-vein thrombosis | 52 (1.8) | 1.3; 2.3 |
| Lower-extremity | 19 (0.7) | 0.4; 1.0 |
| Upper-extremity | 33 (1.1) | 0.8; 1.5 |
| Other venous thromboembolic event | 9 (0.3) | 0.2; 0.6 |
| Arterial thromboembolism | 25 (0.9) | 0.6; 1.3 |
| Stroke or transient ischemic attack† | 18 (0.6) | 0.4; 1.1 |
| Acute myocardial infarction‡ | 4 (0.14) | 0.06; 0.34 |
| Acute lower-limb ischemia | 3 (0.10) | 0.03; 0.29 |
| Catheter-related thrombosis | 111 (3.8) | 3.2; 4.5 |
| Upper-extremity deep-vein thrombosis | 106 (3.8) | — |
| Pulmonary embolism associated with upper-extremity deep-vein thrombosis§ | 5 (0.2) | — |
| Venous thromboembolism excluding catheter-related upper-extremity deep-vein thrombosis|| | 276 (9.6) | 8.7; 10.7 |
| Death | 656 (24.6) | 22.9; 26.3 |
| Patients presenting at least 1 event . | N = 3032 . | |
|---|---|---|
| No. (%) . | 95% CI . | |
| Any thromboembolic event* | 397 (13.8) | 12.6; 15.0 |
| Venous thromboembolism | 378 (13.1) | 12.0; 14.3 |
| Any pulmonary embolism | 142 (5.0) | 4.3; 5.9 |
| Symptomatic | 87 (3.1) | 2.5; 3.9 |
| Fatal | 37 (1.3) | — |
| Incidental (asymptomatic) | 58 (2.0) | 1.6; 2.5 |
| Lower-extremity deep-vein thrombosis | 106 (3.7) | 3.1; 4.4 |
| Proximal | 72 | |
| Distal | 34 | |
| Upper-extremity deep-vein thrombosis | 119 (4.1) | 3.4; 4.8 |
| Superficial-vein thrombosis | 52 (1.8) | 1.3; 2.3 |
| Lower-extremity | 19 (0.7) | 0.4; 1.0 |
| Upper-extremity | 33 (1.1) | 0.8; 1.5 |
| Other venous thromboembolic event | 9 (0.3) | 0.2; 0.6 |
| Arterial thromboembolism | 25 (0.9) | 0.6; 1.3 |
| Stroke or transient ischemic attack† | 18 (0.6) | 0.4; 1.1 |
| Acute myocardial infarction‡ | 4 (0.14) | 0.06; 0.34 |
| Acute lower-limb ischemia | 3 (0.10) | 0.03; 0.29 |
| Catheter-related thrombosis | 111 (3.8) | 3.2; 4.5 |
| Upper-extremity deep-vein thrombosis | 106 (3.8) | — |
| Pulmonary embolism associated with upper-extremity deep-vein thrombosis§ | 5 (0.2) | — |
| Venous thromboembolism excluding catheter-related upper-extremity deep-vein thrombosis|| | 276 (9.6) | 8.7; 10.7 |
| Death | 656 (24.6) | 22.9; 26.3 |
All time-to-event outcomes were estimated by a competitive risk analysis taking into account the competing risk of death. Mortality was estimated by the Kaplan-Meier method.
Some patients experienced >1 event. Other venous thromboembolic events were located as follows: 4 in the portal vein, 2 in the inferior vena cava, 1 in the renal and splanchnic veins, 1 in the splanchnic vein, and 1 in the mesenteric vein.
Four strokes were fatal.
One was fatal.
None was fatal.
Nine patients experienced both a catheter-related thrombotic event and a venous thromboembolic event unrelated to the catheter.
Cumulative estimates of the probability of the composite of any confirmed symptomatic thromboembolic complication, whether venous or arterial, and incidental (asymptomatic) pulmonary embolism during the entire study period. Symptomatic events included pulmonary embolism, upper- or lower-extremity deep- or superficial-vein thrombosis, acute myocardial infarction, stroke or transient ischemic attack, acute lower-limb ischemia, and any other symptomatic event confirmed by an objective test. Incidental pulmonary embolisms were those discovered on scans performed for reasons other than suspected pulmonary embolism. Dotted lines indicate the 95% CI limits (competing risk analysis).
Cumulative estimates of the probability of the composite of any confirmed symptomatic thromboembolic complication, whether venous or arterial, and incidental (asymptomatic) pulmonary embolism during the entire study period. Symptomatic events included pulmonary embolism, upper- or lower-extremity deep- or superficial-vein thrombosis, acute myocardial infarction, stroke or transient ischemic attack, acute lower-limb ischemia, and any other symptomatic event confirmed by an objective test. Incidental pulmonary embolisms were those discovered on scans performed for reasons other than suspected pulmonary embolism. Dotted lines indicate the 95% CI limits (competing risk analysis).
Cumulative estimates of the probability of confirmed symptomatic catheter-related thrombosis during the entire study period. Catheter-related thrombosis was defined as any symptomatic upper-extremity deep-vein thrombosis occurring in the clinical and anatomic setting of the central venous catheter, with or without pulmonary embolism. Dotted lines indicate the 95% CI limits (competing risk analysis).
Cumulative estimates of the probability of confirmed symptomatic catheter-related thrombosis during the entire study period. Catheter-related thrombosis was defined as any symptomatic upper-extremity deep-vein thrombosis occurring in the clinical and anatomic setting of the central venous catheter, with or without pulmonary embolism. Dotted lines indicate the 95% CI limits (competing risk analysis).
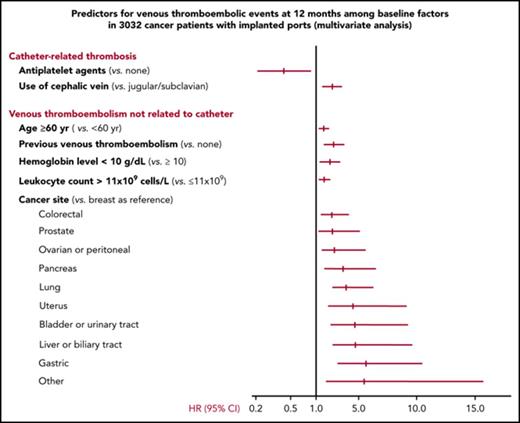
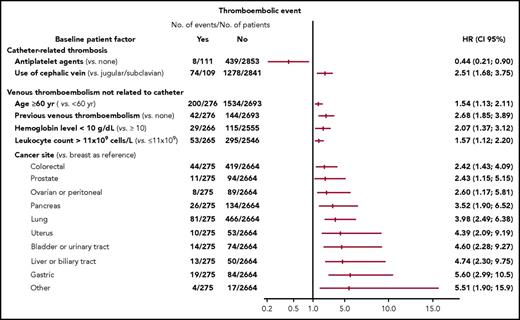
The only independent risk factor for catheter-related thrombosis at 12 months identified by multivariate analysis (Figure 4; supplemental Table 6) was use of the cephalic vein for catheter insertion (hazard ratio: 2.51; 95% CI, 1.68-3.75; P < .0001). Ongoing treatment with an antiplatelet agent at baseline was found to be associated with a decreased 12-month risk of catheter-related thrombosis (hazard ratio: 0.44; 95% CI, 0.21-0.90; P = .024). Independent risk factors for venous thromboembolic events other than catheter-related at 12 months were age 60 years or over, previous venous thromboembolism, certain specific cancer sites, and low hemoglobin level (<10 g/dL) or increased leukocyte count (>11 × 109 cells per L) before chemotherapy initiation (Figure 4; supplemental Table 6).
Risk factors for venous thromboembolic events at 12 months identified by multivariate analysis. HR (95% CI): Hazard ratios and corresponding 95% CIs. Cutoff values for hemoglobin level and leukocyte counts were chosen based on the Khorana score (see supplemental Data). Factors with a hazard ratio of <1 are protective and those with a hazard ratio of >1 are risk factors. Cancer sites in the category “other” include small intestine: 4, soft tissues: 4, muscles: 3, abdominal: 3, cutaneous: 2, vulva: 1 penis: 1, thyroid: 1, thymus: 1, and mediastinum: 1.
Risk factors for venous thromboembolic events at 12 months identified by multivariate analysis. HR (95% CI): Hazard ratios and corresponding 95% CIs. Cutoff values for hemoglobin level and leukocyte counts were chosen based on the Khorana score (see supplemental Data). Factors with a hazard ratio of <1 are protective and those with a hazard ratio of >1 are risk factors. Cancer sites in the category “other” include small intestine: 4, soft tissues: 4, muscles: 3, abdominal: 3, cutaneous: 2, vulva: 1 penis: 1, thyroid: 1, thymus: 1, and mediastinum: 1.
Discussion
This large prospective cohort study of 3032 patients with a solid tumor and an implanted port, followed for 12 months in a real-life practice setting, showed that the risk factors for catheter-related thrombosis differ from those for venous thromboembolism unrelated to the catheter. By 12 months, 3.8% of patients had presented symptomatic catheter-related venous thromboembolism, and 9.6% of patients had experienced venous thromboembolism unrelated to the catheter. Overall, cancer-associated thrombosis occurred in 13.8% of patients. All events were objectively confirmed and centrally adjudicated, and rate assessments integrated the competing risk of death.
The observed event rates fall within the previously reported ranges of 4% to 10% for symptomatic catheter-related thrombosis,12,20,21,34,35 0.5% to 20% for symptomatic venous thromboembolism,16 and 1% to 5% for incidental pulmonary embolism.36 However, these ranges are wide, indicating that comparisons of event rates across studies may be unrealistic owing to the substantial heterogeneity between studies regarding patient populations, outcome definitions, and average lengths of follow-up. We selected a broad range of cancer outpatients comparable in terms of time to cancer diagnosis and need for port implantation. Among other patient-, tumor-, and treatment-related risk factors for thrombosis, insertion of a central venous catheter is a landmark event in the cancer patient’s life, occurring at a period of high thrombotic risk when patients are more likely to be hospitalized and receive thrombogenic chemotherapy during the initial course of their disease. In a recently published single-center study in 1097 cancer outpatients requiring a first port implantation for chemotherapy, rates of events at 12 months, defined as in our study, were as high as those we observed (any venous thromboembolism: 15.3%; pulmonary embolism: 5.5%; catheter-related deep-vein thrombosis: 5.9%; and lower extremity deep-vein thrombosis: 4.6%).37 In our study, consistent with several previous reports,37-42 most thrombotic events occurred during the first 3 months of follow-up. After inclusion, some patients received anticoagulation at prophylactic or therapeutic doses. Although the reasons for initiation and choice of duration of anticoagulant treatment were not recorded in our study, therapeutic anticoagulation was mainly initiated in patients experiencing a thromboembolic event. Prophylactic anticoagulation, likely to have been administered to patients perceived by the investigator as having a higher risk profile, might have decreased the rate of venous thromboembolism events.
Data regarding risk factors for catheter-related thrombosis are limited. In a meta-analysis of individual patient-level data (N = 5636) from 5 randomized trials and 7 prospective cohort studies,21 4 baseline variables were identified as independent predictors of events, namely previous deep-vein thrombosis, use of the subclavian vein for insertion (vs an upper arm vein) and incorrect catheter tip location, whereas implanted ports were found to be protective compared with peripherally implanted central venous catheters. In our study, all patients had implanted ports, and correct catheter tip location was ensured by radiological control; the only independent predictor of events identified was use of the cephalic vein for port implantation, a factor that was not assessed in the meta-analysis.21 In contrast to the meta-analysis, we did not find that previous venous thromboembolism was a risk factor for catheter-related thrombosis, even though 14.8% of our patients had such a medical history (compared with 2% in the meta-analysis). The reason for this may be that mechanical factors are more important than medical background in determining the risk of specific events such as catheter-related thrombosis. Ongoing antiplatelet therapy was found to be protective, but whether such therapy is effective in preventing catheter-related thrombosis remains to be investigated. As no patient included in the ONCOCIP study had a multilumen port, we were unable to assess the impact of the type of port on the occurrence of thrombotic events.
Some of the risk factors for venous thromboembolism other than catheter-related that we identified have been described previously and are included in the most popular risk score for cancer-associated thrombosis developed by Khorana et al,22 these factors comprising the site of cancer and prechemotherapy low hemoglobin level and increased leukocyte count. Conversely, certain additional risk factors we identified, including advanced age and previous venous thromboembolism, had not been previously reported and were not been included in the Khorana score because the relevant data were not available in the original cohort study from which this score was derived. However, age and previous venous thromboembolism have been associated with an increased risk of venous thromboembolism in many studies involving noncancer patients, and it is thus not surprising to find that they are also risk factors for venous thromboembolism in patients with cancer.
The fact that risk factors for catheter-related thrombosis differ from those for venous thromboembolism unrelated to the catheter was an unexpected finding and may have clinical implications. On the one hand, catheter-related thrombosis may affect primarily catheter function/permeability, the rates of serious complications such as pulmonary embolism (including fatal pulmonary embolism) being very low, as shown in our study and by others.13 Thus, prevention of catheter-related thrombosis may focus mainly on mechanical factors, with the objective of protecting catheter function. In this respect, our results suggest that use of the cephalic vein for catheter insertion may be hazardous in this context. In addition, whereas prophylaxis with anticoagulants did not show benefit in preventing catheter-related thrombosis,19,20 our results suggest that antiplatelet therapy may be of value. On the other hand, the occurrence of venous thromboembolism unrelated to the catheter is influenced by various factors8-10 and is associated with significantly increased morbidity and mortality in patients with cancer.1-6 Venous thromboembolism prevention may therefore rely on anticoagulant drugs to improve patient prognosis. Previous placebo-controlled prevention studies of anticoagulants in the general population of outpatients with cancer showed promising results.17,18 However, in these studies the absolute benefit of anticoagulants was small owing to a low rate of events in the placebo groups. This emphasizes the need to develop risk assessment models enabling identification of cancer outpatients most likely to benefit from thromboprophylaxis with anticoagulants.
In conclusion, this large prospective cohort study showed a high rate of thrombotic events in patients with a solid tumor and an implanted port in the real-life practice setting, and identified independent risk factors that may be useful for risk stratification. Importantly, risk factors for catheter-related thrombosis differed from those for venous thromboembolism unrelated to the catheter, suggesting that measures to prevent each type of event may also differ.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors are indebted to Bruno Perpoint, oncologist at the Saint-Etienne University Hospital, who greatly inspired this study but died in January 2000. The authors warmly thank Jean-Yves Darmon (MediBridge, France) who critically reviewed the manuscript during its development.
The ONCOCIP study was supported by unrestricted grants from the Ligue Nationale Contre le Cancer, Paris, France, and from Bayer Healthcare, Loos, France, as well as by regional public funding from the Plateforme Régionale d’Aide à la Recherche Clinique, Auvergne et Rhône-Alpes, supported by the Ligue Nationale Contre le Cancer, Lyon, France, and from the Région Rhône-Alpes (CIBLE 2009), Saint-Etienne, France. The sponsor was a public cancer hospital, the Institut de Cancérologie Lucien Neuwirth, Saint-Priest en Jarez, France.
Authorship
Contribution: A steering committee was responsible for the design, conduct, and reporting of the ONCOCIP study. Data were collected and analyzed by the Centre d’Investigation Clinique CIC1408, Saint-Etienne, France. The database (including adjudicated outcomes) was managed by the Centre d’Investigation Clinique CIC1408, Saint-Etienne, France. L.B., A.B., F.C., H.D., P.F., C.L., S.L., and G.M. served on the steering committee; G.M. chaired the central adjudication committee; A.M. was responsible for the organization of the central adjudication committee; P.F. was the national coordinator of investigators and also enrolled patients; F.D.P., J.-P.J., and L.S. enrolled patients; C.L. was responsible for the management of the study operations; S.L. and E.P. performed the statistical analysis; H.D. and F.C. wrote the first draft of the manuscript; and all authors contributed to designing the study, had full access to the data and analyses, contributed to interpreting the data and developing the report, agreed on the final version of the manuscript, had final responsibility for the report content and decision to publish the findings, and vouch for the report’s accuracy and completeness.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the ONCOCIP (ONCOlogie et Chambres ImPlantables) Study Group appears in “Appendix.”
Correspondence: Hervé Decousus, Service de Médecine Vasculaire et Thérapeutique, Hôpital Nord, CHU de Saint-Etienne, 42055 Saint-Etienne Cedex 2, France; e-mail: herve.decousus@chu-st-etienne.fr.
Appendix
The members of the ONCOCIP Study Group (all in France) were as follows: Steering Committee: H. Decousus (Chair), F. Chauvin (Co-Chair), P. Berthelot, L. Bertoletti, A. Bourmaud, P. Fournel, C. Labruyère, S. Laporte, G. Meyer, P. Mismetti, B. Tardy. Executive Committee: H. Decousus (Chair), F. Chauvin (Co-Chair), A. Bourmaud, C. Labruyère, P. Fournel. Independent Central Adjudication Committee: Members: G. Meyer (Chair), C. Minozzi, F. Scotte; Organization: A. Merah (Coordinator) and S. Ayala. Data Management and Statistical Analysis: E. Presles, S. Laporte; electronic case report form: Clininfo. Operation team (CIC 1408): C. Labruyère (Project Manager); Y. Bach, H. Bada, O. Chacornac, J. Durif, M. Pierre (Clinical Research Assistants). Investigators (by center and in order of the number of patients enrolled): Institut de Cancérologie Lucien Neuwirtz, Saint-Priest en Jarez, France (587): O. Collard, P. Fournel, A. Guillot, P. Jacquin, B.Mery, L. Saban Roche, C. Vassal; Centre Hospitalier d'Annecy, Annecy (336): M. Baconnier, C. Decroisette, P. E. Heudel, E. Maillard, L. Mermet, L. Stefani; Hôpitaux du Léman, Thonon-les-Bains (297): A. Bedjaoui, F. Del Piano, M. Delouane, T. Laurent, K. Mahour Bacha, P. Romand; Hôpital Privé Drôme Ardèche, Valence (206): M. Bosset, M. El Demery, S. Lantheaume, F. Sensenbrenner, H. Barletta; Centre Hospitalier d'Annemasse, Annemasse (204): C. Alliot, J. M. Arimont, J. B. Bouclier, P. Chatellain, F. Huet; Hôpital Privé de la Loire, Saint-Etienne (178): T. Muron; Institut Daniel Hollard, Grenoble (170): L. Dupuy-Brousseau, C. Garnier Tixidre, C. Leyronnas, J. Long; Centre Hospitalo-Universitaire de Saint-Etienne, Saint-Etienne (169): C. Chauleur, N. Mottet, J. M. Phelip; Infirmerie Protestante, Lyon (124): V. Garbit, J. Hartwig, H. Perrier; Centre Hospitalo-Universitaire de Clermont-Ferrand, Clermont-Ferrand (109): P. Merle; Centre Hospitalier Saint-Joseph Saint Luc, Lyon (93): D. Péré-Vergé, S. Vuillermoz-Blas; Hôpital de la Croix Rousse, Lyon (81): R. Chumbi-Flores, G. Dubernard; Polyclinique de Savoie, Annemasse (73): D. Loiseau, M. Sautier; Pôle Santé République, Clermont-Ferrand (68): J. P. Desir, V. Chanet; Clinique de la Sauvegarde, Lyon (57): I. Moullet, P. Ardisson; Clinique Eugène André, Lyon (53): Y. Hammou, E. Staub; Centre Hospitalier de Chambéry, Chambéry (50): I. Cauvin; Centre Hospitalier du Puy-en-Velay, Le Puy-en-Velay (32): B. Monange; Centre Hospitalier de Roanne, Roanne (30): L. Vincent; Clinique Les Portes du Sud, Lyon (25): J. M. Padet; Centre Hospitalier du Pays du Gier, Saint Chamond (19): P. Ibanez Martin; Centre Hospitalier du Forez, Feurs (18): C. Portois; Hôpital Desgenettes, Lyon (16): P. Debourdeau; Hôpital Privé Jean Mermoz, Lyon (13): J. P. Martin; Centre Hospitalier de Montbrison, Montbrison (7): G. Soglu; Centre Hospitalo-Universitaire de Lyon Sud, Lyon (7): V. Trillet-Lenoir; Centre Hospitalier de Villefranche, Villefranche (4): C. Dussopt; Centre Hospitalo-Universitaire de Grenoble, Grenoble (3): M. Mousseau; Clinique Trénel, Vienne (3): I. Graber. Clinical Research Technicians at the investigator sites: Institut de Cancérologie Lucien Neuwirtz, Saint-Priest en Jarez, France: M. Pierre; Centre Hospitalier d'Annecy, Annecy: I. Quint; Hôpitaux du Léman, Thonon-les-Bains: G. Landry; Hôpital Privé Drôme Ardèche, Valence: M. Rohfritsch; Centre Hospitalier d'Annemasse, Annemasse: L. Maximin; Institut Daniel Hollard, Grenoble: L. V. Alcalay; Centre Hospitalo-Universitaire de Saint-Etienne, Saint-Etienne: E. Gleize; Infirmerie Protestante, Lyon: D. Tavan; Centre Hospitalo-Universitaire de Clermont-Ferrand, Clermont-Ferrand: M. Durand; Centre Hospitalier Saint-Joseph Saint Luc, Lyon: S. Armand; Hôpital de la Croix Rousse, Lyon: C. Leclerc; Polyclinique de Savoie, Annemasse: L. Maximin; Clinique de la Sauvegarde, Lyon: R. Sapey; Centre Hospitalier du Puy-en-Velay, Le Puy-en-Velay: F. Torche; Centre Hospitalier du Pays du Gier, Saint Chamond: F. Torche; Centre Hospitalier du Forez, Feurs: F. Torche; Centre Hospitalier de Montbrison, Montbrison: F. Torche.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal