TO THE EDITOR:
Neurotoxicity from anti-CD19 chimeric antigen receptor (CAR) T cells, an effective immunotherapy for refractory B-cell malignancies,1-6 is common and poorly understood. Commonly manifesting as headache, delirium, language disturbances and occasionally seizures and life-threatening cerebral edema, CAR T cell–associated neurotoxicity may in part be due to endothelial activation that leads to increased permeability of the blood–brain barrier and entry of inflammatory cytokines and CAR T cells into the central nervous system (CNS).7 Neurotoxicity is usually observed in association with or after cytokine release syndrome (CRS).6,7 Severe CRS can be life-threatening and is mediated by elevated cytokines including interleukin-6 (IL-6), tumor necrosis factor-α, IL-10, and interferon-γ.6-8 IV tocilizumab, a humanized, monoclonal antibody against membrane-associated and soluble IL-6 receptor (IL-6R), is US Food and Drug Administration approved for the management of severe CRS. Most CRS symptoms improve within hours of a single dose of IV tocilizumab, in contrast to neurotoxicities, which tend not to respond to IL-6R blockade.4,6,7,9
In some patients, neurotoxicity worsens or develops after tocilizumab administration. This may be due to a transient rise in serum IL-6 resulting from decreased clearance via endocytosis by the antagonized IL-6R.6,7 An animal model recently demonstrated that neurotoxicity is at least in part mediated by IL-6,10 and IL-6 can disrupt the blood–brain barrier endothelium.11 One mechanism to explain this phenomenon is that tocilizumab concentration within the brain and cerebrospinal fluid (CSF) may not be sufficient to competitively inhibit the IL-6R. The CSF penetration of tocilizumab is unknown and, as a large monoclonal antibody, is not expected to penetrate the blood–brain barrier, but other routes should be explored. The objective of this study is to determine the pharmacokinetics and CSF exposure of tocilizumab after IV, intranasal, and intraventricular administration in a nonhuman primate (NHP) model.
The National Cancer Institute Institutional Animal Care and Use Committee approved this study. NHP were cared for in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, 8th edition.12
Six adult male rhesus monkeys (Macaca mulatta) ages 13 to 15 years, weighing 10.5 to 14.7 kg, were used. Each NHP was previously implanted with an indwelling, subcutaneous CSF ventricular reservoir in the lateral or fourth ventricle13 and an indwelling subcutaneous IV port in the jugular or femoral vein for drug administration and sample collection. For studies of intraventricular administration, 3 additional NHP with lumbar CSF ports were included14 for CSF collection from a site different from intraventricular drug administration. For intranasal administration, animals were sedated and placed in Mygind position. All NHP were physiologically and neurologically normal, as determined by physical examination and blood chemistries. All NHP were sedated (ketamine, intramuscularly, 10 mg/kg, Vedco Inc.) only during drug administration. Three NHP received 10 mg/kg tocilizumab (human-equivalent dose of 8 mg/kg) administered via IV port in a total volume of 60 mL normal saline over 60 minutes with plasma sampling via the contralateral IV port and ventricular CSF sampling via the CSF reservoir. Three animals received intranasal tocilizumab. Twenty milligrams of tocilizumab in a volume of 1 mL was administered through a mucosal atomization device. Three additional NHP received 50 mg total dose of tocilizumab (human-equivalent dose ∼40 mg), approximately one-half the dose delivered IV, via intraventricular administration in a volume of 0.28 mL normal saline over 1 minute via the CSF ventricular reservoir followed by lumbar CSF sampling via the lumbar port and plasma sampling via an IV port. Serial, paired blood and CSF samples were collected hourly from 0 to 8 hours, then once on days 1 through 3, 6, and 10 postinfusion. Serum was collected in a serum separator collection tube. Serum and CSF were stored at −20°C until analysis.
Free tocilizumab was measured in NHP serum and CSF using a pharmacokinetic bridging enzyme-linked immunosorbent assay. Antitocilizumab capture antibody (HCA252, Bio-Rad) and horseradish peroxidase–conjugated detection antibody (HCA253P, Bio-Rad) were used in this assay following the manufacturer’s published pharmacokinetic bridging enzyme-linked immunosorbent assay protocol in a 96-well format (Bio-Rad).
Plasma and CSF pharmacokinetic parameters were calculated using noncompartmental methods in Microsoft Excel 2017 for Mac, version 15.32. The peak concentration (Cmax) and time to peak concentration (Tmax) were observed values. The area under the concentration–time curve (AUC) was calculated using the linear trapezoidal method to infinity (AUC0-∞). The elimination rate constant (Kel) was determined using the concentration vs time data from Cmax to the terminal time point. The natural log of the concentration was plotted in ascending order against the corresponding time as a semilogarithmic plot and the associated slope is equal to Kel.
The half-life (t1/2) was calculated as natural log of the concentration(2) or 0.693/Kel. Body surface area was calculated as the weight of the animal (in kilograms) divided by 20, which is the weight and species-appropriate Michaelis-Menten constant (Km) used for our model.15 Plasma and CSF clearance after IV administration and plasma clearance after intraventricular administration was calculated as the total dose administered/AUC0-∞/body surface area. CSF clearance after intraventricular administration was calculated as the total dose administered/AUC0-∞.
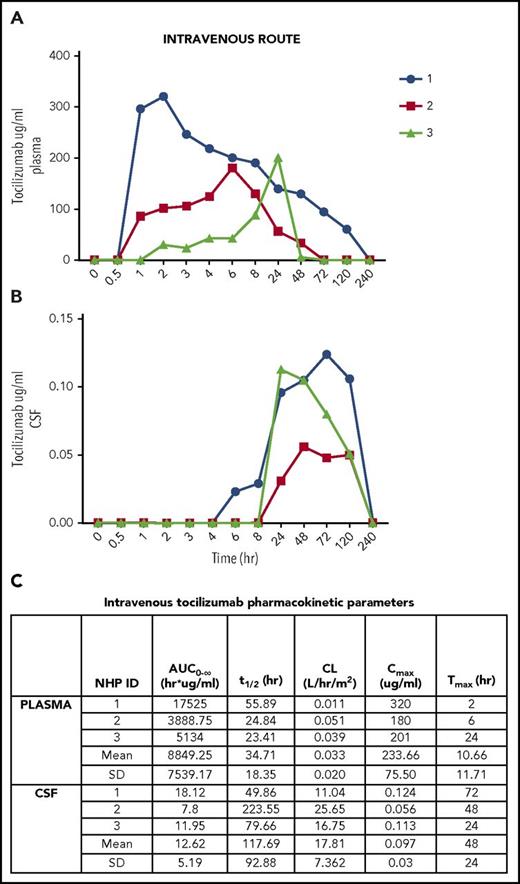
All samples and standards were plated in triplicate and had a coefficient of variation <20%. For the 3 NHP that received IV tocilizumab, the mean plasma Cmax = 233.66 μg/mL (Figure 1A) and mean CSF Cmax = 0.097 μg/mL (Figure 1B). Mean plasma and CSF Tmax were 10 hours and 48 hours, respectively (Figure 1C). Mean plasma and CSF t1/2 were 34 and 117 hours, respectively (Figure 1C). Plasma AUC 0-∞ (mean) = 8849.25 h/μg per milliliter, whereas CSF AUC0-∞ (mean) = 12.62 h/μg per milliliter (Figure 1C). CSF penetration was low, with mean CSF:plasma AUC0-∞ ratio of 0.14%.
Intravenous tocilizumab. (A) Concentration detected in plasma over time. (B) Concentration detected in CSF over time. (C) Pharmacokinetic parameters in plasma and CSF after intravenous administration. CL, clearance; SD, standard deviation.
Intravenous tocilizumab. (A) Concentration detected in plasma over time. (B) Concentration detected in CSF over time. (C) Pharmacokinetic parameters in plasma and CSF after intravenous administration. CL, clearance; SD, standard deviation.
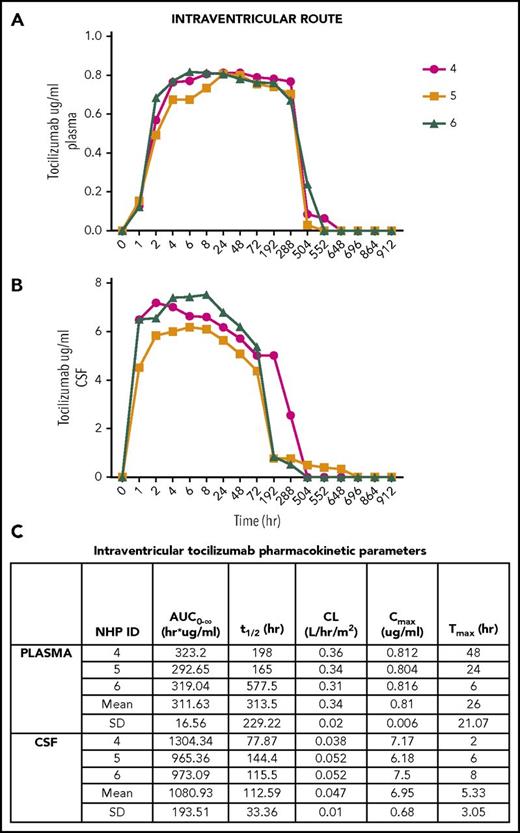
For the 3 NHP treated with intraventricular tocilizumab (Figure 2), the mean plasma Cmax = 0.81 μg/mL (Figure 2A) and mean CSF Cmax = 6.95 μg/mL (Figure 2B). Mean plasma and CSF Tmax were 26 hours and 5 hours, respectively (Figure 2C). Mean plasma and CSF t1/2 were 313 and 112 hours, respectively (Figure 2C). CSF exposure was higher than IV administered tocilizumab with plasma AUC0-∞ (mean) = 311.63 h/μg per milliliter, whereas CSF AUC 0-∞ (mean) = 1080.93 h/μg per milliliter (Figure 2C). The mean CSF:plasma AUC0-∞ ratio was 28.82%. Tocilizumab levels were below the level of detection in the plasma and CSF after intranasal administration in all (n = 3) animals. No animals exhibited any physical examination, blood count, or basic chemistry abnormalities after treatment with tocilizumab.
Intraventricular tocilizumab. (A) Concentration detected in plasma over time. (B) Concentration detected in CSF over time. (C) Pharmacokinetic parameters in plasma and CSF after intraventricular administration.
Intraventricular tocilizumab. (A) Concentration detected in plasma over time. (B) Concentration detected in CSF over time. (C) Pharmacokinetic parameters in plasma and CSF after intraventricular administration.
Tocilizumab completely inhibits the IL-6 receptor at ∼4 μg/mL, the same concentration needed to see clinical improvement in rheumatologic conditions.16-18 Intraventricular, but not IV delivery of tocilizumab, in this study achieves these therapeutic concentrations within the CSF. One important limitation is that this study uses physiologically and neurologically normal NHP with an intact blood–brain barrier at the time of tocilizumab administration. We demonstrated that tocilizumab has very poor CSF penetration when administered IV. Poor CSF penetration of tocilizumab in the setting of elevated CSF IL-6 and the transient rise in plasma IL-6 observed after IV tocilizumab may, in combination, explain the development or exacerbation of neurologic symptoms in patients with CRS. Intranasal delivery is a proposed mechanism to bypass the blood–brain barrier,19 but significant exposure was not achieved in our model using this route of administration. Delivery of tocilizumab directly into the CSF space is feasible and resulted in significant exposure, suggesting a potential role in the management of patients with cytokine-mediated neurotoxicity.
Acknowledgments
This work was supported by intramural funding from the National Institutes of Health National Cancer Institute. D.W.L. receives support from a St. Baldrick’s Foundation Scholar Award.
Authorship
Contribution: A.N., K.E.W., B.C.W., and D.W.L. designed the study; A.N. and C.M.L.M. wrote the manuscript; K.E.W. and D.W.L. reviewed and edited the manuscript; A.N. performed all in vitro experiments; C.M.L.M. and R.C.G. performed all nonhuman primate experiments; C.M.L.M., K.E.W., and A.N. analyzed the data; and N.J. assisted with in vitro experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anandani Nellan, Department of Pediatrics, University of Colorado, Denver Anschutz Medical Center, 13123 East 16th Ave, B115, Aurora, CO 80045; e-mail: anandani.nellan@ucdenver.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal