TO THE EDITOR:
The human proteins rabenosyn-5 and VPS45 form a complex that plays a key role in early endocytosis.1-4 Pathogenic variants in VPS45 cause severe congenital neutropenia (SCN) with impaired neutrophil function, reticulin fibrosis of the bone marrow, and extramedullary hematopoiesis (Online Mendelian Inheritance in Man [OMIM]: 615285).5-8 Patients with a specific VPS45 variant (p.Glu238Lys) also have intellectual disability and bilateral optic nerve hypoplasia.5,6 To date, the only evidence of a potential role for RBSN in human disease is the report of a homozygous missense variant (p.Gly425Arg) in a patient with intellectual disability, seizures, microcephaly, osteopenia, mild reticulin fibrosis of the bone marrow, and transient neutropenia.9
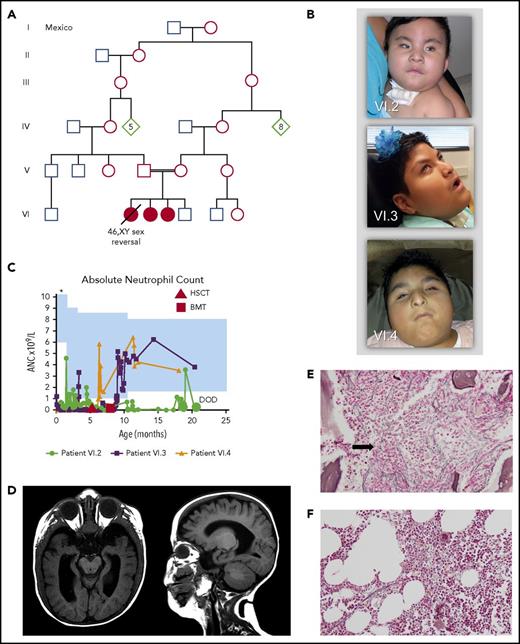
We evaluated 3 siblings born to consanguineous parents for a phenotype of SCN, anemia, thrombocytopenia, reticulin fibrosis of the bone marrow, dysmorphic facial features, osteopenia, hypertriglyceridemia, hepatomegaly, microphthalmia, and optic nerve hypoplasia (Figure 1A-E). Bone marrow examination revealed trilineage hematopoiesis without maturation arrest (supplemental Figure 1, available on the Blood Web site). Myelofibrosis was documented as early as age 4 weeks and was progressive and severe (supplemental Figures 1 and 2). The proband (patient VI.2) had complete 46,XY male-to-female sex reversal and died at age 20 months after multiple infections. At autopsy, she was found to have extensive extramedullary hematopoiesis. The other 2 affected siblings (patients VI.3, and VI.4) are currently ages 9 and 5 years. They underwent unrelated-donor bone marrow or stem cell transplantation at 8 and 6.5 months of age, respectively. Posttransplantation bone marrow examinations have shown a reduction in myelofibrosis (Figure 1F), and their circulating blood counts have remained normal (supplemental Figure 3). Both have severe intellectual disability and are nonambulatory and nonverbal. Clinical presentation is detailed in Table 1, supplemental Figures 1-3, and supplemental Table 1.
Family pedigree and phenotypic features of the patients. (A) Family pedigree indicating consanguinity. Filled symbols represent affected individuals (patients VI.2, VI.3, VI.4). (B) Photographs of patient VI.2 at 11 months, patient VI.3 at 9 years 2 months, and patient VI.4 at 4 years 11 months. Note microphthalmia, beaked nose, and prominent columella. Consent for the publication of images was provided by the patients’ parents. (C) Absolute neutrophil count (ANC) over time for the 3 patients. Shaded blue area represents the normal reference range based on age. The upper limit of normal in the newborn period in our laboratory is 23.5 × 109/L, which is above the range depicted (*). (D) Brain magnetic resonance imaging of patient VI.3 at age 9 months. Note supratentorial volume loss involving the white matter more than the gray matter, ex vacuo ventricular enlargement, and optic nerve hypoplasia. (E) Bone marrow biopsy for patient VI.3 at age 33 days. Reticulin stain, original magnification ×200. Note moderate to severe reticulin fibrosis. Trichrome staining for collagen fibers was not performed. (F) Bone marrow biopsy for patient VI.3 at age 20 months, 1 year after bone marrow transplantation. Reticulin stain, original magnification ×200. Note the reduction in reticulin fibrosis to mild and focal. Trichrome staining for collagen fibers was negative (data not shown). BMT, bone marrow transplantation; DOD, date of death; HSCT, hematopoietic stem cell transplantation.
Family pedigree and phenotypic features of the patients. (A) Family pedigree indicating consanguinity. Filled symbols represent affected individuals (patients VI.2, VI.3, VI.4). (B) Photographs of patient VI.2 at 11 months, patient VI.3 at 9 years 2 months, and patient VI.4 at 4 years 11 months. Note microphthalmia, beaked nose, and prominent columella. Consent for the publication of images was provided by the patients’ parents. (C) Absolute neutrophil count (ANC) over time for the 3 patients. Shaded blue area represents the normal reference range based on age. The upper limit of normal in the newborn period in our laboratory is 23.5 × 109/L, which is above the range depicted (*). (D) Brain magnetic resonance imaging of patient VI.3 at age 9 months. Note supratentorial volume loss involving the white matter more than the gray matter, ex vacuo ventricular enlargement, and optic nerve hypoplasia. (E) Bone marrow biopsy for patient VI.3 at age 33 days. Reticulin stain, original magnification ×200. Note moderate to severe reticulin fibrosis. Trichrome staining for collagen fibers was not performed. (F) Bone marrow biopsy for patient VI.3 at age 20 months, 1 year after bone marrow transplantation. Reticulin stain, original magnification ×200. Note the reduction in reticulin fibrosis to mild and focal. Trichrome staining for collagen fibers was negative (data not shown). BMT, bone marrow transplantation; DOD, date of death; HSCT, hematopoietic stem cell transplantation.
Clinical, imaging, and histopathologic findings in 3 patients with a pathogenic RBSN variant
| . | Patient VI.2 . | Patient VI.3 . | Patient VI.4 . |
|---|---|---|---|
| Phenotype | Female | Female | Female |
| Chromosomes | 46,XY | 46,XX | 46,XX |
| CMA | Normal (BG v.5 and 400 000 oligonucleotide research array) | Normal (BG v.6.4) | Normal (BG v.8.3) |
| Growth | Age, 11 mo; height, 25%; weight, 75%; OFC, 25%-50% | Age, 9 y 2 mo; height, <1%; weight, 76%; OFC, 18% | Age, 4 y 11 mo; height, <1%; weight, 57%; OFC: 5% |
| Facial features | Narrow bi-temporal diameter, metopic prominence, sloping forehead, upslanting palpebral fissures, epicanthal folds, bulbous nose, depressed nasal tip | Prominent nasal bridge, low-set posteriorly rotated ears, retromicrognathia, high-arched palate | Bi-temporal narrowing, tubular nose, prominent columella |
| Hematologic | SCN transiently responsive to intermediate-dose filgrastim, PB NRBCs, progressive anemia and thrombocytopenia, hypercellular bone marrow with severe reticulin fibrosis and myeloid hyperplasia, extramedullary hematopoiesis (liver, spleen, kidneys, lymph nodes) on PME | SCN without maturation arrest, refractory to filgrastim, transfusion-dependent anemia, variable thrombocytopenia including at birth and with infection, PB NRBCs, normocellular bone marrow with moderate to severe reticulin fibrosis and maturing trilineage hematopoiesis | SCN refractory to filgrastim, moderate anemia at birth (variably present subsequently), PB NRBCs, hypercellular bone marrow with moderate to severe reticulin fibrosis and maturing trilineage hematopoiesis |
| Ophthalmologic | Poor visual response, microphthalmia, hypoplastic irides, microcornea, blepharophimosis, absent retinal vessels, aplastic optic nerves | Poor visual response, microphthalmia, microcornea, blepharophimosis, aplastic optic nerves | Poor visual response, microphthalmia, aniridia with iris coloboma, blepharophimosis, dysplastic optic nerves, absent retinal vessels |
| Neurologic | Increased muscle tone in the lower extremities, DTRs 2+, poor head control | Increased muscle tone in the 4 extremities, DTRs 3+, normal electroencephalogram | Joint restriction, hypotonia, diminished strength, muscle atrophy, normal DTRs |
| Brain MRI | Prominent supratentorial ventricles, sulci, and cisterns, widened sylvian fissures, thin corpus callosum, cerebral atrophy, severe optic nerve and chiasm hypoplasia, diffuse expansion of diploic calvarial marrow space | Perinatal intraparenchymal and subarachnoid hemorrhages, supratentorial volume loss, thin corpus callosum, mild myelin maturation delay, hypoplasia of the optic nerve, chiasm, and optic tract, bone enhancement likely related to myelofibrosis | N/A |
| Respiratory | Tracheomalacia | Tracheomalacia | Normal |
| Cardiovascular | Mild cardiomegaly on PME | Small PDA, redundant atrial septum | Small PFO, small secundum ASD |
| Gastrointestinal | Gastrostomy feeding only, gastroesophageal reflux, hepatosplenomegaly with extramedullary hematopoiesis on PME, accessory spleen | Gastrostomy feeding only, mild esophageal dysmotility, hepatomegaly likely related to extramedullary hematopoiesis | Gastrostomy feeding only, hepatomegaly likely related to extramedullary hematopoiesis |
| Orthopedic | Osteopenia, congenital hip dysplasia, tapered fifth finger | Osteopenia, femur fracture, bilateral hip dysplasia, clinodactyly, delayed bone age (2-3 y), advanced bone age (6-7 y) | Delayed bone age (−3 standard deviations below mean), bilateral hip dysplasia |
| Genitourinary | Hypoplastic labia minora and majora, underdeveloped genital tubercle, anteriorly placed anus, streak gonads, uterine didelphys, and double vagina on PME, nephromegaly with extramedullary hematopoiesis on PME | Nephromegaly, likely related to extramedullary hematopoiesis | Normal |
| Endocrine | Mild adrenal cortical lipid depletion and pituitary hypoplasia on PME, elevated FSH and LH for age, normal testosterone | Elevated FSH for age | Normal |
| Audiological | Bilateral mild to moderate high-frequency sensorineural hearing impairment | Normal | N/A |
| Immunologic | Frequent infections (Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Candida albicans, influenza A virus, respiratory syncytial virus) | Normal IgG, IgA, IgM, and IgE, normal CH50 activity, normal numbers and ratios of CD4+ and CD8+ T cells and CD19+ B cells, NK cells and CD4+/CD45RA+ naïve T cells increased in absolute number with normal ratio, CD4+/CD45RO+ memory cells low in absolute number and ratio | Frequent urinary tract infections |
| Metabolic | Hypertriglyceridemia (249-335 mg/dL) | Hypertriglyceridemia (155-1086 mg/dL) | Hypertriglyceridemia (176-296 mg/dL) |
| Development/ cognition | Global developmental delay | At 9 y, severe intellectual disability, nonverbal, nonambulatory | At 5 y, severe intellectual disability, nonverbal, nonambulatory |
| . | Patient VI.2 . | Patient VI.3 . | Patient VI.4 . |
|---|---|---|---|
| Phenotype | Female | Female | Female |
| Chromosomes | 46,XY | 46,XX | 46,XX |
| CMA | Normal (BG v.5 and 400 000 oligonucleotide research array) | Normal (BG v.6.4) | Normal (BG v.8.3) |
| Growth | Age, 11 mo; height, 25%; weight, 75%; OFC, 25%-50% | Age, 9 y 2 mo; height, <1%; weight, 76%; OFC, 18% | Age, 4 y 11 mo; height, <1%; weight, 57%; OFC: 5% |
| Facial features | Narrow bi-temporal diameter, metopic prominence, sloping forehead, upslanting palpebral fissures, epicanthal folds, bulbous nose, depressed nasal tip | Prominent nasal bridge, low-set posteriorly rotated ears, retromicrognathia, high-arched palate | Bi-temporal narrowing, tubular nose, prominent columella |
| Hematologic | SCN transiently responsive to intermediate-dose filgrastim, PB NRBCs, progressive anemia and thrombocytopenia, hypercellular bone marrow with severe reticulin fibrosis and myeloid hyperplasia, extramedullary hematopoiesis (liver, spleen, kidneys, lymph nodes) on PME | SCN without maturation arrest, refractory to filgrastim, transfusion-dependent anemia, variable thrombocytopenia including at birth and with infection, PB NRBCs, normocellular bone marrow with moderate to severe reticulin fibrosis and maturing trilineage hematopoiesis | SCN refractory to filgrastim, moderate anemia at birth (variably present subsequently), PB NRBCs, hypercellular bone marrow with moderate to severe reticulin fibrosis and maturing trilineage hematopoiesis |
| Ophthalmologic | Poor visual response, microphthalmia, hypoplastic irides, microcornea, blepharophimosis, absent retinal vessels, aplastic optic nerves | Poor visual response, microphthalmia, microcornea, blepharophimosis, aplastic optic nerves | Poor visual response, microphthalmia, aniridia with iris coloboma, blepharophimosis, dysplastic optic nerves, absent retinal vessels |
| Neurologic | Increased muscle tone in the lower extremities, DTRs 2+, poor head control | Increased muscle tone in the 4 extremities, DTRs 3+, normal electroencephalogram | Joint restriction, hypotonia, diminished strength, muscle atrophy, normal DTRs |
| Brain MRI | Prominent supratentorial ventricles, sulci, and cisterns, widened sylvian fissures, thin corpus callosum, cerebral atrophy, severe optic nerve and chiasm hypoplasia, diffuse expansion of diploic calvarial marrow space | Perinatal intraparenchymal and subarachnoid hemorrhages, supratentorial volume loss, thin corpus callosum, mild myelin maturation delay, hypoplasia of the optic nerve, chiasm, and optic tract, bone enhancement likely related to myelofibrosis | N/A |
| Respiratory | Tracheomalacia | Tracheomalacia | Normal |
| Cardiovascular | Mild cardiomegaly on PME | Small PDA, redundant atrial septum | Small PFO, small secundum ASD |
| Gastrointestinal | Gastrostomy feeding only, gastroesophageal reflux, hepatosplenomegaly with extramedullary hematopoiesis on PME, accessory spleen | Gastrostomy feeding only, mild esophageal dysmotility, hepatomegaly likely related to extramedullary hematopoiesis | Gastrostomy feeding only, hepatomegaly likely related to extramedullary hematopoiesis |
| Orthopedic | Osteopenia, congenital hip dysplasia, tapered fifth finger | Osteopenia, femur fracture, bilateral hip dysplasia, clinodactyly, delayed bone age (2-3 y), advanced bone age (6-7 y) | Delayed bone age (−3 standard deviations below mean), bilateral hip dysplasia |
| Genitourinary | Hypoplastic labia minora and majora, underdeveloped genital tubercle, anteriorly placed anus, streak gonads, uterine didelphys, and double vagina on PME, nephromegaly with extramedullary hematopoiesis on PME | Nephromegaly, likely related to extramedullary hematopoiesis | Normal |
| Endocrine | Mild adrenal cortical lipid depletion and pituitary hypoplasia on PME, elevated FSH and LH for age, normal testosterone | Elevated FSH for age | Normal |
| Audiological | Bilateral mild to moderate high-frequency sensorineural hearing impairment | Normal | N/A |
| Immunologic | Frequent infections (Pseudomonas aeruginosa, Stenotrophomonas maltophilia, Candida albicans, influenza A virus, respiratory syncytial virus) | Normal IgG, IgA, IgM, and IgE, normal CH50 activity, normal numbers and ratios of CD4+ and CD8+ T cells and CD19+ B cells, NK cells and CD4+/CD45RA+ naïve T cells increased in absolute number with normal ratio, CD4+/CD45RO+ memory cells low in absolute number and ratio | Frequent urinary tract infections |
| Metabolic | Hypertriglyceridemia (249-335 mg/dL) | Hypertriglyceridemia (155-1086 mg/dL) | Hypertriglyceridemia (176-296 mg/dL) |
| Development/ cognition | Global developmental delay | At 9 y, severe intellectual disability, nonverbal, nonambulatory | At 5 y, severe intellectual disability, nonverbal, nonambulatory |
ASD, atrial septal defect; BG, Baylor Genetics Laboratory; CH50, 50% hemolytic complement activity; CMA, chromosomal microarray analysis; DTR, deep tendon reflex; FSH, follicle-stimulating hormone; Ig, immunoglobulin; LH, luteinizing hormone; MRI, magnetic resonance imaging; N/A, not assessed; NK, natural killer; OFC, occipital frontal circumference; PB NRBC, peripheral blood nucleated red blood cell; PDA, patent ductus arteriosus; PFO, patent foramen ovale; PME, postmortem examination; SCN, severe congenital neutropenia.
The parents and a fourth sibling (patient VI.5 who is currently 7 months of age) have no neurologic or ophthalmologic abnormalities. The fourth sibling had transient neonatal thrombocytopenia and anemia and has persistent, mild neutropenia (supplemental Figure 4). The mother has had chronic moderate thrombocytopenia, an elevated immature platelet fraction, and peripheral blood smear with macrothrombocytes among variably sized platelets. These findings have been interpreted as consistent with immune thrombocytopenia. Her complete blood count is otherwise normal. The father’s complete blood count is normal.
This study was approved by the Institutional Review Board for Human Subject Research at Baylor College of Medicine. The parents gave written informed consent before evaluation, counseling, and testing.
Whole-exome sequencing on the proband (patient VI.2) revealed a variant (NM_022340.3:c.289G>C; NP_001289307.1:p.Gly97Arg) in RBSN, the gene encoding rabenosyn-5, which was homozygous in the 3 affected children, heterozygous in each parent and patient VI.5, and predicted to affect messenger RNA splicing and protein function (supplemental Figures 5 and 6A).
To assess splicing, we sequenced complementary DNA libraries from the proband (patient VI.2) and the father. Normally spliced transcripts were not observed in the proband and represented 45% of transcripts in the father. The most common abnormal transcripts had skipping of exon 5 or an alternative exon 5a (supplemental Figure 7). High-throughput RNA sequencing confirmed these observations and revealed abnormally spliced transcripts that retained exon 5 and its neighboring introns (supplemental Figure 6B).
Immunoblotting showed an absence of intact rabenosyn-5 in the affected patients (supplemental Figure 6C). Immunofluorescence staining revealed larger, clustered EEA1-positive endosomes in patient fibroblasts compared with controls (supplemental Figure 6D). These findings suggest that the absence of intact rabenosyn-5 affects the structure and function of early endosomes.
Transferrin uptake and recycling assays revealed quantitative and qualitative differences in cells from the affected patients compared with those from a healthy control. After 30 minutes of uptake, transferrin was localized to a more clustered perinuclear region in cells from the affected patient (supplemental Figure 6E). In addition, total transferrin accumulation was higher and the washout phase was delayed in cells from the affected patient (supplemental Figure 6F). These findings are consistent with a decreased rate of recycling of the transferrin receptor and increased steady-state intracellular accumulation of the ligand in cells harboring the RBSN variant. The perinuclear clustering and the increased transferrin accumulation were reversed in cells transfected with a version of the human RBSN gene that does not have the variant (supplemental Figure 8).
Our observations suggest that RBSN loss of function causes a severe syndromic form of congenital myelofibrosis and confirm that rabenosyn-5 plays a vital role in human development and hematopoiesis. The mechanisms by which RBSN loss of function leads to hematologic and developmental abnormalities are unknown. As previously suggested for VPS45, the neutropenia may result from apoptosis induced by the toxic effects of impaired endosomal trafficking.5,7 It is important to note, however, that other congenital disorders associated with increased neutrophil apoptosis, such as pathogenic variants in ELANE, do not result in congenital myelofibrosis. Thus, additional factors must be at play. One hypothesis is that the myelofibrosis results from impaired trafficking of α-granules and their release from megakaryocytes, resulting in a proinflammatory, profibrotic response. In addition, given that RBSN loss of function is predicted to result in a reduction of β1 integrin expression at the cell surface, as observed for VPS45 deficiency,5 it is tempting to speculate that the optic pathway and other central nervous system effects are the result of abnormal axonal transport of integrin transmembrane proteins during development.
The phenotypic overlap between our patients and those with mutations affecting VPS455-8 suggests the existence of a distinct disorder of early endocytosis caused by pathogenic variants in the genes encoding either member of the rabenosyn-5/VPS45 complex. SCN without maturation arrest, reticulin fibrosis of the bone marrow, and extramedullary hematopoiesis seem to be the hallmarks of the disease. Anemia and thrombocytopenia in our patients were variable and exacerbated by infection and progressive hepatosplenomegaly (supplemental Table 1; supplemental Figure 3). Long-term survival without substantial hematologic or infectious complications was possible after bone marrow or stem cell transplantation, suggesting that the hematopoietic defect is intrinsic to hematopoietic stem and progenitor cells, although a transferable factor from the transplanted cells to the bone marrow cannot be excluded. The neurologic and ophthalmologic abnormalities in the surviving patients have remained stable. Other concordant features in our patients include facial dysmorphism, abnormal bone development, and hypertriglyceridemia. The only sibling homozygous for the RBSN variant who had a 46,XY chromosome complement also had a disorder of sex development. This seems to be part of the syndrome, and we hypothesize that it could be the result of ineffective endocytosis of sex hormones in utero, as has been observed in animal models.10 Confirmation will require additional cases of 46,XY individuals with loss-of-function RBSN variants.
The mother’s hematologic abnormality is consistent with immune thrombocytopenia. This could explain the transient neonatal thrombocytopenia in the heterozygous sibling (patient VI.5), but it would not explain his transient anemia and persistent, mild neutropenia. It is certainly possible that the hematologic manifestations in these 2 individuals represent a milder phenotype caused by the RBSN variant in heterozygous state.
The structural and phenotypic effects of the variant we report are considerably more severe than those of the previously reported RBSN variant (p.Gly425Arg).9 In that patient, intact rabenosyn-5 was detected by immunoblot, the subcellular localization of the protein by immunofluorescence staining was normal, and transferrin endocytosis and recycling assays showed a significantly enhanced rate of recycling suggestive of a gain-of-function effect. However, the phenotypic overlap with our patients suggests that the p.Gly425Arg variant is also pathogenic, leading to a milder phenotype within the same spectrum. Therefore, pathogenic RBSN variants, in homozygous or heterozygous state, should also be considered in cases of unexplained transient neutropenia, particularly when neurologic abnormalities and/or myelofibrosis are also present.
The online version of this article contains a data supplement.
Acknowledgments
The authors thank the family who participated in the study, the health care providers who referred them to us for evaluation, and Cynthia Dunbar for critical review of the manuscript.
This work was supported by the Intramural Research Program of the National Institute of Allergy and Infectious Diseases at the National Institutes of Health (NIH) (L.M.F.) and the Intramural Research Program of the National Human Genome Research Institute at NIH (O.A.S.).
Authorship
Contribution: O.A.S., M.N.B., S.Y., C.S., G.Z., P.P.H., M.G., D.E., D.M.M., R.A.G., A.A.B., S.C., and L.M.F. designed and performed the research; P.L.M., O.A.S., S.B.-S., L.P., R.A.L., A.A.B., D.A.S., and L.M.F. collected data; P.L.M., O.A.S., M.N.B., S.Y., R.A.L., C.S., A.N.M., M.T.E., G.Z., P.P.H., M.G., D.E., D.M.M., R.A.G., A.A.B., D.A.S., S.C., and L.M.F. analyzed and interpreted data; S.C. performed statistical analysis; P.L.M., O.A.S., R.A.L., A.A.B., D.A.S., S.C., and L.M.F. wrote the manuscript; and P.L.M., O.A.S., and L.M.F. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Luis M. Franco, Laboratory of Immune System Biology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, 9000 Rockville Pike, Building 4, Room 138, Bethesda, MD 20892; e-mail: luis.franco@nih.gov.
References
Author notes
P.L.M. and O.A.S. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal