Key Points
CK1α is essential for the survival of PEL cell lines, and its degradation mediates toxicity of IMiDs.
Loss of IRF4 expression is a CK1α-, IKZF1-, and IKZF3-independent arm of IMiD toxicity in PEL cell lines.
Abstract
Primary effusion lymphoma (PEL) is an aggressive cancer with few treatment options. The immunomodulatory drugs (IMiDs) lenalidomide and pomalidomide have recently been shown to kill PEL cell lines, and lenalidomide is in clinical trials against PEL. IMiDs bind to the CRL4CRBN E3 ubiquitin ligase complex, leading to the acquisition of the Ikaros family zinc finger proteins 1 and 3 (IKZF1 and IKZF3), casein kinase 1 α (CK1α), and zinc finger protein 91 (ZFP91) as neosubstrates. IMiDs are effective against multiple myeloma because of degradation of IKZF1 and IKZF3 and the consequent loss of interferon regulatory factor 4 (IRF4) and MYC expression. Lenalidomide is also effective in chromosome 5q deletion–associated myelodysplastic syndrome as a result of degradation of CK1α. An essential IKZF1-IRF4-MYC axis has recently been proposed to underlie the toxicity of IMiDs in PEL. Here, we further investigate IMiD effectors in PEL cell lines, based on genome-wide CRISPR/Cas9 screens for essential human genes. These screens and extensive validation experiments show that, of the 4 neosubstrates, only CK1α is essential for the survival of PEL cell lines. In contrast, IKZF1 and IKZF3 are dispensable, individually or in combination. IRF4 was critical in all 8 PEL cell lines tested, and surprisingly, IMiDs triggered downregulation of IRF4 expression independently of both IKZF1 and IKZF3. Reexpression of CK1α and/or IRF4 partially rescued PEL cell lines from IMiD-mediated toxicity. In conclusion, IMiD toxicity in PEL cell lines is independent of IKZF1 and IKZF3 but proceeds through degradation of the neosubstrate CK1α and downregulation of IRF4.
Introduction
Kaposi sarcoma–associated herpesvirus (KSHV) causes Kaposi sarcoma, a form of multicentric Castleman disease and primary effusion lymphoma (PEL).1-4 PEL is a highly aggressive non-Hodgkin B-cell lymphoma that presents in serous body cavities or, less commonly, as extracavitary tumors.5 The defining feature of PEL is infection of all tumor cells by KSHV. Accordingly, KSHV latent gene expression is essential for the survival and proliferation of PEL-derived cell lines.6-8 Approximately 80% of PEL tumors also carry Epstein-Barr virus.5
PEL is most common in HIV-infected patients, in whom it comprises ∼4% of HIV-related non-Hodgkin lymphomas,9 but also occurs in elderly patients and those treated with transplantation. PEL carries a very poor prognosis10,11 and is currently treated with conventional or modified combinations of several chemotherapy drugs, such as cyclophosphamide, doxorubicin, vincristine, and prednisone (a regimen called CHOP) or related combinations that also include etoposide (EPOCH).12,13 In HIV+ patients, treatment also includes combination antiretroviral therapy. Targeted therapies that exploit specific molecular vulnerabilities in PEL are lacking. The only interventional clinical trial currently recruiting patients with PEL combines the immunomodulatory drug (IMiD) lenalidomide with dose-adjusted EPOCH and the anti-CD20 monoclonal antibody rituximab.14
IMiDs are a family of thalidomide derivatives, including lenalidomide and pomalidomide. Lenalidomide is in use as frontline therapy for multiple myeloma (MM), and pomalidomide is used for treatment of relapsed or refractory cases. Lenalidomide is also approved for mantle cell lymphoma and is effective in myelodysplastic syndrome associated with the haploid deletion of chromosome 5q (del5q-MDS). The molecular target of IMiDs is cereblon (encoded by CRBN),15 the substrate recognition subunit of the cullin-RING E3 ubiquitin ligase complex CRL4CRBN. IMiD-bound CRL4CRBN complexes gain neosubstrates,16-18 which underlies at least some of the observed anticancer activity. In MM cell lines, either deletion of CRBN or disruption of its IMiD binding site eliminates IMiD toxicity.18
In MM, the neosubstrates IKZF1 (Ikaros) and IKZF3 (Aiolos) control a critical IKZF1/IKZF3-IRF4-MYC transcriptional axis.17,18 All tested MM cell lines are addicted to IRF4 and MYC expression, irrespective of oncogenic driver mutation.19 Consistent with this mechanism of action, the reexpression of IRF4 conferred relative IMiD resistance to the MM cell line MM.1S in a competition assay.18 In del5q-MDS, casein kinase 1 α (CK1α; encoded on chromosome 5q by CSNK1A1) is the neosubstrate that likely explains the observed efficacy of lenalidomide.16 In a mouse model, haploid expression of CK1α increased Wnt signaling and provided a proliferative advantage to hematopoietic stem cells, whereas complete inactivation of CK1α triggered a p53-induced loss of viability.20 Accordingly, the enhanced sensitivity to lenalidomide of murine CSNK1A1+/− hematopoietic stem and progenitor cells compared with CSNK1A1+/+ cells was rescued by the heterozygous deletion of p53.16 Therefore, a threshold for minimal CK1α expression in del5q-MDS exists, which sensitizes this disease to lenalidomide-induced CK1α degradation. In addition to these effects, IMiDs have clinically relevant antiangiogenic and immune modulatory outcomes with uncertain molecular mechanisms. A final recently identified neosubstrate, ZPF91, has not been linked to clinical effects of IMiDs to date.21
A recent report showed that PEL-derived cell lines are highly susceptible to lenalidomide and pomalidomide.22 Both drugs trigger loss of IKZF1, IRF4, and MYC expression in PEL cell lines.22,23 Functional knockdown experiments supported the notion that PEL, like MM, requires an IKZF1-IRF4-MYC axis for its survival.22 We recently conducted genome-wide CRISPR/Cas9 knockout screens in a panel of 8 PEL cell lines.24 Resulting data provided an unbiased picture of the gene essentiality landscape of PEL cell lines and identified several candidate drug targets. Surprisingly, however, our screens did not detect the previously reported requirement of PEL cell lines for IKZF122 but instead pointed to a requirement for CK1α expression. Importantly, IRF4 scored among the most confident single gene requirements in all 8 PEL cell lines, consistent with the recently reported role for IRF4 in the PEL cell line BC-3.22 These unexpected findings prompted us to revisit the mechanism of action of IMiDs in PEL. Our results show that IKZF1 and IKZF3 are indeed dispensable for the survival and proliferation of PEL cell lines. In agreement with this finding, the combined inactivation of IKZF1 and IKZF3 did not affect the response of PEL cell lines to IMiDs. Instead, this work identifies the degradation of CK1α and an IKZF1/3-independent downregulation of IRF4 as mechanisms of IMiD toxicity in PEL cell lines.
Methods
Cell lines, lentiviral preparation, and reagents
All parental cell lines were maintained as described recently.24 Lenalidomide and pomalidomide were from Cayman Chemicals (Ann Arbor, MI) and Selleck Chemicals (Houston, TX), respectively, and dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO). Doxycycline (Dox) was from Enzo Life Sciences (Farmingdale, NY). Lentiviruses were prepared as described previously and functionally titrated.24 Cas9-expressing cell lines are described in detail elsewhere.24 Briefly, these cell lines were generated by lentiviral transduction with pLenti-Cas925 at multiplicities of infection (MOIs) between 0.6 and 1.5. For BC-3 and BCBL-1, Cas9-expressing cell clones were obtained by limited-dilution cloning into 96-well round-bottom plates.
Vector design and cloning procedures
Single-guide RNA (sgRNA) sequences and primers are listed in supplemental Table 1 (available on the Blood Web site), other primers and synthetic DNAs in supplemental Table 2, and short hairpin RNA (shRNA) target and hairpin sequences in supplemental Table 3. sgRNAs were designed using published parameters26 and inserted into pLenti-guide puro25 (Addgene #52963) or pLenti SpBsmBI sgRNA Hygro27 (Addgene #62205). sgAAVS1 and sgIRF4 are published controls.24,28,29 Detailed cloning procedures for lentiviral miR-30–embedded shRNA and protein expression can be found in the supplemental Methods.
Generation of knockout cell lines
To inactivate CRBN, BC-3 cells were transduced with the Dox-inducible Cas9 expression vector pCW-Cas928 (Addgene #50661) at an MOI <1. After complete puromycin selection, these cells were transduced again with pLenti SpBsmBI sgRNA Hygro27 (Addgene #52963) carrying a CRBN-specific sgRNA, selected by addition of hygromycin, and Cas9 expression was induced using 1 µg/mL of Dox. Both hygromycin and Dox were withdrawn after 5 days, and cells were plated for limited-dilution cloning. Resulting clones were screened for loss of CRBN expression by western blot. To create IKZF1/IKZF3 double-knockout cell pools, clonal Cas9 expressing BC-3 and BCBL-1 cells were sequentially transduced with and selected for pLenti SpBsmBI sgRNA Hygro27 containing IKZF1 sg1 and pLenti-guide puro25 containing IKZF3 sg1.
Growth curve analyses
PEL cell lines (6 × 105 naïve for shRNA or Cas9 expressing for sgRNAs) were transduced with pZIP-hCMV-ZsGreen-P2A-Puro shRNA vectors or pLenti-guide puro sgRNA vectors at MOIs between 2 and 3. Twenty-four hours later, media were changed, and cells were selected with puromycin for 2 days. Relative live cell numbers were measured every 2 days using the Cell Titer Glo 2.0 kit (Promega, Madison, WI). At each time point, resulting data were used to calculate absolute live cell numbers, based on manual counting of a subset of samples, and cell concentrations were adjusted to 2 × 105 or 3 × 105 cells per mL. Cumulative live cell numbers were calculated by considering the dilution factors at every passage and normalized to the cumulative live cell numbers of control cell pools transduced with the nontargeting shRNA vector (NT4) or sgAAVS1. Other growth curve and time course assays were performed similarly, except that cells were spun at every passage and resuspended in fresh media.
IC50 dose-response assays
Cells were seeded in wells of 24-well plates with 2 × 105 cells per mL (day 0) and treated with the indicated concentrations of lenalidomide or pomalidomide. The negative control well received the same volume of DMSO as the well with the highest drug concentration. The final volume of drug or DMSO added was <0.1% of the total volume in all wells. Cells were split 1:2 and redosed with drug or DMSO on day 3. Cell viability was measured on day 5 using Cell Titer Glo 2.0 (Promega). For 50% inhibitory concentration (IC50) assays after IRF4 overexpression, cells were treated with 1 μg/mL of Dox for 24 hours before seeding cells for the IC50 assay. IC50 values were calculated using GraphPad Prism.
Quantitative western blotting
Quantitative western blotting was performed as reported.24 Briefly, cells were lysed in radioimmunoprecipitation assay buffer and lysates subjected to 6 cycles of sonication (30 seconds on, 30 seconds off) in a 4°C water bath using the Bioruptor Sonication System (Diagenode, Denville, NJ) at the high-intensity setting. Lysates were cleared and resolved in Bolt 4% to 12% Bis-Tris gradient gels (Thermo Fisher Scientific) and transferred to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). Settings for primary antibodies are listed in supplemental Table 4. IRDye 800CW–conjugated secondary antibodies were used and detected with the Odyssey Fc Dual-Mode Imaging System (LI-COR).
Results
Genome-wide CRISPR screens reveal functionally essential IMiD effectors in PEL cells
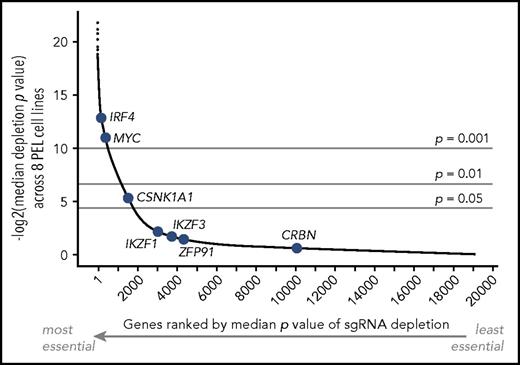
Our laboratory recently conducted genome-wide CRISPR/Cas9 knockout screens for genes that are essential for survival and/or proliferation across a panel of 8 PEL cell lines.24 We analyzed resulting data for the essentiality of candidate IMiD effectors (Figure 1). The screens and validation experiments revealed a critical dependency on IRF4 in all 8 PEL cell lines.24 MYC scored as essential in each of our screens as well as in all similar screens reported to date, presumably because of a housekeeping function.30-32 The screens confirmed that CRBN is indeed nonessential in PEL cell lines, as reported previously22 and also seen in MM.18 Surprisingly, the screens strongly suggested that IKZF1 is nonessential for the proliferation and survival of PEL cell lines. Similarly, IKZF3 and ZFP91 scored as nonessential. In contrast, sgRNAs targeting CSNK1A1 were significantly depleted across PEL cell lines, indicating that CSNK1A1 is essential or a fitness gene in PEL cells. These results therefore point to CK1α, but not IKZF1, as a potential IMiD effector in PEL cell lines. We therefore decided to further investigate the mechanism of IMiD action in PEL cell lines.
Essentiality of IMiD effectors across PEL cell lines. Median significance of essentiality of all genes screened by the Brunello sgRNA library across 8 PEL cell lines. Genes are ranked using median P value scores. The position of genes that have been implicated in IMiD action is indicated in blue, including the IMiD target CRBN, neosubstrates IKZF1, IKZF3, CSNK1A1, and ZFP91, and downstream effectors IRF4 and MYC. Only IRF4, MYC, and CSNK1A1 score as essential across PEL cell lines.
Essentiality of IMiD effectors across PEL cell lines. Median significance of essentiality of all genes screened by the Brunello sgRNA library across 8 PEL cell lines. Genes are ranked using median P value scores. The position of genes that have been implicated in IMiD action is indicated in blue, including the IMiD target CRBN, neosubstrates IKZF1, IKZF3, CSNK1A1, and ZFP91, and downstream effectors IRF4 and MYC. Only IRF4, MYC, and CSNK1A1 score as essential across PEL cell lines.
IMiDs induce the CRBN-mediated degradation of CK1α, ZFP91, IKZF1, and IKZF3 in PEL cell lines
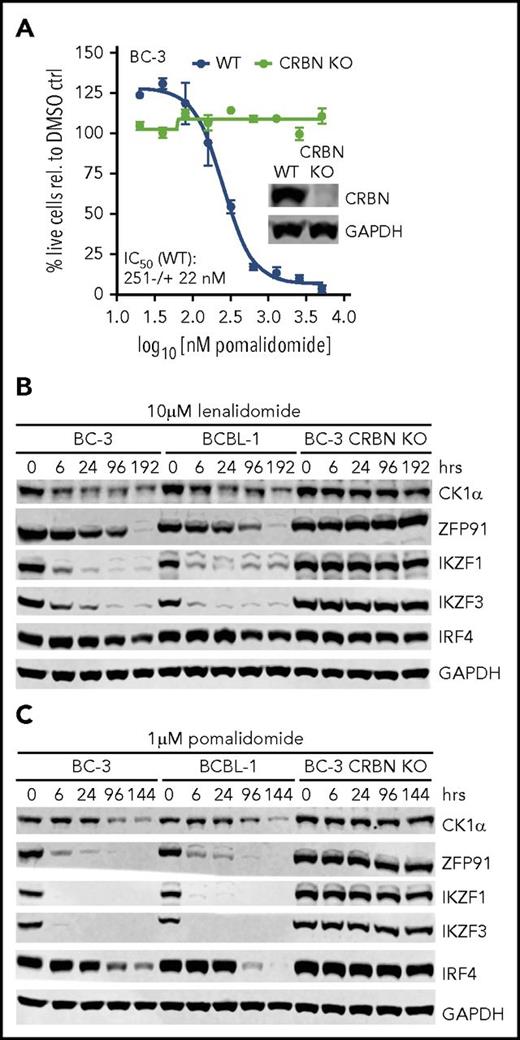
We first tested whether IMiDs induce the degradation of CK1α and the other neosubstrates in PEL cell lines. As a specificity control and to confirm that the acquisition of neosubstrates by IMiD-bound CRL4CRBN underlies IMiD toxicity in PEL cell lines, we created a clonal CRBN knockout BC-3 line using CRISPR/Cas9 gene editing. The IC50 for pomalidomide in unmodified BC-3 was very close to reported data.22 At saturating concentrations, lenalidomide and pomalidomide triggered a complete loss of viability 9 to 10 days and 5 to 8 days into treatment, respectively (Figure 2A; supplemental Figure 1A). As expected, CRBN knockout BC-3 cells proliferated normally, and inactivation of CRBN provided complete resistance to pomalidomide (Figure 2A; supplemental Figure 1A). Treatment of naïve BC-3 or BCBL-1 cells with either lenalidomide or pomalidomide indeed resulted in the rapid degradation of CK1α (Figure 2B-C; supplemental Figure 1B). These experiments furthermore showed degradation of the neosubstrate ZFP91 and confirmed the recently reported degradation of the neosubstrates IKZF1 and IKZF3 and the delayed loss of IRF4.22 None of these proteins were downregulated in CRBN knockout cells (Figure 2B-C; supplemental Figure 1B). Similar, but delayed, responses were observed using lower concentrations of these drugs (supplemental Figure 2). Together, these results suggest that the neosubstrate CK1α is indeed a novel candidate for an IMiD effector in PEL cell lines.
Treatment of PEL cell lines with lenalidomide and pomalidomide leads to degradation of neosubstrates IKZF1, IKZF3, ZFP91, and CK1α in a CRBN-dependent manner. (A) IC50 curves in parental BC-3 or a BC-3 clone with CRISPR-inactivated CRBN (n = 3; error bars represent standard error of the mean). Inset: western blot analysis of BC-3 and the CRBN knockout (KO) clone. (B-C) Quantitative western blot analysis of CK1α, ZFP91, IKZF1, IKZF3, and IRF4 expression at the indicated time points after treatment of BC-3, BCBL-1, or BC-3 CRBN KO cells with 10 μM of lenalidomide (B) or 1 μM of pomalidomide (C). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading control. Supplemental Figure 1 shows growth curve and quantitative analysis. WT, wild type.
Treatment of PEL cell lines with lenalidomide and pomalidomide leads to degradation of neosubstrates IKZF1, IKZF3, ZFP91, and CK1α in a CRBN-dependent manner. (A) IC50 curves in parental BC-3 or a BC-3 clone with CRISPR-inactivated CRBN (n = 3; error bars represent standard error of the mean). Inset: western blot analysis of BC-3 and the CRBN knockout (KO) clone. (B-C) Quantitative western blot analysis of CK1α, ZFP91, IKZF1, IKZF3, and IRF4 expression at the indicated time points after treatment of BC-3, BCBL-1, or BC-3 CRBN KO cells with 10 μM of lenalidomide (B) or 1 μM of pomalidomide (C). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as loading control. Supplemental Figure 1 shows growth curve and quantitative analysis. WT, wild type.
CK1α is essential in most PEL cell lines
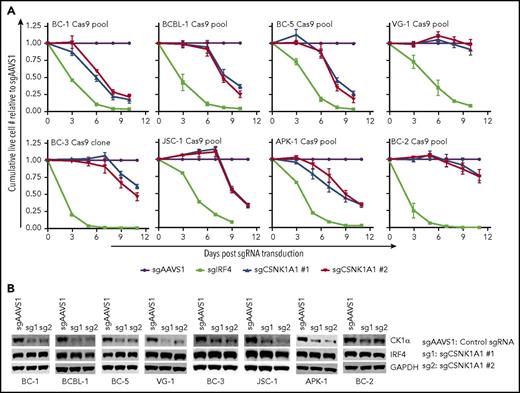
A role for CK1α in PEL has not been reported to date. To confirm the requirement for CK1α in PEL cell lines (Figure 1), a panel of 8 Cas9-expressing PEL cell lines was transduced with individual lentiviral sgRNAs targeting CK1α, a validated positive control guide against IRF4,24 or a negative control guide against the noncoding locus AAVS1.28 Absolute live cell numbers were monitored over time. As expected,22,24 sgRNA-mediated inactivation of IRF4 triggered rapid cell death in all 8 cell lines (Figure 3A; supplemental Figure 3A). The inactivation of CK1α resulted in a significant loss of live cell numbers in 6 of 8 cell lines tested (Figure 3A-B). Only VG-1 and BC-2 remained viable despite relatively robust inactivation of CK1α. Although CK1α is consequently dispensable in these 2 cell lines, both cell lines responded to IMiD treatment with the downregulation of the highly essential IRF4 (supplemental Figure 3B). IRF4 protein levels were not affected in CK1α knockout cells, suggesting that cell death after loss of CK1α expression in the CK1α-dependent PEL cell lines is independent of IRF4 downregulation.
CK1α is essential for the survival of a majority of PEL cell lines. (A) Growth curve analyses of a panel of 8 Cas9-expressing PEL cell lines after transduction of 2 independent sgRNAs targeting CK1α. All cell lines were Cas9-transduced cell pools, except for BC-3/Cas9, which is a Cas9-expressing clone (described in “Methods”). sgIRF4 was included as a positive control and confirms that IRF4 is essential in all 8 PEL cell lines (supplemental Figure 3A). Cumulative live cell counts were normalized to those from cells transduced with the negative control guide sgAAVS1. Final reduction in live cell numbers after CK1α inactivation was statistically significant, except for BC-2 and VG-1 (n ≥ 3; all error bars represent standard error of the mean). (B) Representative western blot analyses confirm efficient CRISPR-induced inactivation of CK1α and demonstrate lack of regulation of IRF4. GAPDH served as loading control. Samples for westerns were taken at the end of the growth curves, except for BC-1, BC-2, and BC-5 (day 6).
CK1α is essential for the survival of a majority of PEL cell lines. (A) Growth curve analyses of a panel of 8 Cas9-expressing PEL cell lines after transduction of 2 independent sgRNAs targeting CK1α. All cell lines were Cas9-transduced cell pools, except for BC-3/Cas9, which is a Cas9-expressing clone (described in “Methods”). sgIRF4 was included as a positive control and confirms that IRF4 is essential in all 8 PEL cell lines (supplemental Figure 3A). Cumulative live cell counts were normalized to those from cells transduced with the negative control guide sgAAVS1. Final reduction in live cell numbers after CK1α inactivation was statistically significant, except for BC-2 and VG-1 (n ≥ 3; all error bars represent standard error of the mean). (B) Representative western blot analyses confirm efficient CRISPR-induced inactivation of CK1α and demonstrate lack of regulation of IRF4. GAPDH served as loading control. Samples for westerns were taken at the end of the growth curves, except for BC-1, BC-2, and BC-5 (day 6).
IKZF1, IKZF3, and ZFP91 are dispensable in PEL cells
These findings suggest that degradation of CK1α and loss of IRF4 expression are independent mechanisms of IMiD toxicity in PEL. Although the neosubstrate IKZF1 has recently been implicated upstream of IRF4 in PEL cell lines, our gene essentiality screens failed to detect an essential role for IKZF1. To resolve this discrepancy, we directly tested whether IKZF1 and/or IKZF3 are essential in PEL cell lines using individual inactivation by CRISPR/Cas9. sgRNAs against IKZF1 (Figure 4A) or IKZF3 (Figure 4B) led to a robust loss of target gene expression as confirmed by western blotting. Neither loss of IKZF1 nor loss of IKZF3, however, measurably affected live cell numbers over time. Furthermore, the inactivation of IKZF1 did not affect IKZF3 expression, or vice versa, in either cell line. Finally, there was no significant change in IRF4 expression in sgIKZF1- or sgIKZF3-transduced cells. Similar results were obtained in an independent approach using IKZF1- or IKZF3-specific miR-30–embedded lentiviral shRNAs (Figure 4C). Thus, neither our CRISPR screens, which employ 4 independent sgRNAs in a pooled format, our CRISPR/Cas9-based validation, using 2 individually delivered sgRNAs, nor shRNA-induced knockdown suggested an essential role for IKZF1 or IKZF3 upstream of IRF4 in PEL cell lines. Finally, we confirmed that individual inactivation of ZFP91 in BC-3 and BCBL-1 did not affect viability or IRF4 expression (Figure 4D). Our results strongly support the notion that IKZF1, IKZF3, and ZFP91 are individually nonessential in PEL cells and are therefore unlikely candidates for effectors of IMiD toxicity in PEL.
IKZF1, IKZF3, and ZFP91 are dispensable for the survival of PEL cell lines. (A-B) Growth curve analyses of Cas9-expressing BC-3 or BCBL-1 cell clones after transduction with lentiviral sgRNA vectors targeting IKZF1 (A) or IKZF3 (B). Two independent sgRNAs (sg1 and sg2) were designed per target; sgRNAs targeting AAVS1 and IRF4 were used as negative and positive controls, respectively (n = 3; error bars represent standard error of the mean [SEM]). Representative western blot analyses below the growth curves confirm the efficient inactivation of the targeted genes and demonstrate lack of regulation of IRF4. GAPDH served as loading control. (C) Growth curve analyses of naïve BC-3 or BCBL-1 cells after transduction with lentiviral shRNA vectors targeting IKZF1 or IKZF3. The nontargeting shRNA NT4 served as negative control (n = 3; error bars represent SEM). Representative western blot analyses below the growth curves assess the degree of shRNA-mediated target knockdown and IRF4 expression. GAPDH served as loading control. (D) Same as panels A and B, except that sgRNAs against ZFP91 were used (n = 3; error bars represent SEM). KO, knockout.
IKZF1, IKZF3, and ZFP91 are dispensable for the survival of PEL cell lines. (A-B) Growth curve analyses of Cas9-expressing BC-3 or BCBL-1 cell clones after transduction with lentiviral sgRNA vectors targeting IKZF1 (A) or IKZF3 (B). Two independent sgRNAs (sg1 and sg2) were designed per target; sgRNAs targeting AAVS1 and IRF4 were used as negative and positive controls, respectively (n = 3; error bars represent standard error of the mean [SEM]). Representative western blot analyses below the growth curves confirm the efficient inactivation of the targeted genes and demonstrate lack of regulation of IRF4. GAPDH served as loading control. (C) Growth curve analyses of naïve BC-3 or BCBL-1 cells after transduction with lentiviral shRNA vectors targeting IKZF1 or IKZF3. The nontargeting shRNA NT4 served as negative control (n = 3; error bars represent SEM). Representative western blot analyses below the growth curves assess the degree of shRNA-mediated target knockdown and IRF4 expression. GAPDH served as loading control. (D) Same as panels A and B, except that sgRNAs against ZFP91 were used (n = 3; error bars represent SEM). KO, knockout.
IKZF1/3 double-knockout cells are viable and exhibit unchanged sensitivity to IMiDs
Given that IKZF1 and IKZF3 are not individually essential in PEL cell lines, we reasoned they might functionally compensate for each other’s loss. This idea is supported by the observation that expression of degradation-resistant versions of either IKZF1 or IKZF3 can confer partial resistance against IMiDs in an MM cell line,17,18 suggesting a degree of functional redundancy between these proteins. We therefore inactivated both proteins in Cas9-expressing BC-3 or BCBL-1 cells by sequential transduction of lentiviruses expressing the targeting sgRNAs. The almost complete loss of both IKZF1 and IKZF3 expression was confirmed by western blotting (Figure 5A). IRF4 expression was not altered in these cells. Double-knockout cell pools were viable and grew similarly to WT BC-3 and BCBL-1 cells (Figure 5B).
IKZF1 and IKZF3 double-knockout (DKO) cells are viable and susceptible to IMiD-induced toxicity and IRF4 downregulation. (A) Western blot analyses confirm efficient inactivation of IKZF1 and IKZF3 in Cas9-expressing BC-3 and BCBL-1 sequentially transduced with targeting sgRNAs. IRF4 expression was not altered, and GAPDH was the loading control. (B) Growth curve analyses of cell lines described in panel A (n = 3; error bars represent standard error of the mean [SEM]). (C) Pomalidomide IC50 curves of cell lines described in panel A. The BC-3 CRBN knockout (KO) clone was included as an additional control (n = 3; error bars represent SEM). (D) Growth curve analyses after pomalidomide treatment of the cell lines described in panel A; live cell numbers are plotted relative to DMSO-treated controls (n = 3; error bars represent SEM). (E) Representative western blot analysis of IKZF1, IRF4, and MYC expression at all time points analyzed in panel D. GAPDH served as loading control.
IKZF1 and IKZF3 double-knockout (DKO) cells are viable and susceptible to IMiD-induced toxicity and IRF4 downregulation. (A) Western blot analyses confirm efficient inactivation of IKZF1 and IKZF3 in Cas9-expressing BC-3 and BCBL-1 sequentially transduced with targeting sgRNAs. IRF4 expression was not altered, and GAPDH was the loading control. (B) Growth curve analyses of cell lines described in panel A (n = 3; error bars represent standard error of the mean [SEM]). (C) Pomalidomide IC50 curves of cell lines described in panel A. The BC-3 CRBN knockout (KO) clone was included as an additional control (n = 3; error bars represent SEM). (D) Growth curve analyses after pomalidomide treatment of the cell lines described in panel A; live cell numbers are plotted relative to DMSO-treated controls (n = 3; error bars represent SEM). (E) Representative western blot analysis of IKZF1, IRF4, and MYC expression at all time points analyzed in panel D. GAPDH served as loading control.
Importantly, the loss of both IKZF1 and IKZF3 did not provide resistance to pomalidomide (Figure 5C). Pomalidomide toxicity in IKZF1/3 double-knockout cells was also not delayed compared with WT cells (Figure 5D). Intriguingly, pomalidomide triggered comparable downregulation of IRF4 and MYC in WT and IKZF1/3 double-knockout BC-3 and BCBL-1 cells. In sum, our data indicate that IKZF1 and IKZF3 are simultaneously dispensable in PEL. Importantly, IMiD-induced downregulation of IRF4 and MYC is independent of IKZF1 and IKZF3 in PEL cells.
Loss of IRF4 and loss of CK1α both contribute to IMiD-mediated toxicity
The experiments presented so far suggest at least 2 independent mechanisms for toxicity of IMiDs in PEL cells: (1) the direct IMiD-induced degradation of CK1α by CRL4CRBN and (2) the indirect downregulation of oncogenic IRF4. We next tested whether reexpression of either CK1α or IRF4 conferred at least partial resistance to IMiD-mediated toxicity in PEL cells.
Because ectopic CK1α is still susceptible to ubiquitination by IMiD-bound CRL4CRBN, we sought to construct an IMiD-resistant mutant of CK1α. On the basis of a recent structure and in vitro data,33 G40N-mutant CK1α should be unable to bind IMiD-bound CRL4CRBN. G40N-mutant CK1α was indeed resistant to lenalidomide- or pomalidomide-induced degradation in 293T cells (supplemental Figure 4). In BCBL-1 cells, exogenous Flag-tagged CK1α reproducibly reduced endogenous CK1α expression, suggesting that overall expression of CK1α is tightly regulated (Figure 6A). Pomalidomide treatment of these cells confirmed that Flag-G40N CK1α is indeed resistant to CRL4CRBN degradation, which became evident at day 6 of treatment. Delayed degradation of Flag-tagged WT CK1α compared with the endogenous protein is likely due to ongoing expression of the protein from the lentiviral vector in the presence of pomalidomide. Despite these differences in expression later into pomalidomide treatment, both WT and G40N CK1α conferred equally modest increases in IC50 values for pomalidomide (Figure 6B). This result can likely be explained by the loss of IRF4, which was unaffected by reexpression of CK1α and preceded differences in WT and G40N CK1α expression (Figure 6A).
Reexpression of CK1α or IRF4 rescues PEL cells from IMiD-mediated toxicity. (A) Representative western blots of BCBL-1 cells transduced with constitutive lentiviral vectors expressing GFP, WT, or G40N CK1α. Cells were treated for the indicated duration with 1 µM of pomalidomide. The asterisk in the anti-CK1α blot marks a nonspecific band. GAPDH served as loading control. (B) Pomalidomide IC50 values of BCBL-1 cells analyzed in panel A (n = 3; error bars represent standard error of the mean [SEM]). (C) Representative western blots demonstrate efficient loss of endogenous, but not exogenous, IRF4 expression after treatment with 1 µM of pomalidomide. GAPDH served as loading control. (D) Pomalidomide IC50 values of naïve BC-3 and BCBL-1 and cell lines transduced with a Dox-inducible IRF4 expression vector (n = 3; error bars represent SEM). (E) Pomalidomide IC50 values of naïve BCBL-1 cells and cell lines transduced with a Dox-inducible IRF4 expression vector and constitutive lentiviral vectors expressing ZsGreen or G40N CK1α (n = 3; error bars represent SEM). (F) Representative western blots for IRF4 and CK1α of cells analyzed in panel E. The dotted line indicates where the image was cropped to remove irrelevant lanes. GAPDH served as a loading control. *P < .05, **P < .01, ***P < .001 by unpaired t test.
Reexpression of CK1α or IRF4 rescues PEL cells from IMiD-mediated toxicity. (A) Representative western blots of BCBL-1 cells transduced with constitutive lentiviral vectors expressing GFP, WT, or G40N CK1α. Cells were treated for the indicated duration with 1 µM of pomalidomide. The asterisk in the anti-CK1α blot marks a nonspecific band. GAPDH served as loading control. (B) Pomalidomide IC50 values of BCBL-1 cells analyzed in panel A (n = 3; error bars represent standard error of the mean [SEM]). (C) Representative western blots demonstrate efficient loss of endogenous, but not exogenous, IRF4 expression after treatment with 1 µM of pomalidomide. GAPDH served as loading control. (D) Pomalidomide IC50 values of naïve BC-3 and BCBL-1 and cell lines transduced with a Dox-inducible IRF4 expression vector (n = 3; error bars represent SEM). (E) Pomalidomide IC50 values of naïve BCBL-1 cells and cell lines transduced with a Dox-inducible IRF4 expression vector and constitutive lentiviral vectors expressing ZsGreen or G40N CK1α (n = 3; error bars represent SEM). (F) Representative western blots for IRF4 and CK1α of cells analyzed in panel E. The dotted line indicates where the image was cropped to remove irrelevant lanes. GAPDH served as a loading control. *P < .05, **P < .01, ***P < .001 by unpaired t test.
To assess rescue by reexpression of IRF4, BC-3 and BCBL-1 cells were transduced with a Dox-inducible lentiviral expression vector for IRF4. Cells were left uninduced or treated with Dox for 24 hours, to allow exogenous IRF4 expression, and then challenged with pomalidomide. Cells expressing exogenous IRF4 were significantly more resistant to IMiDs than either parental or uninduced cells, as indicated by three- or fourfold increases in pomalidomide IC50 values in BCBL-1 or BC-3 cells, respectively (Figure 6D). Pomalidomide triggered the loss of endogenous, but not exogenous, IRF4 expression in BC-3 and BCBL-1 (Figure 6C). This confirms that IRF4 is not a neosubstrate of CRL4CRBN 17,18 and excludes IMiD regulation of IRF4 at the posttranslational level. These results furthermore directly demonstrate that downregulation of IRF4 significantly contributes to the toxicity of IMiDs in PEL cells.
Finally, we assessed rescue by the combined reexpression of degradation-resistant G40N CK1α and Dox-inducible IRF4 (Figure 6E-F). Reexpression of both proteins indeed conferred significantly greater rescue than reexpression of each factor alone. However, resistance to IMiDs was not complete, hinting at the existence of other essential genes that are regulated by IMiDs in PEL.
Discussion
This study investigates the mechanism of action of IMiDs in PEL cells, using data from both genome-wide CRISPR/Cas9 knockout screens and the individual inactivation of the IMiD CRL4CRBN neosubstrates. It has been shown that lenalidomide, but not pomalidomide, triggers degradation of CK1α in a del5q-MDS cell line.16 However, the affinity of pomalidomide-bound CUL4CRBN to CK1α is reduced only approximately twofold compared with lenalidomide-bound CUL4CRBN. Both drugs induce CUL4CRBN-dependent CK1α ubiquitination in vitro, with only slight preference for lenalidomide.33 In our study, both drugs triggered the degradation of all 4 neosubstrates in PEL cell lines, further suggesting that differences between lenalidomide and pomalidomide in neosubstrate degradation are not absolute. As reported previously,22 both lenalidomide and pomalidomide treatments also inhibited IRF4 expression in PEL cells.
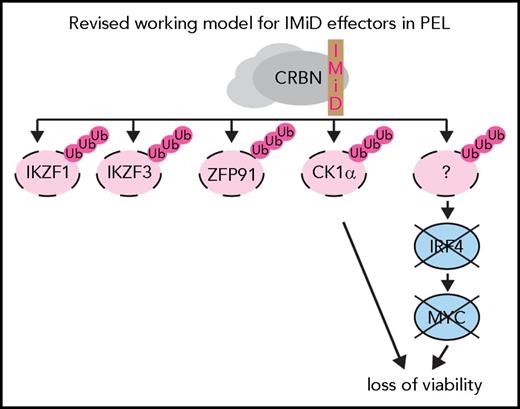
The CK1 family of serine/threonine kinases has 6 members in humans (α, γ1, γ2, γ3, δ, and ε).34 On the basis of our genome-wide knockout screens, only CK1α is essential in a large majority of PEL cell lines.24 Partial rescue by reexpression of CK1α shows that the IMiD-induced degradation of CK1α contributes significantly to IMiD-induced cell death in PEL cell lines. The inactivation of CK1α did not affect IRF4 expression, suggesting that it represents an independent arm of IMiD-induced toxicity (Figure 7).
CK1α is a multifunctional protein, with roles in the inhibition of tumor suppressor p53 and Wnt signaling, in epidermal progenitor cell maintenance, and in cancer.20,35-39 Published CRISPR screens from 16 cancer cell types suggest that CK1α is essential in most cancer cell types screened to date.24,30-32,40 CK1α and other CK1 family members are currently being investigated as potential therapeutic targets in other cancers, including chronic lymphocytic leukemia,41 acute myeloid leukemia,35 and MM.42,43 Interestingly, at least in some cases, a modest reduction in CK1α expression of approximately twofold was sufficient to trigger a significant loss of viability,35,42 suggesting that the critical threshold for CK1α expression differs between settings and does not necessarily mirror del5q-MDS. Although p53-null mutant leukemia cells were unaffected by CK1α inhibition,35 CK1α remained essential in p53-mutant MM cell lines.42 This essentiality of CK1α raises the question of whether it contributes to IMiD toxicity in MM as well. Most PEL cell lines, including BC-2 and VG-1, which did not respond to inactivation of CK1α, retain WT p53.44,45 Future studies should therefore address the mechanisms underlying CK1α essentiality in PEL. Such studies will be of particular interest, because KSHV encodes gene products that modulate p53 and Wnt signaling.46
The individual inactivation of IKZF1, IKZF3, or ZFP91 using CRISPR/Cas9 did not negatively affect PEL cell survival or proliferation. IKZF1 and IKZF3 double-knockout PEL cells were viable and proliferated normally, excluding a redundancy between these 2 genes. Furthermore, IKZF1 and IKZF3 double-knockout BC-3 or BCBL-1 cells were as sensitive to pomalidomide as parental cells. These conclusions are additionally supported by genome-wide CRISPR screens in a panel of 8 PEL cell lines and shRNA knockdown experiments. Our study therefore does not support a role of IKZF1 as an essential IMiD effector in PEL, as recently proposed by Gopalakrishnan et al.22 This reported interpretation was based on experiments using 2 distinct U6 promoter–driven shRNAs in the PEL cell lines BC-3 and BCBL-1, which triggered a progressive loss of viability between 4 and 8 days into the experiment.22 Western blot analyses confirmed a reduction of IKZF1 expression, but reported reductions in IRF4 and MYC expression in these samples were <40% and did not correlate with degree of IKZF1 knockdown, which would be expected if IKZF1 were indeed essential for the expression of IRF4.22 Although the sources of IKZF1 shRNA toxicity in this study are unclear, toxicity from U6-driven shRNAs has been reported previously, and controlling for such toxicity is difficult.47,48 This and other limitations of U6-driven shRNAs can be overcome by an updated shRNA design as used here.49,50
Revised model for mechanism of action of IMiDs in PEL cells. Lenalidomide and pomalidomide trigger inconsequential degradation of neosubstrates IKZF1, IKZF3 and ZFP91. The degradation of the neosubstrate CK1α and the IKZF1- and IKZF3-independent loss of IRF4 expression upon IMiD treatment represent separate arms of IMiD toxicity.
Revised model for mechanism of action of IMiDs in PEL cells. Lenalidomide and pomalidomide trigger inconsequential degradation of neosubstrates IKZF1, IKZF3 and ZFP91. The degradation of the neosubstrate CK1α and the IKZF1- and IKZF3-independent loss of IRF4 expression upon IMiD treatment represent separate arms of IMiD toxicity.
Our results strongly support a requirement for IRF4, which was demonstrated by Gopalakrishnan et al22 in BC-3 cells. Genome-wide CRISPR screens identified IRF4 as among the top PEL-specific single-gene requirements, a finding we confirmed in BC-3 and BCBL-1 cells using CRISPR/Cas9 and 3 independent sgRNAs.24 In the current study, we have extended this result to a panel of 8 PEL cell lines. Our unpublished data furthermore confirm that IRF4 is essential for expression of MYC in PEL cell lines.22 Therefore, PEL cell lines, like MM cell lines, are invariably addicted to IRF4.19 Regulation of IRF4 in PEL, however, differs from that in MM cell lines.17,18 Importantly, the loss of IRF4 and MYC expression after IMiD treatment of PEL cell lines was unaffected by the absence of IKZF1 and IKZF3. In line with previous reports that IRF4 is not a CRL4CRBN neosubstrate,17,18 ectopically expressed IRF4 is not susceptible to IMiD-induced degradation in PEL cells. Therefore, IMiD-induced downregulation of IRF4 likely occurs at the levels of transcription or messenger RNA processing and function. Our results raise the important question of the identity of the IMiD-sensitive regulators upstream of IRF4 in PEL cell lines. Although we have excluded redundancies between IKZF1 and IKZF3, at least in BC-3 and BCBL-1, synthetic lethality of IKZF1 and IKZF3 with ZFP91 remains possible. Alternatively, a novel IMiD neosubstrate upstream of IRF4 may exist. Such a factor could conceivably also contribute to the action of IMiDs in other cancers of the hematopoietic lineage. Importantly, we demonstrate that reexpression of IRF4 partially rescues sensitivity of PEL cell lines to pomalidomide, which directly demonstrates that IRF4 contributes to IMiD toxicity in PEL cells. Several observations suggest that downregulation of IRF4 may be the primary mechanism of IMiD toxicity in PEL cell lines; the phenotype of CRISPR-induced inactivation of CK1α was delayed and relatively subtle compared with inactivation of IRF4. In addition, rescue from IMiD toxicity after reexpression of CK1α was less efficient than that after reexpression of IRF4. Loss of IRF4 expression proceeded despite restoration of CK1α and, importantly, before differences in the stability of WT and G40N-mutant CK1α in pomalidomide-treated cells were evident. Finally, cell lines that did not respond to inactivation of CK1α in our study are still sensitive to IMiDs.22 Importantly, reexpression of CK1α and IRF4 together did not result in a complete rescue of IMiD toxicity, suggesting that additional effectors remain to be discovered.
In summary, we show that IMiD-induced repression of IRF4 in PEL cell lines is independent of IKZF1 and IKZF3 and describe a second, CK1α-dependent mechanism of IMiD toxicity in PEL cells. Overall, this study supports the further exploration of IMiDs as a viable treatment strategy in PEL and underscores the need for studies of the mechanisms of IMiD action.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Northwestern University NUSeq Core Facility and the University of Chicago Genomics Facility for their Illumina sequencing services and John Crispino, Rich Longnecker, and Marc Mendillo and members of the Gottwein Laboratory for their feedback on this manuscript.
This study was supported by National Institutes of Health, National Cancer Institute Grant R21 CA210904, by Searle and Zell Scholar Awards from the Robert H. Lurie Comprehensive Cancer Center (E.G.), and by Chicago Biomedical Consortium Postdoctoral Award PDR-061 (M.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Authorship
Contribution: A.P. and E.G. designed the study, analyzed data, and wrote the manuscript; A.P. conducted all experiments; and M.M. provided CRISPR data (generated with help from A.P.), several sgRNA constructs, and feedback on the study and manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Eva Gottwein, Department of Microbiology-Immunology, Northwestern University Feinberg School of Medicine, 320 E Superior St, Searle Building, Room 3-525, Chicago, IL 60611; e-mail: e-gottwein@northwestern.edu.


![Figure 4. IKZF1, IKZF3, and ZFP91 are dispensable for the survival of PEL cell lines. (A-B) Growth curve analyses of Cas9-expressing BC-3 or BCBL-1 cell clones after transduction with lentiviral sgRNA vectors targeting IKZF1 (A) or IKZF3 (B). Two independent sgRNAs (sg1 and sg2) were designed per target; sgRNAs targeting AAVS1 and IRF4 were used as negative and positive controls, respectively (n = 3; error bars represent standard error of the mean [SEM]). Representative western blot analyses below the growth curves confirm the efficient inactivation of the targeted genes and demonstrate lack of regulation of IRF4. GAPDH served as loading control. (C) Growth curve analyses of naïve BC-3 or BCBL-1 cells after transduction with lentiviral shRNA vectors targeting IKZF1 or IKZF3. The nontargeting shRNA NT4 served as negative control (n = 3; error bars represent SEM). Representative western blot analyses below the growth curves assess the degree of shRNA-mediated target knockdown and IRF4 expression. GAPDH served as loading control. (D) Same as panels A and B, except that sgRNAs against ZFP91 were used (n = 3; error bars represent SEM). KO, knockout.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/6/10.1182_blood-2018-01-828418/4/m_blood828418f4.jpeg?Expires=1769142430&Signature=tTWxVBiIvv5RQtqW~zE-d2QiufrtG70pIn6SgZVFp4XOWfv7tts2wrPcTAfkukQMwcSZomY5DF3d3nZDIq-i3HjB0S2oqqhOGRDsWFAKhWu4nCJao552SxpexLtNXAgPe8EDVKBw83AGoEXgb0s1lbNeXXPGV2j2UkslOpP0y-hDsMpJVlpwZCwUWji0BFWwFOkhvG9aqirF1mzMHkUfScRiakFRtl3jLwsh1DtIhsQcbsuHjn8S76~VAY495YWajoqi1BcpGFQ1a51RHtAHDIuyerZX5KrNC4rIyJSgvCpRMQWgjNL36ZWcVE0MjmWGL2NZVJz6aCcAhSgFtfewow__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. IKZF1 and IKZF3 double-knockout (DKO) cells are viable and susceptible to IMiD-induced toxicity and IRF4 downregulation. (A) Western blot analyses confirm efficient inactivation of IKZF1 and IKZF3 in Cas9-expressing BC-3 and BCBL-1 sequentially transduced with targeting sgRNAs. IRF4 expression was not altered, and GAPDH was the loading control. (B) Growth curve analyses of cell lines described in panel A (n = 3; error bars represent standard error of the mean [SEM]). (C) Pomalidomide IC50 curves of cell lines described in panel A. The BC-3 CRBN knockout (KO) clone was included as an additional control (n = 3; error bars represent SEM). (D) Growth curve analyses after pomalidomide treatment of the cell lines described in panel A; live cell numbers are plotted relative to DMSO-treated controls (n = 3; error bars represent SEM). (E) Representative western blot analysis of IKZF1, IRF4, and MYC expression at all time points analyzed in panel D. GAPDH served as loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/6/10.1182_blood-2018-01-828418/4/m_blood828418f5.jpeg?Expires=1769142430&Signature=Ov-eqmRPGj6ZxbHn7glQdH9xMBuKhX3-nFLI3~bUXpJJ2zNs~fZIpH~ogYHxR2Jf5ezNYRD5ENN-p0z8nMS8JedzmU0hMq6eJb4X-W1DDUL1JydVfTgnHlLuYQ0PtcObCs-2Kt7RCqAea9-hPh7eNSLPwmSpEzBWjFY40M0eiF-oLSZiEAz1BmD9e0a4VYn3-0Nepy49hOva65SfX2tkrwuSTsIosXFX41-aWdP8iJcmE08gU5U4KbJk~bva-9hzEeQgJHwhmKrxbdPs70JkNZ0GuATl4IFkpH6LQn1Dujgn4gEbHMRU0DqeT5Ejy~oNC-nwAbDLj9wiVBPt3148mQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. Reexpression of CK1α or IRF4 rescues PEL cells from IMiD-mediated toxicity. (A) Representative western blots of BCBL-1 cells transduced with constitutive lentiviral vectors expressing GFP, WT, or G40N CK1α. Cells were treated for the indicated duration with 1 µM of pomalidomide. The asterisk in the anti-CK1α blot marks a nonspecific band. GAPDH served as loading control. (B) Pomalidomide IC50 values of BCBL-1 cells analyzed in panel A (n = 3; error bars represent standard error of the mean [SEM]). (C) Representative western blots demonstrate efficient loss of endogenous, but not exogenous, IRF4 expression after treatment with 1 µM of pomalidomide. GAPDH served as loading control. (D) Pomalidomide IC50 values of naïve BC-3 and BCBL-1 and cell lines transduced with a Dox-inducible IRF4 expression vector (n = 3; error bars represent SEM). (E) Pomalidomide IC50 values of naïve BCBL-1 cells and cell lines transduced with a Dox-inducible IRF4 expression vector and constitutive lentiviral vectors expressing ZsGreen or G40N CK1α (n = 3; error bars represent SEM). (F) Representative western blots for IRF4 and CK1α of cells analyzed in panel E. The dotted line indicates where the image was cropped to remove irrelevant lanes. GAPDH served as a loading control. *P < .05, **P < .01, ***P < .001 by unpaired t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/6/10.1182_blood-2018-01-828418/4/m_blood828418f6.jpeg?Expires=1769142430&Signature=yrJx2pH39jcVY39twpJUjPax7iDKvGhCgRMMiPhdHBnt2vdxUwkonKJMbBSIqlE-2Bys8xS-crCMGLMxagw3ZDLRpwqA05aPVeITEj9pXif54YFbS-deLrI4OqF2NpHR5Pc4WR3WusrexTFFRNH0OJ4PE9rHg2qdmovhd91mUSfpv1ztp9lZyHaLDyEZBdU~ersAIQnp6cbTuCGyCeR1Xn3TxwA6C-D1hVqhR-TUap-ET2FkqPTf2Vf9JIhdcWs3bdAC4sHMXOsDVhoCyBJHTV9jpyybHU3YtWzKm~y~JnY6FiKUV4KtMXfXCZpkNcjpJvq463GTR3Le0sN9oC49tg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal