Key Points
SVd is safe and tolerable in patients with relapsed or refractory MM.
SVd produced high response rates with an ORR of 84% in PI-nonrefractory and 43% in PI-refractory patients.
Abstract
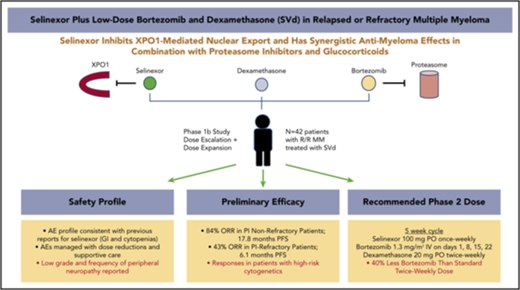
Selinexor is an oral inhibitor of the nuclear export protein exportin 1. Preclinical studies demonstrated synergistic antimyeloma activity between selinexor and proteasome inhibitors (PI) through suppression of NF-κB signaling and nuclear retention of tumor suppressor proteins. We tested selinexor in combination with low-dose bortezomib and dexamethasone (SVd) for the treatment of relapsed or refractory multiple myeloma (MM). The primary objectives of this study were to determine the safety profile, overall response rate (ORR), and a recommended phase 2 dose (RP2D) of SVd. We enrolled 42 patients to receive selinexor (60, 80, or 100 mg orally) plus bortezomib (1.3 mg/m2 subcutaneously) and dexamethasone (20 mg orally) once or twice weekly in 21- or 35-day cycles. Patients had a median of 3 (range 1-11) prior lines of therapy, and 50% were refractory to a PI. Treatment-related grade 3 or 4 adverse events reported in ≥10% of patients were thrombocytopenia (45%), neutropenia (24%), fatigue (14%), and anemia (12%). Incidence (4 patients, 10%) and grade (≤2) of peripheral neuropathy were low. The ORR for the entire population was 63%: 84% ORR for PI nonrefractory and 43% for PI-refractory patients. The median progression-free survival for all patients was 9.0 months; 17.8 months for PI nonrefractory, and 6.1 months for PI refractory. SVd treatment produced high response rates in patients with relapsed or refractory MM, including borezomib-refractory MM, with no unexpected side effects. The RP2D is selinexor (100 mg once weekly), bortezomib (1.3 mg/m2 once weekly for 4 weeks), and dexamethasone (40 mg once weekly) per 35-day cycle. This trial was registered at www.clinicaltrials.gov as #NCT02343042.
Introduction
Survival outcomes for patients with multiple myeloma (MM) have significantly improved over the past 2 decades due primarily to the introduction of novel classes of drugs such as proteasome inhibitors (PIs) (bortezomib, carfilzomib, and ixazomib), immunomodulatory agents (IMiDs) (thalidomide, lenalidomide, and pomalidomide), monoclonal antibodies targeting CD38 (daratumumab, isatuximab) and SLAMF7 (elotuzumab), and HDAC inhibitors (panobinostat).1-8 In particular, combinations of these therapies have augmented the depth of responses, and rates of progression-free survival (PFS) as well as overall survival (OS).6-11 Despite these additions to the MM armamentarium, myeloma cells invariably acquire resistance, and nearly all patients develop disease that is refractory to available therapies. Therefore, the discovery of therapeutics with novel mechanisms of action to overcome drug resistance remains a high priority.
Bortezomib, a first-in-class PI, is a standard antimyeloma therapy administered in combination with low-dose dexamethasone (Vd). Vd has been tested in combination with panobinostat, pegylated liposomal doxorubicin, lenalidomide, and more recently, daratumumab for the treatment of patients with MM. In randomized trials, these combinations demonstrated improved response rates and PFS vs Vd alone.6,8,12,13 These regimens, however, often require a twice-weekly dosing of bortezomib and are therefore cumbersome for patients and their direct caregivers. Moreover, this dosing frequency of bortezomib induces significant toxicity, including sensory and motor neuropathy often leading to treatment interruptions, dose reductions, and ultimately, loss of therapeutic efficacy. Importantly, patients with bortezomib-refractory disease are often excluded from trials where Vd is the backbone in a combination regimen, and hence, it is unclear whether myeloma can be resensitized to bortezomib when combined with novel agents. Therefore, identifying therapies that synergize with low-dose weekly bortezomib will improve the convenience and side effects of a bortezomib-based regimen and may resensitize the disease to this class of drugs.
Exportin 1 (XPO1/CRM1) is a nuclear export protein that is overexpressed in many cancers, including MM.14 Elevated levels of XPO1 promote the nuclear export, cytoplasmic localization, and functional inactivation of tumor suppressor proteins (TSPs), allowing cancer cells to evade apoptotic and antiproliferative signals. In addition, XPO1 mediates the nuclear export of multiple oncoprotein messenger RNAs (mRNAs) carried by eukaryotic initiation factor 4E (eIF4E), thus facilitating ribosomal translation of these mRNAs in the cytoplasm.15,16 Importantly, a genome-wide RNA interference screen identified XPO1 as an essential gene required for myeloma cell survival and proliferation.17 Furthermore, elevated XPO1 expression in patients with myeloma has been correlated with poor survival and increased bone lesions.18 These findings lead to the evaluation of XPO1 inhibitors for the treatment of MM.
Selinexor is an oral, slowly reversible, covalent inhibitor of XPO1-mediated nuclear export. Treatment of cancer cells with selinexor forced nuclear retention of TSPs (p53, Rb, FOXO1, survivin and IκB) and blocked the export of eIF4E-bound oncoprotein mRNAs (c-Myc, cyclin D1, Bcl-6, Mdm2, and Pim), leading to growth inhibition and apoptosis.14,19 In nonclinical studies, selinexor demonstrated single-agent antimyeloma activity and synergy with PIs by limiting NF-κB transcriptional activity and inducing a ribosomal stress response.20-22 Importantly, selinexor exhibited significant cytotoxicity and synergized with PIs in PI-resistant MM cell lines and in a nonobese diabetic/severe combined immunodeficiency-γ xenograft mouse model.18,23 In addition, selinexor showed promising efficacy in combination with other antimyeloma therapies, including corticosteroids, IMiDs, and anti-CD38 antibodies.24-28 In a phase 1, first-in-human study, selinexor plus low-dose dexamethasone (Sd) demonstrated an overall response rate (ORR) of up to 50% at the recommended phase 2 dose (RP2D) in patients with heavily pretreated MM.24 Further studies testing Sd in patients with quad-refractory (bortezomib, carfilzomib, lenalidomide, and pomalidomide refractory) and penta-refractory (quad-refractory plus anti-CD38 antibody refractory) MM demonstrated a 21% ORR and median duration of response of 5 months.25
Based on the activity of Sd in patients with heavily pretreated MM and the preclinical synergy with antimyeloma agents, including PIs, we evaluated the combination of Sd with approved therapies for MM as part of the multiarmed Selinexor Treatment of Myeloma Patients trial. Here, we report results from the phase 1 and 2 portions of the selinexor plus bortezomib and dexamethasone (SVd) arm, which was designed to deliver a convenient and potent antimyeloma regimen with reduced side effects, including lesser rates of peripheral neuropathy.
Methods
Study design and oversight
These data are part of an ongoing, multicenter, open-label, phase 1b/2 study to test the safety and efficacy of selinexor in combination with approved therapies for the treatment of relapsed or refractory MM. Here, we report the dose-escalation and dose-expansion phases of the SVd arm. The primary objectives of the study were to determine the maximum tolerated dose (MTD) for once-weekly and twice-weekly selinexor dose cohorts and identify a RP2D, as well as to determine the ORR, duration of response, and clinical benefit rate (CBR). The study enrolled patients 18 years of age or older with MM that was progressing after ≥1 prior therapeutic regimen. Prior treatment with bortezomib or PI, including refractory disease, was permitted; however, patients could not be refractory to bortezomib in their most recent line of therapy. Disease refractory to carfilzomib or ixazomib in the last prior line of therapy was permitted. Refractory disease was defined as per International Myeloma Working Group (IMWG) guidelines (lack of response while on therapy or disease progression 2 months of completing therapy). A full list of inclusion and exclusion criteria can be found in supplemental Table 1, available on the Blood Web site. Patients were encouraged to take a prophylactic 5-HT3 antagonist (8 mg ondansetron or equivalent) to prevent nausea, starting before the first dose of selinexor and continuing as needed. Patients were required to receive standard prophylaxis for herpes zoster, but not for other opportunistic infections. The study protocol was approved by the institutional review board or an independent ethics committee at each participating center and was in accordance with the Declaration of Helsinki, the International Conference on Harmonization-Good Clinical Practice, and local laws. All patients provided written informed consent prior to enrollment. All authors reviewed the data for accuracy and collaborated in the preparation of the manuscript.
Treatment
In the dose-escalation phase, patients were randomized to 1 of 2 treatment cohorts to receive selinexor once weekly or twice weekly in 21- or 35-day cycles, depending on the bortezomib dosing schedule. The starting doses of selinexor and Vd were selected based on the RP2D from previously published work. Dose escalation proceeded unless a dose limiting toxicity (DLT) was observed. DLTs were evaluated only in patients enrolled during the dose-escalation phase over their first cycle of treatment. DLTs were defined as any of the following: (1) missing ≥25% of scheduled doses, a dose-reduction, or discontinuation due to treatment-related adverse events (AEs) in the first cycle; (2) occurrence of grade ≥3 nausea, vomiting, dehydration, diarrhea, or fatigue lasting >3 days despite optimal supportive care medications; (3) any other grade 3 or 4 nonhematologic toxicity; (4) febrile neutropenia, grade 4 neutropenia lasting >7 days, and grade ≥3 thrombocytopenia with clinically significant bleeding, petechiae, or purpura. Electrolyte abnormalities that were reversible and asymptomatic, alopecia, alanine aminotransferase, aspartate aminotransferase, or alkaline phosphatase elevations from disease in the setting of baseline grade 2 levels were not considered a DLT. If DLTs were cleared, the highest prespecified dose level was considered the MTD for the cohort. An overview of the dose levels and treatment schedules may be found in Table 1.
Treatment schedule and dose
| Dose-escalation phase . | Dose level . | Patients (n) . | Selinexor, mg PO . | Dexamethasone, mg PO . | Bortezomib, mg/m2 SC . |
|---|---|---|---|---|---|
| Days 1, 8, 15, 22, 29 . | Days 1, 8, 15, 22, 29 . | Days 1, 8, 15, 22 . | |||
| Cohort 1. Once-weekly selinexor in a 35-d cycle | 1 2 | 4 6 | 80 100 | 40 40 | 1.3 1.3 |
| Dose-escalation phase . | Dose level . | Patients (n) . | Selinexor, mg PO . | Dexamethasone, mg PO . | Bortezomib, mg/m2 SC . |
|---|---|---|---|---|---|
| Days 1, 8, 15, 22, 29 . | Days 1, 8, 15, 22, 29 . | Days 1, 8, 15, 22 . | |||
| Cohort 1. Once-weekly selinexor in a 35-d cycle | 1 2 | 4 6 | 80 100 | 40 40 | 1.3 1.3 |
| . | . | . | Days 1, 8, 15 . | Days 1, 8, 15 . | Days 1, 4, 8, 11 . |
|---|---|---|---|---|---|
| Cohort 1. Once-weekly selinexor in a 21-d cycle | 3 | 3 | 80 | 40 | 1.3 |
| . | . | . | Days 1, 8, 15 . | Days 1, 8, 15 . | Days 1, 4, 8, 11 . |
|---|---|---|---|---|---|
| Cohort 1. Once-weekly selinexor in a 21-d cycle | 3 | 3 | 80 | 40 | 1.3 |
| . | . | . | Days 1, 3, 8, 10, 15, 17, 22, 24 . | Days 1, 3, 8, 10, 15, 17, 22, 24, 29, 31 . | Days 1, 8, 15, 22 . |
|---|---|---|---|---|---|
| Cohort 2. Twice-weekly selinexor in a 35-d cycle | 1 2 | 3 6 | 60 80 | 20 20 | 1.3 1.3 |
| . | . | . | Days 1, 3, 8, 10, 15, 17, 22, 24 . | Days 1, 3, 8, 10, 15, 17, 22, 24, 29, 31 . | Days 1, 8, 15, 22 . |
|---|---|---|---|---|---|
| Cohort 2. Twice-weekly selinexor in a 35-d cycle | 1 2 | 3 6 | 60 80 | 20 20 | 1.3 1.3 |
| . | . | . | Days 1, 8, 15, 22, 29 . | Days 1, 8, 15, 22, 29 . | Days 1, 8, 15, 22 . |
|---|---|---|---|---|---|
| RP2D | 20 | 100 | 40 | 1.3 |
| . | . | . | Days 1, 8, 15, 22, 29 . | Days 1, 8, 15, 22, 29 . | Days 1, 8, 15, 22 . |
|---|---|---|---|---|---|
| RP2D | 20 | 100 | 40 | 1.3 |
Treatment schedule and starting dose for patients treated with SVd. During the dose-escalation phase, patients were randomized to receive selinexor once weekly or twice weekly in 21- or 35-d cycles, depending on the bortezomib dosing schedule (cohort 1 or 2). Dose escalation proceeded after DLTs were cleared at dose level 1. Based on the opinions of the investigators, the side effects of SVd, when bortezomib was given twice weekly in 21-d cycles (cohort 1, dose level 3), were not sustainable for long-term treatment, and therefore, this dosing schedule was abandoned. The dose-expansion phase enrolled an additional 20 patients at the RP2D to better understand the safety and efficacy of SVd in a once-weekly schedule.
PO, orally; SC, subcutaneously.
Study assessments
Safety was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events, v4.03. Response was evaluated using IMWG response criteria.29 All patients who received at least 1 dose of therapy were considered evaluable for safety.
Statistics
Sample sizes for the dose-escalation phase were based on a standard 3+3 design. The dose-expansion phase was designed to test the null hypothesis that the true ORR was ≤0.30 against a 1-sided alternative that the true ORR was ≥0.55, where the first 10 patients treated at the RP2D were considered the first stage of the 2-stage design and the study arm would be terminated if ≤2 patients respond. If ≥3 patients responded in stage 1, an additional 10 patients would be enrolled. If ≥9 out of the 20 patients responded, the treatment would be accepted as promising for further study. This design achieved 80% power at a 1-sided 0.10 significance level.
Results
Demographics
Between 14 October 2015 and 28 February 2017, 42 patients were enrolled to receive SVd. The median age was 64 years (range 43-75), and patients had received a median of 3 prior lines of therapy (range 1-11). Twenty-one patients (50%) had MM that was refractory to a prior PI (bortezomib, carfilzomib, or ixazomib), and 19 patients (45%) had MM that was refractory to both a PI and an IMiD (lenalidomide, pomalidomide, or thalidomide). The median time interval between prior PI exposure and enrollment for patients with PI-refractory MM was 8.2 months (range 0.7-76.1 months). High-risk cytogenetics, which included del(17p), t(4;14), and t(14;16), were identified by fluorescence in situ hybridization in 4/24 (17%) patients. A full listing of patient characteristics at baseline can be found in Table 2 and supplemental Table 2.
Characteristics of patients at screening
| Patient characteristics at screening . | All patients(n = 42) . |
|---|---|
| Age | |
| Median (range), n (%) | 64 (43-75) |
| ≤65 | 22 (52) |
| 66-74 | 19 (45) |
| ≥75 | 1 (3) |
| Sex, n (%) | |
| Male | 23 (55) |
| Female | 19 (45) |
| Race, n (%) | |
| White | 37 (88) |
| Black or African American | 2 (5) |
| Hispanic | 1 (2) |
| Other | 2 (5) |
| ECOG performance status, n (%) | |
| 0 | 6 (14) |
| 1 | 35 (83) |
| 2 | 1 (2) |
| ISS disease stage, n (%) | |
| I | 15 (36) |
| II | 9 (21) |
| III | 11 (26) |
| Unknown | 7 (17) |
| Median time since initial diagnosis of MM | |
| Years (range) | 5 (1-19) |
| Number of prior lines of therapy, n (%) (range) | 3 (1-11) |
| 1 | 6 (14) |
| 2 | 8 (19) |
| 3 | 9 (21) |
| 4 | 3 (7) |
| ≥5 | 16 (38) |
| Types of prior therapies, n (%) | |
| Bortezomib | 36 (86) |
| Carfilzomib | 7 (17) |
| Ixazomib | 3 (7) |
| Lenalidomide | 22 (52) |
| Pomalidomide | 19 (45) |
| Thalidomide | 5 (12) |
| Anti-CD38 antibody | 5 (12) |
| Glucocorticoid | 42 (100) |
| Alkylating agent | 38 (90) |
| Anthracyline | 7 (17) |
| Autologous stem cell transplant | 30 (71) |
| Disease refractory to, n (%) | |
| PI | 21 (50) |
| Immunomodulatory drug | 34 (81) |
| Anti-CD38 antibody | 5 (12) |
| PI and immunomodulatory drug | 19 (45) |
| Cytogenetics, n (%) | |
| Standard risk | 20 (48) |
| High risk | |
| del(17p)° | 3 (7) |
| del(4;14)° | 1 (2) |
| del(14;16)° | 0 (0) |
| Unknown | 18 (43) |
| History of peripheral neuropathy, n (%) | |
| No | 22 (52) |
| Yes | 20 (48) |
| Ongoing peripheral neuropathy at screening, n (%) | |
| Grade 1 | 18 (43) |
| Grade 2 | 1 (2) |
| Unknown grade | 1 (2) |
| Patient characteristics at screening . | All patients(n = 42) . |
|---|---|
| Age | |
| Median (range), n (%) | 64 (43-75) |
| ≤65 | 22 (52) |
| 66-74 | 19 (45) |
| ≥75 | 1 (3) |
| Sex, n (%) | |
| Male | 23 (55) |
| Female | 19 (45) |
| Race, n (%) | |
| White | 37 (88) |
| Black or African American | 2 (5) |
| Hispanic | 1 (2) |
| Other | 2 (5) |
| ECOG performance status, n (%) | |
| 0 | 6 (14) |
| 1 | 35 (83) |
| 2 | 1 (2) |
| ISS disease stage, n (%) | |
| I | 15 (36) |
| II | 9 (21) |
| III | 11 (26) |
| Unknown | 7 (17) |
| Median time since initial diagnosis of MM | |
| Years (range) | 5 (1-19) |
| Number of prior lines of therapy, n (%) (range) | 3 (1-11) |
| 1 | 6 (14) |
| 2 | 8 (19) |
| 3 | 9 (21) |
| 4 | 3 (7) |
| ≥5 | 16 (38) |
| Types of prior therapies, n (%) | |
| Bortezomib | 36 (86) |
| Carfilzomib | 7 (17) |
| Ixazomib | 3 (7) |
| Lenalidomide | 22 (52) |
| Pomalidomide | 19 (45) |
| Thalidomide | 5 (12) |
| Anti-CD38 antibody | 5 (12) |
| Glucocorticoid | 42 (100) |
| Alkylating agent | 38 (90) |
| Anthracyline | 7 (17) |
| Autologous stem cell transplant | 30 (71) |
| Disease refractory to, n (%) | |
| PI | 21 (50) |
| Immunomodulatory drug | 34 (81) |
| Anti-CD38 antibody | 5 (12) |
| PI and immunomodulatory drug | 19 (45) |
| Cytogenetics, n (%) | |
| Standard risk | 20 (48) |
| High risk | |
| del(17p)° | 3 (7) |
| del(4;14)° | 1 (2) |
| del(14;16)° | 0 (0) |
| Unknown | 18 (43) |
| History of peripheral neuropathy, n (%) | |
| No | 22 (52) |
| Yes | 20 (48) |
| Ongoing peripheral neuropathy at screening, n (%) | |
| Grade 1 | 18 (43) |
| Grade 2 | 1 (2) |
| Unknown grade | 1 (2) |
ECOG, Eastern Cooperative Oncology Group; ISS, International Staging System.
As of the date of data cutoff, 7 patients (17%) were still receiving treatment, 22 (52%) had discontinued due to progressive disease (PD), 8 patients (19%) had discontinued due to AEs of any causality, 5 patients (12%) had withdrawn for other reasons (2 patients citing difficulty with travel time, 2 patient decision, and 1 physician’s decision for noncompliance with study procedures). One early death was reported in the study population in which a patient died after 18 days on therapy due to a stroke with concurrent pneumonia deemed by the investigator as unrelated to treatment.
Safety
SVd was tested at 3 dose levels of once-weekly or twice-weekly selinexor (60, 80, and 100 mg) and/or once-weekly (n = 39 patients) or twice-weekly (n = 3 patients) bortezomib (1.3 mg/m2 subcutaneously) in 21- or 35-day cycles. Patients received a median of 225 days (range 18-707 days) of therapy. Treatment-related AEs occurring in ≥10% of patients can be found in Table 3. The most frequently reported nonhematologic AEs of any grade included nausea (62%), fatigue (60%), decreased appetite (60%), diarrhea (43%), vomiting (31%), weight decrease (19%), and blurred vision (19%). These AEs were primarily grade 1 or 2 and were reversible with dose interruption. Twelve of the 13 patients who reported vomiting had corresponding nausea. Seven of the 42 patients (17%) on study reported nausea with vomiting and diarrhea. A majority of patients (89%) received prophylactic supportive care of ondansetron, prochlorperazine, aprepitant, or granisetron to control nausea. Fourteen patients (33%) required >1 antinausea medication while on study. In addition to the 40 mg of weekly Vd in their regimen, 9 patients (21%) received an additional appetite stimulant of either megestrol acetate, metoclopramide, or dronabinol.
Treatment-related AEs occurring in ≥10% of patients
| AE term . | All patients (n = 42) . | Dose escalation patients (n = 16) . | RP2D patients (n = 26) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All doses, n (%) . | 60, 80 mg selinexor QW/BIW + 1.3 mg/m2 bortezomib QW/BIW, n (%) . | 100 mg selinexor QW + 1.3 mg/m2 bortezomib QW, n (%) . | |||||||||||||
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Total . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Total . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Total . | |
| Nausea | 7 (17) | 17 (40) | 2 (5) | — | 26 (62) | 2 (13) | 3 (19) | 2 (13) | — | 7 (44) | 5 (19) | 14 (54) | — | — | 19 (73) |
| Fatigue | 3 (7) | 16 (38) | 6 (14) | — | 25 (60) | — | 9 (56) | — | — | 9 (56) | 3 (12) | 7 (27) | 6 (23) | — | 16 (62) |
| Decreased appetite | 13 (31) | 11 (26) | 1 (2) | — | 25 (60) | 5 (31) | 3 (19) | 1 (6) | — | 9 (56) | 8 (31) | 8 (31) | — | — | 16 (62) |
| Thrombocytopenia | — | 2 (5) | 7 (17) | 12 (29) | 21 (50) | — | 1 (6) | 4 (25) | 7 (44) | 12 (75) | — | 1 (4) | 3 (12) | 5 (19) | 9 (35) |
| Diarrhea | 11 (26) | 4 (10) | 3 (7) | — | 18 (43) | 4 (25) | 3 (19) | 2 (13) | — | 9 (56) | 7 (27) | 1 (4) | 1 (4) | — | 9 (35) |
| Vomiting | 9 (21) | 3 (7) | 1 (2) | — | 13 (31) | 2 (13) | 2 (13) | 1 (6) | — | 5 (31) | 7 (27) | 1 (4) | — | — | 8 (31) |
| Neutropenia | 1 (2) | — | 9 (21) | 1 (2) | 11 (26) | — | — | 4 (25) | 1 (6) | 5 (31) | 1 (4) | — | 5 (19) | — | 6 (23) |
| Anemia | 1 (2) | 2 (5) | 5 (12) | — | 8 (19) | — | 1 (6) | 4 (25) | — | 5 (31) | 1 (4) | 1 (4) | 1 (4) | — | 3 (12) |
| Weight decreased | 2 (5) | 6 (14) | — | — | 8 (19) | 1 (6) | 4 (25) | — | — | 5 (31) | 1 (4) | 2 (8) | — | — | 3 (12) |
| Blurred vision | 6 (14) | 2 (5) | — | — | 8 (19) | 2 (13) | — | — | — | 2 (13) | 4 (15) | 2 (8) | — | — | 6 (23) |
| Altered taste | 5 (12) | 1 (2) | — | — | 6 (14) | 2 (13) | — | — | — | 2 (13) | 3 (12) | 1 (4) | — | — | 4 (15) |
| Dehydration | — | 5 (12) | — | — | 5 (12) | — | 2 (13) | — | — | 2 (13) | — | 3 (12) | — | — | 3 (12) |
| Cataract | — | 5 (12) | — | — | 5 (12) | — | 3 (19) | — | — | 3 (19) | — | 2 (8) | — | — | 2 (8) |
| Confusion | 1 (2) | 3 (7) | 1 (2) | — | 5 (12) | — | 1 (6) | 1 (6) | — | 2 (13) | 1 (4) | 2 (8) | — | — | 3 (12) |
| Peripheral edema | 4 (10) | 1 (2) | — | — | 5 (12) | 3 (19) | — | — | — | 3 (19) | 1 (4) | 1 (4) | — | — | 2 (8) |
| Hyponatremia | 2 (5) | — | 2 (5) | — | 4 (10) | 1 (6) | — | 1 (6) | — | 2 (13) | 1 (4) | — | 1 (4) | — | 2 (8) |
| Peripheral neuropathy* | 2 (5) | 2 (5) | — | — | 4 (10) | 2 (13) | 1 (6) | — | — | 3 (19) | — | 1 (4) | — | — | 1 (4) |
| AE term . | All patients (n = 42) . | Dose escalation patients (n = 16) . | RP2D patients (n = 26) . | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All doses, n (%) . | 60, 80 mg selinexor QW/BIW + 1.3 mg/m2 bortezomib QW/BIW, n (%) . | 100 mg selinexor QW + 1.3 mg/m2 bortezomib QW, n (%) . | |||||||||||||
| Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Total . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Total . | Grade 1 . | Grade 2 . | Grade 3 . | Grade 4 . | Total . | |
| Nausea | 7 (17) | 17 (40) | 2 (5) | — | 26 (62) | 2 (13) | 3 (19) | 2 (13) | — | 7 (44) | 5 (19) | 14 (54) | — | — | 19 (73) |
| Fatigue | 3 (7) | 16 (38) | 6 (14) | — | 25 (60) | — | 9 (56) | — | — | 9 (56) | 3 (12) | 7 (27) | 6 (23) | — | 16 (62) |
| Decreased appetite | 13 (31) | 11 (26) | 1 (2) | — | 25 (60) | 5 (31) | 3 (19) | 1 (6) | — | 9 (56) | 8 (31) | 8 (31) | — | — | 16 (62) |
| Thrombocytopenia | — | 2 (5) | 7 (17) | 12 (29) | 21 (50) | — | 1 (6) | 4 (25) | 7 (44) | 12 (75) | — | 1 (4) | 3 (12) | 5 (19) | 9 (35) |
| Diarrhea | 11 (26) | 4 (10) | 3 (7) | — | 18 (43) | 4 (25) | 3 (19) | 2 (13) | — | 9 (56) | 7 (27) | 1 (4) | 1 (4) | — | 9 (35) |
| Vomiting | 9 (21) | 3 (7) | 1 (2) | — | 13 (31) | 2 (13) | 2 (13) | 1 (6) | — | 5 (31) | 7 (27) | 1 (4) | — | — | 8 (31) |
| Neutropenia | 1 (2) | — | 9 (21) | 1 (2) | 11 (26) | — | — | 4 (25) | 1 (6) | 5 (31) | 1 (4) | — | 5 (19) | — | 6 (23) |
| Anemia | 1 (2) | 2 (5) | 5 (12) | — | 8 (19) | — | 1 (6) | 4 (25) | — | 5 (31) | 1 (4) | 1 (4) | 1 (4) | — | 3 (12) |
| Weight decreased | 2 (5) | 6 (14) | — | — | 8 (19) | 1 (6) | 4 (25) | — | — | 5 (31) | 1 (4) | 2 (8) | — | — | 3 (12) |
| Blurred vision | 6 (14) | 2 (5) | — | — | 8 (19) | 2 (13) | — | — | — | 2 (13) | 4 (15) | 2 (8) | — | — | 6 (23) |
| Altered taste | 5 (12) | 1 (2) | — | — | 6 (14) | 2 (13) | — | — | — | 2 (13) | 3 (12) | 1 (4) | — | — | 4 (15) |
| Dehydration | — | 5 (12) | — | — | 5 (12) | — | 2 (13) | — | — | 2 (13) | — | 3 (12) | — | — | 3 (12) |
| Cataract | — | 5 (12) | — | — | 5 (12) | — | 3 (19) | — | — | 3 (19) | — | 2 (8) | — | — | 2 (8) |
| Confusion | 1 (2) | 3 (7) | 1 (2) | — | 5 (12) | — | 1 (6) | 1 (6) | — | 2 (13) | 1 (4) | 2 (8) | — | — | 3 (12) |
| Peripheral edema | 4 (10) | 1 (2) | — | — | 5 (12) | 3 (19) | — | — | — | 3 (19) | 1 (4) | 1 (4) | — | — | 2 (8) |
| Hyponatremia | 2 (5) | — | 2 (5) | — | 4 (10) | 1 (6) | — | 1 (6) | — | 2 (13) | 1 (4) | — | 1 (4) | — | 2 (8) |
| Peripheral neuropathy* | 2 (5) | 2 (5) | — | — | 4 (10) | 2 (13) | 1 (6) | — | — | 3 (19) | — | 1 (4) | — | — | 1 (4) |
Treatment-related treatment emergent AEs occurring in ≥10% of patients (n = 42). A subject with an AE coding to the same System Organ Class or Preferred Term on >1 occasion is only counted once for the System Organ Class and Preferred Term at the highest grade. Dashes (—) indicate no event occurred.
BIW, twice weekly; QW, once weekly.
Includes the terms peripheral neuropathy, peripheral sensory neuropathy, sensory neuropathy, and hypoesthesia. Neuralgia, decreased vibratory sense, polyneuropathy, sensory loss, amyotrophy, peripheral motor neuropathy, peripheral sensorimotor neuropathy, sensory disturbance, and toxic neuropathy were not reported in this study.
Hematologic grade ≥3 AEs included thrombocytopenia (50%), neutropenia (26%), and anemia (19%). Clinical sequelae of these hematologic AEs were low as bleeding (5%, both cases grade 1) and febrile neutropenia (5%) were uncommon. Nonhematologic grade 3 AEs included fatigue (14%), diarrhea (7%), nausea (5%), hyponatremia (5%), decreased appetite (2%), confusion (2%), and vomiting (2%). Herpes zoster reactivation was not reported in the study population. Six patients had pneumonia, which was considered unrelated or unlikely related to SVd, while on study. All cases of pneumonia resolved with hospital care except for the early death previously described. Of the 8 patients who discontinued study due to AEs, 5 (12%) were for gastrointestinal or constitutional side effects (ie, nausea, anorexia, vomiting, fatigue), 2 (5%) were for cardiac events, and 1 (2%) was for a macular hole.
Peripheral neuropathy was reported in 4 patients (10%): 2 cases of grade 1 and 2 cases of grade 2. Two of the 4 patients had preexisting neuropathy at screening from prior exposure to bortezomib. The median time to onset of peripheral neuropathy was 12 weeks (range 5-37 weeks). Dose reductions in bortezomib were required by 2 of these 4 patients. Supportive care agents for neuropathic pain, typically pregabalin, were required by 3 patients.
There were 7 serious adverse events (SAEs) considered by the investigator to be at least possibly related to SVd treatment, which were reported in 6 patients (supplemental Table 3). SAEs included 2 cases of febrile neutropenia, and 1 case each of nausea with vomiting, diarrhea, abdominal pain, full-thickness macular hole in left eye, and pulmonary embolism. All patients recovered from the SAE with the exception of the patient with full-thickness macular hole in left eye.
Twenty-one (50%) of the 42 total patients had a dose reduction in selinexor, Vd, and/or bortezomib while on study. Twenty patients (48%) required a dose reduction in selinexor, 5 patients (12%) in Vd, and 5 patients (12%) in bortezomib. Dose reductions in selinexor were most commonly caused by cytopenias, nausea, or fatigue, and occurred more frequently at higher doses (supplemental Table 4). After the first cycle of treatment, each of the 3 patients treated with twice-weekly bortezomib (21-day cycle) required a dose reduction and schedule change to once-weekly bortezomib in a 35-day cycle, primarily due to cytopenias, fatigue, diarrhea, and decreased appetite. Based on the opinions of the investigators, the side effects of SVd, when bortezomib was given twice weekly in 21-day cycles, were not sustainable for long-term treatment, and therefore, this dosing schedule was abandoned.
There were no DLTs reported during the dose-escalation phase, and an MTD, as predefined in the protocol, was established at the highest dose level for each cohort. Based on long-term tolerability and efficacy (described in “Efficacy”), the RP2D of SVd was established at 100 mg selinexor once weekly plus 40 mg Vd once weekly and 1.3 mg/m2 bortezomib once weekly in 35-day cycles. The toxicity profile of the RP2D is summarized in Table 3. With selinexor and bortezomib given once weekly at the RP2D, no grade 3 or 4 nausea or vomiting was observed, and the only grade 3 gastrointestinal AE reported was diarrhea (n = 1). Eight of the 26 patients (30%) treated at the RP2D experienced grade 1 or 2 nausea with vomiting that was typically limited in duration (up to 2 days after treatment administration). Grade 3 nonhematologic AEs at the RP2D included fatigue (23%), and along with thrombocytopenia (grade 3; 12% and grade 4; 19%), these 2 AEs were the predominant reason for treatment interruption or dose reduction. The selection of the RP2D dose and schedule is further supported by the lower rate of consent withdrawal due to AEs (15%) compared with the rate for the dose-escalation schedules (25%) (supplemental Table 5).
Efficacy
Response was evaluable for 40 patients treated during the dose-escalation and expansion phases. Two patients were considered nonevaluable for response based on consent withdrawal prior to any disease assessment and early death deemed unrelated to SVd therapy. The ORR for the 40 evaluable patients was 63% (95% confidence interval [CI]: 47% to 76%), which included 3 (8%) complete responses (CRs), 9 (23%) very good partial responses (VGPRs), and 13 (33%) partial responses (PRs) (Table 4). An additional 7 (18%) patients had a minimal response (MR) for a CBR of 80% (95% CI: 65% to 90%). PD while on therapy occurred in only 1 patient (5%). The ORR for patients treated at the RP2D was 58% (95% CI: 35% to 71%). Two of the 4 patients with documented high-risk cytogenetics at screening responded to therapy, including 1 CR t(4;14) and 1 VGPR del(17p), and were maintained on study for 17.8 and 23.0+ months, respectively.
Best overall response
| Patients . | n . | ORR (≥ PR), n (%) . | CBR (≥ MR), n (%) . | CR, n (%) . | VGPR, n (%) . | PR, n (%)* . | MR, n (%) . | SD, n (%) . | PD, n (%) . |
|---|---|---|---|---|---|---|---|---|---|
| All patients | 40 | 25 (63) | 32 (80) | 3 (8) | 9 (23) | 13 (33) | 7 (18) | 7 (18) | 1 (3) |
| Dose escalation patients | 16 | 11 (69) | 13 (81) | 2 (13) | 4 (25) | 5 (31) | 2 (13) | 2 (13) | 1 (3) |
| RP2D patients | 24 | 14 (58) | 19 (79) | 1 (4) | 5 (21) | 8 (33) | 5 (21) | 5 (21) | — |
| PI nonrefractory | 19 | 16 (84) | 18 (95) | 2 (11) | 5 (26) | 9 (47) | 2 (11) | 1 (5) | — |
| PI refractory | 21 | 9 (43) | 14 (67) | 1 (5) | 4 (19) | 4 (19) | 5 (24) | 6 (29) | 1 (5) |
| PI and IMiD refractory | 19 | 8 (42) | 12 (63) | — | 4 (21) | 4 (21) | 4 (21) | 6 (32) | 1 (5) |
| Patients . | n . | ORR (≥ PR), n (%) . | CBR (≥ MR), n (%) . | CR, n (%) . | VGPR, n (%) . | PR, n (%)* . | MR, n (%) . | SD, n (%) . | PD, n (%) . |
|---|---|---|---|---|---|---|---|---|---|
| All patients | 40 | 25 (63) | 32 (80) | 3 (8) | 9 (23) | 13 (33) | 7 (18) | 7 (18) | 1 (3) |
| Dose escalation patients | 16 | 11 (69) | 13 (81) | 2 (13) | 4 (25) | 5 (31) | 2 (13) | 2 (13) | 1 (3) |
| RP2D patients | 24 | 14 (58) | 19 (79) | 1 (4) | 5 (21) | 8 (33) | 5 (21) | 5 (21) | — |
| PI nonrefractory | 19 | 16 (84) | 18 (95) | 2 (11) | 5 (26) | 9 (47) | 2 (11) | 1 (5) | — |
| PI refractory | 21 | 9 (43) | 14 (67) | 1 (5) | 4 (19) | 4 (19) | 5 (24) | 6 (29) | 1 (5) |
| PI and IMiD refractory | 19 | 8 (42) | 12 (63) | — | 4 (21) | 4 (21) | 4 (21) | 6 (32) | 1 (5) |
The ORR and CBR were applied for all evaluable patients (n = 40). Two patients did not have sufficient disease measurements after 1 cycle of treatment and were therefore considered nonevaluable.
Includes 1 unconfirmed PR.
The ORR for the 19 patients with disease naive to (n = 4) or exposed but not refractory to a PI (n = 15) was 84% (95% CI: 62% to 94%), including 2 CRs (11%), 5 VGPRs (26%), and 9 PRs (47%). The CBR was 95% (CI: 75% to 99%) with the remaining patient having stable disease (SD). For patients with disease refractory to a PI in a previous line of therapy (n = 21), the ORR was 43% (95% CI: 24% to 63%), including 1 CR (5%), 4 VGPRs (19%), and 4 PRs (19%). The CBR was 67% (95% CI: 45% to 83%), with 6 (29%) patients having SD. The median time to first response was 1.2 months (interquartile range: 1.2-1.7 months) (Figure 1).
Time on study. Swim lane plot of time on study for the 25 patients who achieved an objective response (≥ PR). Y-axis indicates refractoriness (naive [Naï], relapsed [Rel], or refractory [Ref]) of the patient’s myeloma to a PI (bortezomib, carfilzomib, or ixazomib). Arrows indicate patient is still on study. × indicates that patient is off study due to PD, adverse event (WC-AE), death, travel issues, or decreased quality of life (WC-QoL).
Time on study. Swim lane plot of time on study for the 25 patients who achieved an objective response (≥ PR). Y-axis indicates refractoriness (naive [Naï], relapsed [Rel], or refractory [Ref]) of the patient’s myeloma to a PI (bortezomib, carfilzomib, or ixazomib). Arrows indicate patient is still on study. × indicates that patient is off study due to PD, adverse event (WC-AE), death, travel issues, or decreased quality of life (WC-QoL).
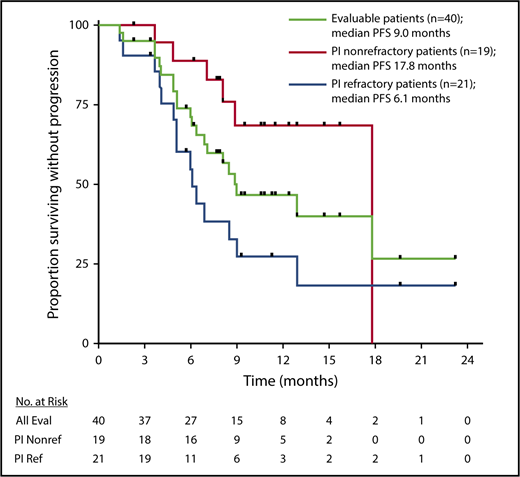
The median PFS for evaluable patients (n = 40) was 9.0 months, 17.8 months for patients who were naive or relapsed to a PI (n = 19), and 6.1 months for patients who were refractory to a PI (n = 21) (Figure 2).
PFS. Kaplan-Meier estimates of PFS for response evaluable (Eval) patients (n = 40) treated with SVd, patients not refractory (Nonref) to a PI (n = 19) at screening, and patients refractory (Ref) to a PI (n = 21) at screening. Black ticks indicate time points where patients were censored.
PFS. Kaplan-Meier estimates of PFS for response evaluable (Eval) patients (n = 40) treated with SVd, patients not refractory (Nonref) to a PI (n = 19) at screening, and patients refractory (Ref) to a PI (n = 21) at screening. Black ticks indicate time points where patients were censored.
Of the 40 patients with at least 1 disease assessment after cycle 1 day 1, 38 patients (95%) had a reduction in M-spike levels while on study (supplemental Figure 1). All patients whose myeloma was not refractory to a prior PI had a reduction in their M-spike from baseline.
Discussion
Inhibition of XPO1 represents a novel and rational approach in cancer therapy in view of the essential role nuclear exporters play in the shuttling of large proteins (in particular TSPs), ribosomal subunits, and oncogenic mRNAs across the nuclear membrane. Indeed, targeting XPO1 has proven to be cytotoxic to a wide variety of transformed cells, including MM, and XPO1 inhibitors have shown marked synergy with PIs.20,23 In the current work, we explored the feasibility and efficacy of selinexor in combination with bortezomib and low-dose Vd.
The observed ORR of 63% is encouraging, in particular considering that 50% of the patients had MM refractory to a PI and had received a median of 3 (1-11) prior lines of therapy, including 38% who received ≥5 lines of therapy. Importantly, the high responses (84%) seen in PI-relapsed/naive patients support the nonclinical synergy observed between XPO1 inhibitors and PIs. Furthermore, although we recognize the need for further validation, these response rates are consistent with recent results of the CASTOR and ENDEAVOR trials, despite the use of reduced-intensity, once-weekly bortezomib in the current study.2,6
Patients who had PI-refractory disease in this trial showed a 43% ORR and 67% CBR. In a study performed by the IMWG and a more recent meta-analysis of the efficacy of bortezomib re-treatment, the response rate to bortezomib-based “re-treatment” in bortezomib-refractory patients was ∼22%, compared with 43% observed in this trial.30,31 In 2 recent phase 2 trials, promising responses were also observed in patients with myeloma refractory to bortezomib when bortezomib (given twice weekly at the dose of 1.3 mg/m2) was reintroduced in combination with nelfinavir or venetoclax.32,33 Although in single-arm, nonrandomized trials, the effect of clonal tiding and reemergence of a PI-sensitive clone could not be fully excluded, it is important to note that in the current study bortezomib was given at the low dose of 1.3 mg/m2 SC once weekly. By study design, only 3 patients (7%) received treatment with twice-weekly bortezomib, and the bortezomib frequency was reduced to once weekly in these patients after treatment of ≤3 weeks. Furthermore, responses to selinexor and Vd in PI-refractory disease (albeit in a more heavily pretreated quad- and penta-refractory population) were seen in 21% of the patients.25 Therefore, it is improbable that the observed responses in the current study in PI-refractory patients are a result of either selinexor or bortezomib alone, and rather, support the nonclinical synergy reported between these 2 drug classes. Last, it is important to note that responses were sustained over time with an overall median PFS of 9.0 months, including a median PFS of 17.8 months in PI-relapsed/naive patients.
The addition of weekly 1.3 mg/m2 SC bortezomib did not impact the tolerability of selinexor, as no unexpected or increased toxicities were reported. Furthermore, no major end organ toxicities were observed even after prolonged dosing, and gastrointestinal AEs were reversible and manageable with dose reductions and supportive care. Of note, although nausea and vomiting were frequently reported in our study, they were manageable with the prophylactic use of antiemetics on the day of treatment and in some patients for the following 2 days. Furthermore, the gastrointestinal AEs typically resolved within 1 to 2 days of selinexor and bortezomib administration with no residual long-term effects. With the exception of peripheral neuropathy, the rates and grades of AEs observed in the current study, including nausea, anorexia, fatigue, and thrombocytopenia, are mostly consistent with those previously reported with the combination of selinexor and Vd, supporting the lack of cumulative or additive toxicity incurred by the addition of low-dose weekly bortezomib.24,25 Of particular interest, and despite the fact that 86% of the patients were previously exposed to bortezomib, the overall rate of peripheral neuropathy was minimal with only 5% of the patients experiencing grade 2 neuropathy and no grade 3 reported. At the RP2D, the doses of bortezomib and Vd are 40% and 25% less than the standard twice-weekly Vd regimen, respectively, allowing for the continued administration of Vd without incurring cumulative toxicities or loss of efficacy.
The treatment algorithm in patients with myeloma is rapidly evolving and now requires the combination with PIs and IMiDs in the induction regimen with therapy often continued until disease progression. Therefore, there is a growing unmet need for novel classes of drugs for patients with dual-refractory (PI and IMiD) myeloma. Targeting XPO1 with selinexor represents a novel approach to effectively treat MM through suppression of multiple oncogenic pathways independent of the proteasome-ubiquitin pathway or the Cullin ring 4–cereblon E3 ligase activity. In the current trial, we demonstrated the feasibility, safety, and clinical efficacy of SVd in patients with relapsed and refractory MM. Based on these encouraging results, a randomized phase 3 trial “BOSTON” comparing the SVd to Vd is ongoing (ClinicalTrials.gov: #NCT03110562).
Some of these data were previously presented at the 59th annual meeting of the American Society of Hematology, Atlanta, GA, 9-11 December 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients who participated in this trial and their families, as well as the coinvestigators, nurses, and study coordinators at each of the sites.
This study was sponsored by Karyopharm Therapeutics.
Authorship
Contribution: N.J.B., H.S., D.W., M.S., S.L., R.K., C.P.V., C.G., A.D.C., P.N., and D.R. enrolled patients, interpreted the results, and wrote the manuscript; M.K. and S.S. designed the study, interpreted the results, provided reagents, and reviewed the manuscript; T.J.U. analyzed the data, interpreted the results, and wrote the manuscript; J.J. and J.-R.S.-M. collected data, analyzed data, and reviewed the manuscript; J.S. collected data, analyzed data, provided reagents, and reviewed the manuscript; and C.C. enrolled patients, interpreted the results, and reviewed the manuscript.
Conflict-of-interest disclosure: D.W. has received consultancy fees and honoraria from Amgen, Celgene, Janssen, Sanofi, and Takeda. S.L. is Chief Scientific Advisor and a shareholder of Caelum Biosciences and sits on the Advisory Boards of Janssen and Bayer. R.K. is a stockholder in Karyopharm Therapeutics. C.P.V. has received honoraria from Johnson & Johnson, Celgene, Amgen, and Takeda. M.K. is CEO of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. S.S. is CSO of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. T.J.U. is an employee of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. J.J. is an employee of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. J.-R.S.-M. is an employee of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. J.S. is an employee of Karyopharm Therapeutics and a stockholder in Karyopharm Therapeutics. The remaining authors declare no competing financial interests.
Correspondence: Nizar J. Bahlis, University of Calgary, Charbonneau Cancer Research Institute, Divisions of Hematology and Oncology, 1403 29th St NW Room C210, Calgary, AB T2N 2T9, Canada; e-mail: nbahlis@ucalgary.ca.

![Figure 1. Time on study. Swim lane plot of time on study for the 25 patients who achieved an objective response (≥ PR). Y-axis indicates refractoriness (naive [Naï], relapsed [Rel], or refractory [Ref]) of the patient’s myeloma to a PI (bortezomib, carfilzomib, or ixazomib). Arrows indicate patient is still on study. × indicates that patient is off study due to PD, adverse event (WC-AE), death, travel issues, or decreased quality of life (WC-QoL).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/132/24/10.1182_blood-2018-06-858852/6/m_blood858852f1.png?Expires=1765887144&Signature=h19a2C97G-ZTpYfJxu7VdRnud~TSLfMa6yRqlVw1CS8Ao5SjXgRoRCfnN~CQLQ~Bre~Z1Q0iWMAjlP~Z5UzVPUoQ-UBTqCJuRHVK0JEHrwB7uithWXx1elaHX1k3PRj0Q40gUpjIi06o5hIl5ntsRUpyvlZIvjzpMbSxOOid3bKoe3uzP4KHmrW9legfHm8EPRKUUF877XtJWx2x4-HSDEfuwIoIQ~rAttcU-wchj53zzoz45-q8dMaa7mMDKy9zs1l6sQZdWVznK7N3raIAPsttZbgn~LBO7VnJobvF~SZXgrWUysXKiS62CCDzu3mB6aXtJaEP0w4xXyhfmWUZhA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)