Key Points
IGF-1 has the ability to promote megakaryocyte differentiation, PPF, and platelet release.
The effect of IGF-1 on thrombopoiesis is mediated primarily by AKT activation with the assistance of SRC-3.
Abstract
It is known that insulin-like growth factor-1 (IGF-1) also functions as a hematopoietic factor, although its direct effect on thrombopoiesis remains unclear. In this study, we show that IGF-1 is able to promote CD34+ cell differentiation toward megakaryocytes (MKs), as well as the facilitation of proplatelet formation (PPF) and platelet production from cultured MKs. The in vivo study demonstrates that IGF-1 administration accelerates platelet recovery in mice after 6.0 Gy of irradiation and in mice that received bone marrow transplantation following 10.0 Gy of lethal irradiation. Subsequent investigations reveal that extracellular signal-regulated kinase 1/2 (ERK1/2) and Akt activation mediate the effect of IGF-1 on thrombopoiesis. Notably, Akt activation induced by IGF-1 is more apparent than that of ERK1/2, compared with that of thrombopoietin (TPO) treatment. Moreover, the effect of IGF-1 on thrombopoiesis is independent of TPO signaling because IGF-1 treatment can also lead to a significant increase of platelet counts in homozygous TPO receptor mutant mice. Further analysis indicates that the activation of Akt triggered by IGF-1 requires the assistance of steroid receptor coactivator-3 (SRC-3). Therefore, our data reveal a distinct role of IGF-1 in regulating thrombopoiesis, providing new insights into TPO-independent regulation of platelet generation.
Introduction
Insulin-like growth factor-1 (IGF-1) is a 70-aa basic polypeptide with an insulin-like structure, and its receptor, IGF-1-receptor (IGF1R), is widely distributed in bones, the liver, muscles, and intestines.1 Thus, IGF-1 is a well-documented cytokine in cell growth and survival, oncogenic transformation, and cancer progression.2-4 Apart from the diverse effects of IGF-1 on various tissues, IGF-1 can also be released by stromal cells in the bone marrow (BM), and distant organs including the liver and other tissues, and functions as a systemic regulator on hematopoiesis.5 IGF-1 has been reported to be not only a crucial mediator of endosteal niche reorganization, consequently facilitating hematopoietic stem cell (HSC) engraftment, but also plays important roles in erythrocytosis and lymphocytopoiesis.6-8 However, the exact role and underlying mechanisms of IGF-1 in regulating thrombopoiesis have not been well illustrated, although some studies have shown that IGF-1 is involved in megakaryocytopoiesis.9-12
It is known that IGF-1 mediates its actions through structurally similar receptors, including insulin receptor (INSR) and IGF-1-receptor (IGF1R), which form homodimers or heterodimers to recognize its ligands.13,14 The 2 receptors consist of an extracellular α-subunit and a transmembrane β-subunit monomer.15 Upon IGF-1 binding, the 2 receptors are auto-phosphorylated and insulin receptor substrates 1 and 2 (IRS-1 and IRS-2) are recruited, resulting in the activation of the 2 most prominent downstream signaling pathways, the phosphoinositide 3-kinase (PI3K)-Akt pathway and the MEK–extracellular signal-regulated kinase 1/2 (ERK1/2) pathway.16,17 PI3K-AKT has been reported to enhance protein synthesis and cellular metabolism via mammalian target of rapamycin (mTOR) and to increase survival via p53, NF-κB, BAD/Bcl2, and FOXOs, whereas MEK-ERK1/2 activation mainly leads to increased cellular expansion.3,18
Platelets are produced by megakaryocytes (MKs), which originate from HSCs and then undergo several consecutive molecular and biological stages that are unique to this cell lineage, including MK differentiation, expansion, maturation, proplatelet formation (PPF) and platelet release.19 Therefore, the output of platelet production will be compromised if any stage is influenced, and various hematopoietic cytokines have been reported to regulate different steps of thrombopoiesis through activation of MEK-ERK1/2, PI3K-Akt or JAK-STAT signaling pathways.20 Thus far, thrombopoietin (TPO) is considered to be the most influential cytokine in this process.21 However, TPO-deficient mice or mice deficient in the TPO receptor c-Mpl, even with only 10% of circulating platelets, appear healthy and show no symptoms of spontaneous hemorrhage.22 Therefore, alternative regulators exist regarding megakaryocytopoiesis rather than TPO, and these regulators are likely to play a supplementary role and to be essential to maintain hemostasis.23 Nevertheless, whether IGF-1 serves as another alternative effector on thrombopoiesis needs to be illustrated more completely.
In the present study, we systematically demonstrated the efficiency of IGF-1 regarding CD34+ cell expansion, MK differentiation, and PPF, as well as platelet release, which worked through both ERK1/2 and Akt signaling. Further studies revealed that this facilitation of IGF-1 was not a result of merely acting through TPO signaling, and required the assistance of steroid receptor coactivator-3 (SRC-3). Collectively, our data more extensively reveal that the role of IGF-1 in thrombopoiesis mainly acts through the Akt signal pathway.
Materials
Animals
C57BL/6J-Mplhlb219/J mice were purchased from The Jackson Laboratory. Mice deficient in SRC-3 (SRC3−/−) were kindly offered by Jianming Xu (Molecular and Cellular Biology Laboratory, Baylor College of Medicine, Houston, TX). Wild-type (WT) C57BL/6 mice were obtained from the Institute of Zoology (Chinese Academy of Sciences, Beijing, China). All mice were handled in accordance with the guidelines of the Third Military Medical University Animal Care and Use Committee.
Human MK culture
Human primary MKs were induced and cultured as previously reported.24,25 Briefly, human cord blood was isolated for CD34+ cells using an immunomagnetic bead separation system (EasySep, Human CD34 Positive Selection kit; Stem Cell Technologies, Vancouver, BC, Canada). After each isolation, CD34+ cells were loaded for purity assessment by flow cytometry, and the purity usually averaged from 92% to 95%. The isolated CD34+ cells were then cultured in serum-free medium (StemSpan SFEM; Stem Cell Technologies) in the presence of 20 ng/mL recombinant human (rh) TPO (rhTPO) or 20 ng/mL recombinant human (rh) stem cell factor (rhSCF; Peprotech, Rocky Hill, NJ) at a density of 4 × 104/mL.
Mouse MK culture
Briefly, mouse BM cells were flushed from the femur and tibia, and were then sorted for c-kit+ cells by flow cytometry after staining with c-kit antibody (eBioscience, San Diego, CA). After each sorting, c-kit+ cells were then cultured in serum-free medium (StemSpan SFEM; Stem Cell Technologies) with or without 20 ng/mL rhTPO in the presence of 10 ng/mL recombinant mouse (rm) interleukin 3 (rmIL3) (Peprotech) for subsequent use.
RT-PCR analysis
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA). RNA was used to produce complementary DNA (cDNA) using the PrimeScript RT-PCR kit (DRR014A) (Takara, Tokyo, Japan) according to the manufacturer’s instructions. Real-time polymerase chain reaction (RT-PCR) was then performed using the iQ5 system (Bio-Rad, Hercules, CA). Each reaction was performed in triplicate and normalized relative to human or mouse glyceraldehyde-3-phosphate dehydrogenase (GAPDH). The primers used were as follows: IGF-1R, forward: 5′-TACAACATCACCGACCCG-3′, reverse: 5′-AAAGACGAAGTTGGAGGC-3; INSR, forward: 5′-GCGACACTTCACGGGCTAT-3′, reverse: 5′-CAGACCATTGGGCTCCTT-3′; GAPDH, forward: 5′-AACGGATTTGGTCGTATTG-3′, reverse: 5′-GGAAGATGGTGATGGGATT-3′.
Measurement of MK differentiation
Human cord blood–derived cells were harvested and labeled with fluorescein isothiocyanate (FITC)-conjugated anti-CD41, and allophycocyanin (APC)-conjugated anti-CD42b (Biolegend, San Diego, CA) after culture. Mouse BM-derived cells were collected and labeled with APC-conjugated anti-CD41 (Biolegend), and FITC-conjugated anti-CD42b (Emfret Analytics, Eibelstadt, Germany) after culture. Both cell types were labeled with control isotype antibodies (Biolegend). After incubation at room temperature (RT) for 30 minutes in the dark followed washing with phosphate-buffered saline (PBS) twice, cell-associated immunofluorescence was finally analyzed after staining 4′, 6 diamidino-2-phenylindole (DAPI) (Sigma-Aldrich, St. Louis, MO) with a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA).
Western blotting
Primary MKs were collected after different treatments, and were then lysed using ice-cold lysis buffer containing 1% protease inhibitors (Roche Applied Science, Penzberg, Germany). Proteins were fractionated by sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis and were transferred to polyvinylidene difluoride (PVDF) membrane. Protein expression was detected by incubation with phospho-ERK1/2, ERK1/2, phospho-Akt, Akt (Cell Signaling Technology, Danvers, MA), IR, IGF-1R, IRS-1, IRS-2, phospho-Rac1/Cdc42 S71, Rac1/Cdc42 (Abcam, Cambridge, MA), or GAPDH (Beyotime, Haimen, China) antibodies. The inhibitors NVP-ADW742, U0126 or LY294002 (MedChem Express, Monmouth Junction, NJ) were pretreated for 20 minutes when used.
Immunoprecipitation
Primary MKs were treated with rhIGF-1 (Peprotech, Rocky Hill, NJ) at 37°C, and were then lysed by the addition of 2×immunoprecipitation buffer (10 mM Tris, 1% Nonidet P-40, 5 mM EDTA, 1 mM Na3VO4, 0.5 mM phenylmethanesulfonyl fluoride, 150 mM NaCl, pH 7.4) containing a protease inhibitor cocktail (Roche Applied Science, Penzberg, Germany), and rotated at 4°C for 1 hour. An IGF-1R antibody or control IgG was added to the lysates, and incubated at 4°C overnight with constant rotation. This procedure was followed by incubation with protein A/G–agarose at 4°C for 3 hours for antigen-antibody complex capture. Next, the samples were washed with 1×immunoprecipitation buffer 3 times, boiled in 2× SDS loading buffer, and analyzed by western blotting with the appropriate antibodies.
MK ploidy analysis
Ploidy was assessed as previously reported.9 Briefly, cultured MKs were collected and incubated with a FITC-conjugated anti-CD41 antibody for 30 minutes in the dark. After washing with PBS and permeabilizing with ice-cold methanol, the pellets were treated with a trypsin inhibitor/RNase buffer, and were then labeled with propidium iodide (PI) (Invitrogen, Carlsbad, CA). Finally, the CD41+ cells were gated for further analysis with a BD FACSCanto II flow cytometer (BD Biosciences, San Jose, CA). The ploidy of cultured MKs were further investigated by a modified Wright-Giemsa method.
Determination of culture-derived platelets
Platelets generated from human or mouse MKs were assayed as previously described.15,16 Briefly, culture-derived MKs were centrifuged at 200g for 5 minutes for MK harvest, and the supernatants were recentrifuged at 1300g for 10 minutes for platelet collection. Thereafter, the pellets containing MKs and platelets were mixed and labeled with CD41, CD42b antibodies and PI. Finally, the CD41+CD42b+PI- particles were gated for analysis after washing.
Platelet activation analysis
Briefly, culture-derived platelets were stimulated with rhIGF-1 for 5 minutes before stimulation with 0.5 μM ADP (Sigma, St Louis, MO) for 5 minutes at RT. The pellets were then stained with a phycoerythrin-CD62p antibody (eBioscience, San Diego, CA) in the dark for 20 minutes at RT followed by flow cytometry analysis.
Immunofluorescence microscopy
For confocal microscopy, MKs were spun onto glass slides, fixed with 10% formalin and permeabilized with 0.25% Triton X-100. Tubulin was then stained with an anti-β1-tubulin antibody (Sigma, St Louis, MO), and the nuclei was stained with DAPI. Images were captured using a laser confocal microscope (LSM 800; ZEISS, Copenhagen, Germany).
Histology
Briefly, femurs and tibias from mice were fixed in 10% formaldehyde and were then embedded in paraffin. Staining with hematoxylin and eosin (H&E) was performed by the standard operating procedures after the tissues were sliced into 6-mm-thick sections. The sections were photographed with a microscope (Olympus Optical, Tokyo, Japan).
Measurement of serum IGF-1
Mice were killed by a sodium pentobarbital injection. Whole blood was collected for serum IGF-1 concentration analysis using an ELISA kit (Invitrogen, Carlsbad, CA) following the manufacturer's instructions. The results are presented as the means ± standard deviation.
In vivo IGF-1 administration
Mice were injected intraperitoneally with saline or rhIGF-1 (1 mg/kg) twice daily for 7 consecutive days. Blood was collected from the tail vein for the platelet count assay. Three randomly selected mice from each group were killed, and their femurs and tibias were obtained for histologic analysis.
Hematological analysis
Twenty microliters of peripheral blood was collected from the tail vein and was then dissolved in a 1% EDTA solution. Complete blood numbers were counted automatically using a hematology analyzer (Sysmex XT-2000iV, Kobe, Japan).
Statistical analysis
The results are expressed as the means ±SEM from at least 3 independent experiments. The data were analyzed using Student t test for 2 groups and 1-way analysis of variance (ANOVA) for multiple groups. P value less than .05 was considered statistically significant.
Results
IGF-1 signaling exists during megakaryocytopoiesis
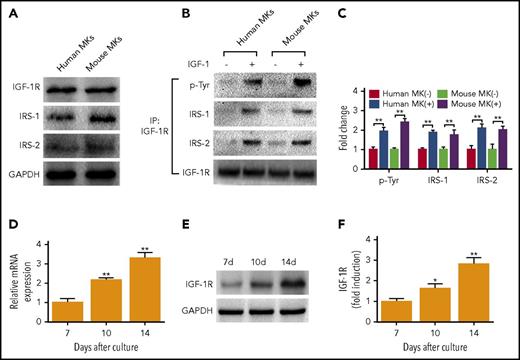
First, to determine whether the IGF-1 signal pathway is correlated with thrombopoiesis, we detected and found that both human and mouse MKs expressed IGF-1R, and IRS-1 and 2 (Figure 1A). Then, we observed that rhIGF-1 stimulated tyrosine phosphorylation of IGF-1R, as well as the association of IGF-1R with IRS-1 and IRS-2 (Figure 1B-C), suggesting that IGF-1 signaling is present in MKs. Next, we detected mRNA transcripts of IGF-1R in human MKs at day 7, 10, and 14, and the expression of IGF-1R was shown to gradually increase with the culture days in human MKs (Figure 1D). This expression profile was also confirmed by western blot analysis (Figure 1E-F). In addition, we found that another binding site of IGF-1, INSR, also exhibited a similar expression tendency as that of IGF-1R, although it showed slight affinity with IGF-126 (supplemental Figure 1A-C, available on the Blood Web site). Thus, these data reveal the existence of IGF-1 signaling in MKs, and a gradually increased expression of its binding sites during the progression of megakaryocytopoiesis.
MKs express functional IGF-1R. (A) Immunoblotting analysis of IGF-1R, IRS-1, and IRS-2 in human and mouse MKs. (B-C) Association of IRS-1 and IRS-2 with IGF-1R in human and mouse MKs after treatment with or without 100 ng/mL rhIGF-1 for 15 minutes. Lysates were subjected to immunoprecipitation with antibody against IGF-1R, and were immunoblotted with antibodies against p-Tyr, IRS-1, IRS-2, or IGF-1R. (D-F) Real-time PCR (RT-PCR) (D) and immunoblot analysis of IGF-1R expression (E-F) in human MKs at the same counts, primary MKs were cultured from CD34+ cells cultured with 20 ng/mL TPO for 7, 10 and 14 days. Human MKs or mouse MKs were purified after being labeled with CD41 antibody. *P < .05, **P < .01, vs the expression level at day 7.
MKs express functional IGF-1R. (A) Immunoblotting analysis of IGF-1R, IRS-1, and IRS-2 in human and mouse MKs. (B-C) Association of IRS-1 and IRS-2 with IGF-1R in human and mouse MKs after treatment with or without 100 ng/mL rhIGF-1 for 15 minutes. Lysates were subjected to immunoprecipitation with antibody against IGF-1R, and were immunoblotted with antibodies against p-Tyr, IRS-1, IRS-2, or IGF-1R. (D-F) Real-time PCR (RT-PCR) (D) and immunoblot analysis of IGF-1R expression (E-F) in human MKs at the same counts, primary MKs were cultured from CD34+ cells cultured with 20 ng/mL TPO for 7, 10 and 14 days. Human MKs or mouse MKs were purified after being labeled with CD41 antibody. *P < .05, **P < .01, vs the expression level at day 7.
IGF-1 stimulation promotes MK differentiation through ERK1/2 and Akt signaling
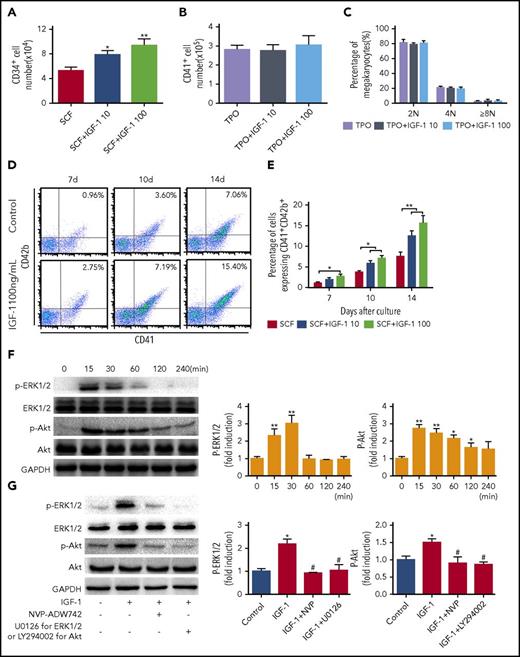
To detect whether IGF-1 exerts a direct role in thrombopoiesis, human cord blood-derived CD34+ cells were first cultured with rhIGF-1 in the presence of rhSCF. Consistent with the results from other reports,27 our data also showed that rhIGF-1 exposure increased the numbers of CD34+ cells after culture for 7 days (Figure 2A). However, it seemed that IGF-1 had no obvious synergistic effect with rhTPO on MK expansion (Figure 2B). Moreover, IGF-1 had no significant influence on polyploidization of MKs, as revealed by DNA content and cell size analysis, as well as Wright-Giemsa staining (Figure 2C; supplemental Figures 2B-C, 3A-B). Interestingly, specific markers of MK differentiation, CD41 and CD42b, were gradually increased with the cultured days after rhIGF-1 treatment, suggesting that IGF-1 has the ability to promote MK differentiation (Figure 2D-E). We next detected the 2 important positive regulators, ERK1/2 and Akt, during megakaryocytopoiesis after IGF-1 treatment. As shown, rapid and time-dependent phosphorylation of both Akt and ERK1/2 was observed after rhIGF-1 stimulation, which was effectively inhibited by pretreatment with an IGF-1R/INSR tyrosine kinase inhibitor (NVP-ADW742), Akt inhibitor (LY294002), or ERK1/2 inhibitor (U0126) in MKs (Figure 2F-G). Consequently, pretreatment with these inhibitors blocked the differentiation of MKs induced by rhIGF-1 (supplemental Figure 3D). These results demonstrate that IGF-1 stimulation can enhance the differentiation of MKs through IGF-1R–mediated ERK1/2 and Akt activations.
IGF-1 promotes the population of CD34+cells and the differentiation of MKs. (A) Flow cytometric analysis of the numbers of CD34+ cells after stimulation with 10 or 100 ng/mL rhIGF-1 for 7 days in the presence of 20 ng/mL rhSCF. (B) Flow cytometric analysis of CD41+ cell counts after 10 or 100 ng/mL rhIGF-1 treatment of 7 days in the presence of 20 ng/mL rhTPO. (C) DNA content assay in CD41+ cells after CD34+ cells stimulated with 10 or 100 ng/mL rhIGF-1 in the presence of 20 ng/mL rhTPO for 7 days. (D-E) CD41+CD42b+ expression on CD34+ cells treated with or without rhIGF-1 for 7, 10 and 14 days in the presence of 20 ng/mL rhSCF. (F) Western blot analysis of ERK1/2 and Akt phosphorylation in MKs after exposure to 100 ng/mL rhIGF-1 for different times. (G) Phosphorylation of p-ERK1/2 and p-Akt in MKs pretreated with 0.5 μM NVP-ADW742, 10 μM U0126 or 20 μM LY294002 followed by rhIGF-1 treatment of 15 minutes. *P < .05, **P < .01, vs the control; #P < .05, ##P < .01, vs the IGF-1-treated group.
IGF-1 promotes the population of CD34+cells and the differentiation of MKs. (A) Flow cytometric analysis of the numbers of CD34+ cells after stimulation with 10 or 100 ng/mL rhIGF-1 for 7 days in the presence of 20 ng/mL rhSCF. (B) Flow cytometric analysis of CD41+ cell counts after 10 or 100 ng/mL rhIGF-1 treatment of 7 days in the presence of 20 ng/mL rhTPO. (C) DNA content assay in CD41+ cells after CD34+ cells stimulated with 10 or 100 ng/mL rhIGF-1 in the presence of 20 ng/mL rhTPO for 7 days. (D-E) CD41+CD42b+ expression on CD34+ cells treated with or without rhIGF-1 for 7, 10 and 14 days in the presence of 20 ng/mL rhSCF. (F) Western blot analysis of ERK1/2 and Akt phosphorylation in MKs after exposure to 100 ng/mL rhIGF-1 for different times. (G) Phosphorylation of p-ERK1/2 and p-Akt in MKs pretreated with 0.5 μM NVP-ADW742, 10 μM U0126 or 20 μM LY294002 followed by rhIGF-1 treatment of 15 minutes. *P < .05, **P < .01, vs the control; #P < .05, ##P < .01, vs the IGF-1-treated group.
IGF-1 promotes PPF through Akt-Rac1/Cdc42-mediated tubulin cytoskeleton reorganization
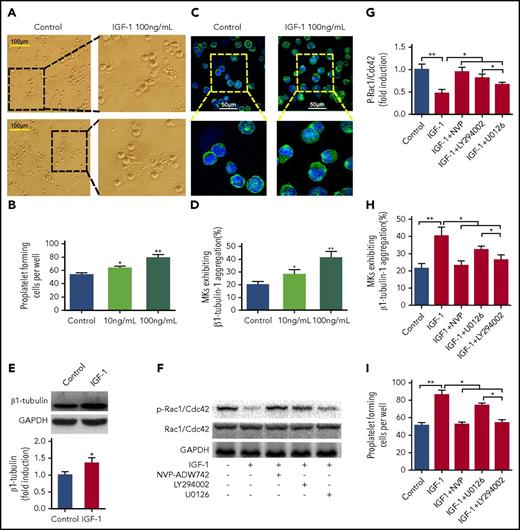
Next, we investigated the effects of IGF-1 on PPFs. As observed under an inverted microscope, rhIGF-1 treatment induced typical PPFs from MKs, characterized by threadlike cytoplasmic extensions with the formation of pseudopods and bead-like nubs (Figure 3A-B). Additionally, obvious aggregation and increased protein expression of β1-tubulin were detected in these MKs after rhIGF-1 stimulation (Figure 3C-E). Because Rac1/Cdc42 GTPase was reported to regulate the rearrangement of the MK tubulin cytoskeleton,28 the phosphorylation of Rac1/Cdc42 at serine 71 was the negative indicative of active Rac1/Cdc42.29,30 Our further data showed that rhIGF-1 led to an evident increase in the activation of Rac1/Cdc42 GTPases in mature MKs, which was effectively inhibited by pretreatment with the ERK1/2 inhibitor, especially by the Akt inhibitor (Figure 3F-G). Correspondingly, pretreatment with U0126, particularly with LY294002, significantly abolished the aggregation of β1-tubulin and PPF induced by rhIGF-1 in a similar manner to that treated with NVP-ADW742 (Figure 3H-I). Therefore, the above data demonstrate that IGF-1 promotes PPF from MKs mainly through Akt-Rac1/Cdc42-mediated tubulin cytoskeleton rearrangement.
IGF-1 promotes proplatelet formation. CD34+ cells cultured with rhTPO for 10 days were purified for MKs, which were used in subsequent experiments. (A-B) Typical PPF from MKs captured by phase contrast imaging after treatment with 10 or 100 ng/ml rhIGF-1 for 4 days. (C-D) Tubulin cytoskeleton distribution in MKs after rhIGF-1 treatment. MKs were stained with β1-tubulin (green) and DAPI (blue). (E) Immunoblot analysis of β1-tubulin expression in MKs treated with or without rhIGF-1. (F-G) Phospho-Rac1/Cdc42 expression in MKs blocked with 0.5 μM NVP-ADW742, 10 μM LY294002, or 20 μM U0126 and then treated with rhIGF-1 for 15 minutes. (H-I) Number of MKs exhibiting β1-tubulin aggregation (H) and PPFs (I) pre-blocked with 0.5 μM NVP-ADW742, 10 μM LY294002, or 20 μM U0126 for 20 minutes followed by rhIGF-1 treatment. *P < .05, **P < .01.
IGF-1 promotes proplatelet formation. CD34+ cells cultured with rhTPO for 10 days were purified for MKs, which were used in subsequent experiments. (A-B) Typical PPF from MKs captured by phase contrast imaging after treatment with 10 or 100 ng/ml rhIGF-1 for 4 days. (C-D) Tubulin cytoskeleton distribution in MKs after rhIGF-1 treatment. MKs were stained with β1-tubulin (green) and DAPI (blue). (E) Immunoblot analysis of β1-tubulin expression in MKs treated with or without rhIGF-1. (F-G) Phospho-Rac1/Cdc42 expression in MKs blocked with 0.5 μM NVP-ADW742, 10 μM LY294002, or 20 μM U0126 and then treated with rhIGF-1 for 15 minutes. (H-I) Number of MKs exhibiting β1-tubulin aggregation (H) and PPFs (I) pre-blocked with 0.5 μM NVP-ADW742, 10 μM LY294002, or 20 μM U0126 for 20 minutes followed by rhIGF-1 treatment. *P < .05, **P < .01.
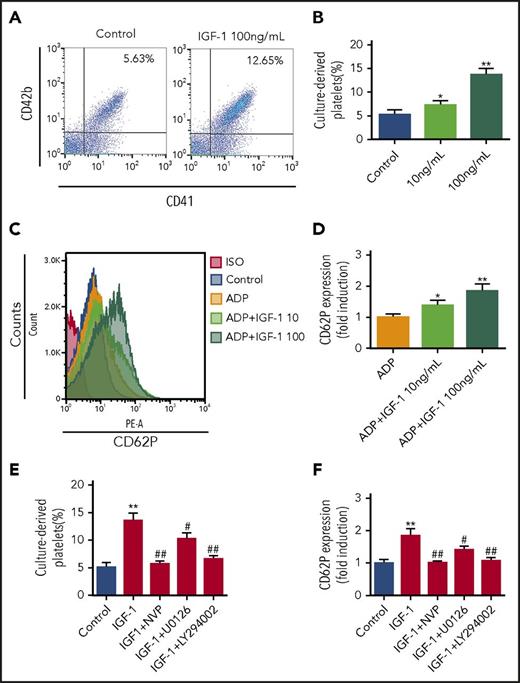
IGF-1 facilitates platelet release and platelet activation mainly through the Akt signal pathway
To further observe whether IGF-1 plays a direct role in platelet production, we next detected the culture-derived platelets produced from MKs using a method established by us and other researchers in previous studies by flow cytometry.24,25 As shown in Figure 4A-B, rhIGF-1 stimulation induced a significant increase in platelet release following cord blood-derived CD34+ cells treatment with rhTPO. We further showed that rhIGF-1 potentiated platelet activation, because an increase of CD62p expression was detected on these pellets after rhIGF-1 stimulation (Figure 4C-D). Nevertheless, it was shown that pretreatment with an ERK1/2 inhibitor, especially with an Akt inhibitor, potently inhibited platelet production and activation induced by rhIGF-1 as effectively as IGF-1R blockade (Figure 4E-F). Therefore, these results collectively suggest that IGF-1 facilitates platelet production and its activation primarily via Akt signal pathway.
IGF-1 induces platelet production and augments platelet activation. (A-B) Flow cytometric analysis of platelet production from MKs induced by IGF-1. CD34+ cells were treated with rhTPO for 10 days followed by rhIGF-1 treatment of 4 days. (C-D) Flow cytometric analysis of the activation of culture-derived platelets. Platelets were stimulated with 10 or 100 ng/mL rhIGF-1 for 5 minutes before stimulation with 0.5 μM ADP for 5 minutes, and were then subjected to analysis after CD62p staining. (E) Platelet production from MKs in CD34+ cells cultured with 20 ng/mL rhTPO for 10 days and then treated with 100 ng/mL rhIGF-1 with or without 0.5 μM NVP-ADW742, 10 μM U0126 or 20 μM LY294002 for another 4 days. (F) Activation analysis of platelets. Culture-derived platelets were first pretreated with 0.5 μM NVP-ADW742, 10 μM U0126 or 20 μM LY294002 for 20 minutes and were then stimulated with rhIGF-1 for 5 minutes following stimulation with 0.5 μM ADP for 5 minutes. *P < .05, **P < .01, vs the control; #P < .05, ##P < .01, vs the IGF-1-treated group.
IGF-1 induces platelet production and augments platelet activation. (A-B) Flow cytometric analysis of platelet production from MKs induced by IGF-1. CD34+ cells were treated with rhTPO for 10 days followed by rhIGF-1 treatment of 4 days. (C-D) Flow cytometric analysis of the activation of culture-derived platelets. Platelets were stimulated with 10 or 100 ng/mL rhIGF-1 for 5 minutes before stimulation with 0.5 μM ADP for 5 minutes, and were then subjected to analysis after CD62p staining. (E) Platelet production from MKs in CD34+ cells cultured with 20 ng/mL rhTPO for 10 days and then treated with 100 ng/mL rhIGF-1 with or without 0.5 μM NVP-ADW742, 10 μM U0126 or 20 μM LY294002 for another 4 days. (F) Activation analysis of platelets. Culture-derived platelets were first pretreated with 0.5 μM NVP-ADW742, 10 μM U0126 or 20 μM LY294002 for 20 minutes and were then stimulated with rhIGF-1 for 5 minutes following stimulation with 0.5 μM ADP for 5 minutes. *P < .05, **P < .01, vs the control; #P < .05, ##P < .01, vs the IGF-1-treated group.
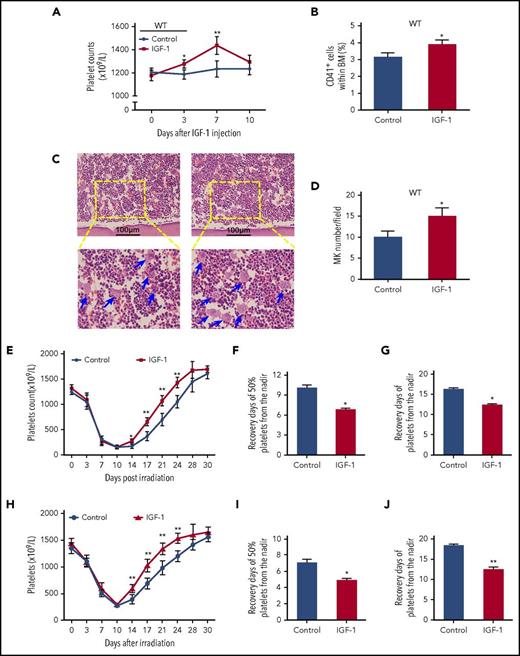
IGF-1 stimulates thrombopoiesis in vivo and promotes platelet recovery in irradiated mice
Subsequently, C57BL/6 mice were injected with rhIGF-1 to verify its role in thrombopoiesis promotion in vivo. As expected, rhIGF-1 treatment induced a gradual elevation of the peripheral platelet counts (Figure 5A). Additionally, the numbers of MKs in the BM were also significantly increased after rhIGF-1 administration as analyzed by flow cytometry and H&E staining (Figure 5B-D). Next, we evaluated this in vivo effect of rhIGF-1 in mice with thrombopenia after irradiation since the 10th day (the day when the platelet level hit the lowest level). As shown, rhIGF-1 administration significantly increased platelet recovery in the irradiated mice, and the days needed for 50% and 100% recovery of platelet loss from the nadir moved up earlier about 3.28 ± 0.14 and 4.12 ± 0.33 days, respectively (Figure 5E-G). Additionally, the same administration with rhIGF-1 led to a similar elevation of the platelet level in mice that received BM transplantation (BMT) following a 10.0Gy lethal irradiation (Figure 5H-J). Therefore, these results indicate that IGF-1 administration also facilitates platelet production in vivo.
IGF-1 administration significantly elevates the platelet levels in vivo and thereby promotes platelet recovery in irradiated mice. (A-B) Peripheral platelet levels (A) and CD41+ cells in BM (B) in C57BL/6 mice injected with saline or rhIGF-1 for 7 days. (C-D) H&E staining for MK quantification in the BM from C57BL/6 mice after rhIGF-1 injection. Ten high-power fields per sample were counted. (E) Peripheral platelet counts in C57BL/6 mice irradiated with 6.0Gy total-body irradiation following saline or rhIGF-1 treatment. (F-G) Days that is essential for 50% (F) or 100% (G) recovery of platelet loss from the nadir in irradiated mice with saline or rhIGF-1 injection. (H) Peripheral platelet counts in mice that received BM transplantation (BMT) following a 10.0Gy of irradiation (I-J) Days needed for 50% (I) or 100% (J) recovery of platelet loss from the nadir in grafted mice after rhIGF-1 administration. *P < .05, **P < .01.
IGF-1 administration significantly elevates the platelet levels in vivo and thereby promotes platelet recovery in irradiated mice. (A-B) Peripheral platelet levels (A) and CD41+ cells in BM (B) in C57BL/6 mice injected with saline or rhIGF-1 for 7 days. (C-D) H&E staining for MK quantification in the BM from C57BL/6 mice after rhIGF-1 injection. Ten high-power fields per sample were counted. (E) Peripheral platelet counts in C57BL/6 mice irradiated with 6.0Gy total-body irradiation following saline or rhIGF-1 treatment. (F-G) Days that is essential for 50% (F) or 100% (G) recovery of platelet loss from the nadir in irradiated mice with saline or rhIGF-1 injection. (H) Peripheral platelet counts in mice that received BM transplantation (BMT) following a 10.0Gy of irradiation (I-J) Days needed for 50% (I) or 100% (J) recovery of platelet loss from the nadir in grafted mice after rhIGF-1 administration. *P < .05, **P < .01.
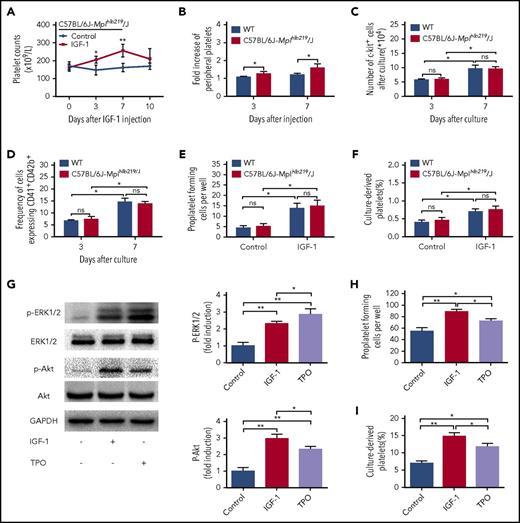
The action of IGF-1 on thrombopoiesis is not a result of merely working through TPO signaling
To more deeply understand IGF-1-induced thrombopoiesis, we performed the same injection of rhIGF-1 in C57BL/6J-Mplhlb219/J mice, which are defective in TPO-dependent thrombopoiesis because of a homozygous c-Mpl mutation. Interestingly, the same treatment led to a more distinct increase in platelets both on day 3 and day 7 (Figure 6A-B). However, the in vitro data showed that nearly all stages in the progression of thrombopoiesis affected by IGF-1 were not significantly influenced when c-Mpl was nonfunctional (Figure 6C-F; supplemental Figure 4A-B). Therefore, we then investigated the signaling alterations in MKs, and the data interestingly showed that a more visible elevation of Akt phosphorylation was detected after rhIGF-1 treatment, compared with that a higher level of ERK1/2 phosphorylation was observed after TPO stimulation (Figure 6G). Considering that TPO alone exerts no significant role in the terminal differentiation of MKs, and that TPO/c-Mpl signaling may even negatively regulate platelet shedding,31,32 we finally investigated and found that rhIGF-1 induced more PPF and platelet production from mouse-derived MKs than those from rhTPO treatment (Figure 6H-I; supplemental Figure 5). Collectively, the above results suggest that IGF-1 mainly exerts its role on MK maturation, PPF and platelet production during the late stages of thrombopoiesis, primarily through Akt signaling.
IGF-1 acts independent of TPO signal pathway to promote thrombopoiesis. (A) Peripheral platelet counts in C57BL/6J-Mplhlb219/J and WT mice injected with saline or rhIGF-1 for 7 days. (B) Fold induction of platelet counts on days 3 and 7 in C57BL/6J-Mplhlb219/J and WT mice after rhIGF-1 injection. Each group contained 6 to 7 mice. (C-D) Number of c-kit+ cells (C) and CD41+CD42b+ expression on c-kit+ cells (D) after stimulation with or without 100 ng/mL rhIGF-1 for 3 and 7 days. (E-F) Proplatelet-forming MKs (E) and culture-derived platelets (F) from c-kit+ cells cultured with or without 100 ng/mL rhIGF-1 for 8 days, c-kit+ cells were isolated from C57BL/6J-Mplhlb219/J or WT mice and were then cultured in the presence of 10 ng/mL rmIL-3. (G) western blot analysis of ERK1/2 and Akt phosphorylation in mouse-derived MKs from WT C57BL/6 mice after exposure to rhIGF-1 or rhTPO for 15 minutes. (H-I) Proplatelet-forming cells (H) and culture-derived platelets (I) from WT mouse-derived MKs cultured with rhIGF-1 or rhTPO for 3 days. Mouse-derived MKs were cultured from WT c-kit+ cells stimulated with rhTPO for 5 days in the presence of 10 ng/mL rmIL-3. *P < .05, **P < .01.
IGF-1 acts independent of TPO signal pathway to promote thrombopoiesis. (A) Peripheral platelet counts in C57BL/6J-Mplhlb219/J and WT mice injected with saline or rhIGF-1 for 7 days. (B) Fold induction of platelet counts on days 3 and 7 in C57BL/6J-Mplhlb219/J and WT mice after rhIGF-1 injection. Each group contained 6 to 7 mice. (C-D) Number of c-kit+ cells (C) and CD41+CD42b+ expression on c-kit+ cells (D) after stimulation with or without 100 ng/mL rhIGF-1 for 3 and 7 days. (E-F) Proplatelet-forming MKs (E) and culture-derived platelets (F) from c-kit+ cells cultured with or without 100 ng/mL rhIGF-1 for 8 days, c-kit+ cells were isolated from C57BL/6J-Mplhlb219/J or WT mice and were then cultured in the presence of 10 ng/mL rmIL-3. (G) western blot analysis of ERK1/2 and Akt phosphorylation in mouse-derived MKs from WT C57BL/6 mice after exposure to rhIGF-1 or rhTPO for 15 minutes. (H-I) Proplatelet-forming cells (H) and culture-derived platelets (I) from WT mouse-derived MKs cultured with rhIGF-1 or rhTPO for 3 days. Mouse-derived MKs were cultured from WT c-kit+ cells stimulated with rhTPO for 5 days in the presence of 10 ng/mL rmIL-3. *P < .05, **P < .01.
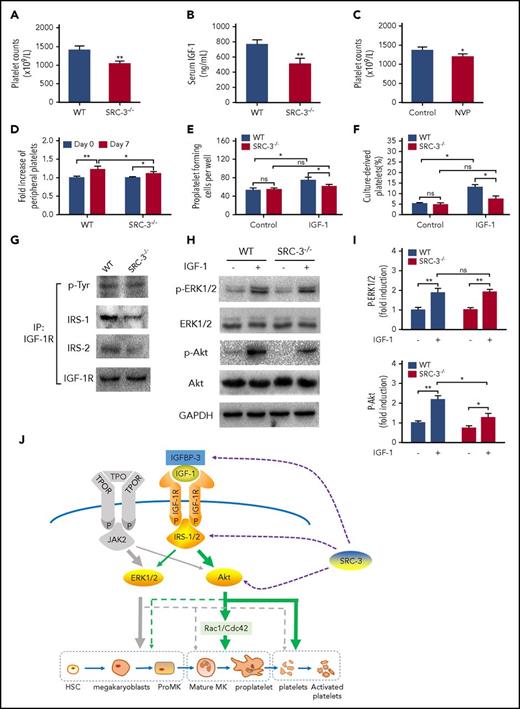
Thrombopoiesis promoted by IGF-1 requires the assistance of SRC-3
Our data above suggest that IGF-1 has a potent role in platelet production. Interestingly, we observed a decrease in the number of peripheral platelets along with a lower level of serum IGF-1 in SRC-3−/− mice (Figure 7A-B), which is also consistent with the previous report.33 We then demonstrated that IGF-1R blockade resulted in a decrease in peripheral platelet count in WT mice (Figure 7C). However, IGF-1 treatment only partly recovered the platelet reduction in SRC-3−/− mice (Figure 7D). These results suggest that there may be a requirement of SRC-3 in IGF-1-mediated thrombopoiesis. To determine how this process is affected by SRC-3, we first measured and found that PPF and culture-derived platelets were significantly decreased in SRC-3−/− MKs in the thrombopoiesis progression facilitated by IGF-1 (Figure 7C-F; supplemental Figure 6A-D). Western blot and immonoprecipitation data revealed that both IRS-1 and IRS-2 were significantly affected after IGF-1R was activated, although the expression of IGF-1R and its phosphorylation were not influenced after SRC-3 was knocked out (Figure 7G; supplemental Figure 7). Further studies found that phosphorylation of Akt, but not of ERK1/2, was decreased in SRC-3−/− MKs after exogenous rhIGF-1 treatment (Figure 7H-I). Therefore, these data extensively suggest that multiple components of IGF-1 signaling are significantly influenced when SRC-3 is abolished in MKs, hampering the late stages of thrombopoiesis progression facilitated by IGF-1.
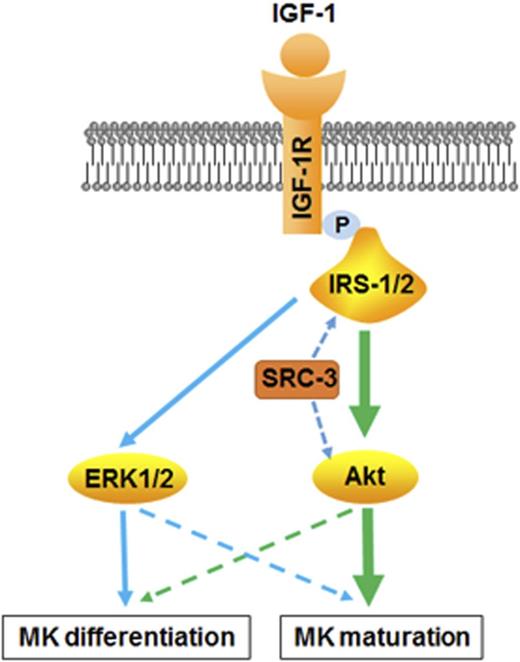
Thrombopoiesis promoted by IGF-1 requires the involvement of SRC-3. (A-B) Peripheral platelet counts (A) and serum IGF-1 levels (B) in SRC-3−/− mice or WT mice. (C) Peripheral platelet counts in WT C57BL/6 mice that were injected intraperitoneally with NVP-ADW742 (10 mg/kg i.p. twice daily) for 7 days. (D) Fold increase of peripheral platelet counts in WT or SRC-3−/− mice injected with saline or rhIGF-1 for 7 days. (E-F) Proplatelet-forming cells (E) and culture-derived platelets (F) from mouse-derived MKs cultured with or without rhIGF-1 for 3 days. Mouse-derived MKs were cultured from SRC-3−/− or WT c-kit+ cells stimulated with rhTPO for 5 days in the presence of 10 ng/mL rmIL-3. (G) Association between IGF-1R and IRS-1or IRS-2 in mouse-derived MKs from SRC-3−/− or WT mice after treatment with 100 ng/mL rhIGF-1. Lysates were subjected to immunoprecipitation with antibody against IGF-1R and were immunoblotted with antibodies against p-Tyr, IRS-1, IRS-2, or IGF-1R. (H-I) western blot analysis of the activation of ERK1/2 and Akt signal pathways in mouse-derived MKs after exposure to rhIGF-1 or rhTPO for 15 minutes. (J) Schematic illustration of the role of IGF-1 in thrombopoiesis. IGF-1 mainly acts through ERK1/2 and Akt, especially via the Akt signal pathway, thereby facilitating thrombopoiesis. Notably, this promotion is more than a result of working through TPO signaling, and requires the assistance of SRC-3. *P < .05, **P < .01.
Thrombopoiesis promoted by IGF-1 requires the involvement of SRC-3. (A-B) Peripheral platelet counts (A) and serum IGF-1 levels (B) in SRC-3−/− mice or WT mice. (C) Peripheral platelet counts in WT C57BL/6 mice that were injected intraperitoneally with NVP-ADW742 (10 mg/kg i.p. twice daily) for 7 days. (D) Fold increase of peripheral platelet counts in WT or SRC-3−/− mice injected with saline or rhIGF-1 for 7 days. (E-F) Proplatelet-forming cells (E) and culture-derived platelets (F) from mouse-derived MKs cultured with or without rhIGF-1 for 3 days. Mouse-derived MKs were cultured from SRC-3−/− or WT c-kit+ cells stimulated with rhTPO for 5 days in the presence of 10 ng/mL rmIL-3. (G) Association between IGF-1R and IRS-1or IRS-2 in mouse-derived MKs from SRC-3−/− or WT mice after treatment with 100 ng/mL rhIGF-1. Lysates were subjected to immunoprecipitation with antibody against IGF-1R and were immunoblotted with antibodies against p-Tyr, IRS-1, IRS-2, or IGF-1R. (H-I) western blot analysis of the activation of ERK1/2 and Akt signal pathways in mouse-derived MKs after exposure to rhIGF-1 or rhTPO for 15 minutes. (J) Schematic illustration of the role of IGF-1 in thrombopoiesis. IGF-1 mainly acts through ERK1/2 and Akt, especially via the Akt signal pathway, thereby facilitating thrombopoiesis. Notably, this promotion is more than a result of working through TPO signaling, and requires the assistance of SRC-3. *P < .05, **P < .01.
Discussion
Although our understanding of the molecular basis of thrombopoiesis has progressed substantially since the primary regulator, TPO, was discovered, the precise cell-signaling events underlying the regulation of thrombopoiesis are incompletely understood because no excessive bleeding is observed in TPO−/− or c-Mpl−/−mice.22 In this study, based on our previous finding concerning the regulation of GH in platelet production,24 we further demonstrated that its main mediator, IGF-1, exerted multiple roles on different steps, especially the late stages of thrombopoiesis, primarily through Akt signal pathways. Interestingly, we also discovered that IGF-1 was a nonnegligible regulator of thrombopoiesis, which requires the assistance of SRC-3. These findings not only deepen the understanding of the effects of IGF-1 on hematopoiesis, but also provide new insights into the treatment of thrombopenia caused by irradiaiton.
It is known that the process of thrombopoiesis includes several consecutive stages.34,35 Previous studies including our own work have indicated an involvement of IGF-1 signaling in megakaryocytopoiesis.9-12,27 However, the distinct role and molecular mechanism of IGF-1 signaling in the whole process of thrombopoiesis are not fully uncovered. Here, we showed that rhIGF-1 stimulated platelet production by promoting MK differentiation, PPF and platelet release. In addition, we also found that rhIGF-1 could expand CD34+ cells, but displayed a weak effect on MK proliferation. Our results suggest a stage-specific role of IGF-1 in regulating thrombopoiesis, which is probably ascribed to the different expression levels of IGF-1R on the cells at different stages of thrombopoiesis (Figure 1D-F).36 Interestingly, the effects of IGF-1 seem partially similar to the action of GH, which also regulates the late stage of thrombopoiesis, including the facilitation of PPF and platelet release.24 Considering the potency of IGF-1 in promoting thrombopoiesis, we then administrated rhIGF-1 in radiation-injured mice from day 10 when platelets hit the lowest level post irradiation, and observed that it promoted platelet recovery. Interestingly, the platelet recovery in solely irradiated mice is slower than that in irradiated mice receiving BMT, which is probably due to the different number and quality of the residual cells after radiation and the transplanted cells. In consistent with our previous finding that IGF-1 can elevate platelet counts through anti-apoptosis effect after irradition,12 we speculate that IGF-1 can act via both ways to accelerate the quick recovery of platelets after irradiation. Moreover, we think that there is a possibility of the synergistic use of IGF-1 with other cytokines which act on the early and middle stages of thrombopoiesis (eg, TPO) for the treatment of severe thrombocytopenia.
Our studies showed that both the ERK1/2 and Akt signal pathways were effectively activated by IGF-1 or TPO in thrombopoiesis. However, the focus of these 2 signal pathways on thrombopoiesis appears to be different because the Akt pathway is the major target of IGF-1, whereas TPO stimulation mainly concentrates on the activation of ERK1/2 signaling. As a result, TPO is observed to mainly affect MK proliferation and differentiation, whereas IGF-1 treatment primarily acts on MK maturation, PPF and platelet production. However, the lack of TPO effect will seriously influence the output of platelet production even if it is long-term cultured with IGF-1 as it is shown in Figure 6. Therefore, it infers that activation of ERK1/2 signaling may be primarily responsible for the early stages of megakaryocytopoiesis, whereas phosphorylation of the Akt pathway mainly contributed to the late steps of thrombopoiesis (Figure 7J). This speculation is also consistent with studies from other researchers that showed that p-Akt is gradually increased during the progression of megakaryocyte differentiation, whereas the level of p-ERK1/2 is gradually decreased.37 It is puzzling that both ERK1/2 and Akt are phosphorylated in a similar manner, but ERK1/2 activation by IGF-1 stimulation exerts no obvious role on MK proliferation, suggesting that the effect of this short activation may be dominated by the prolonged activation of Akt, which merely results in MK differentiation after IGF-1 treatment. In this study, we also found that IGF-1 promoted PPFs through activation of Rac1/Cdc42, which facilitates tubulin cytoskeleton rearrangement to elongate and form new bulges at proplatelet ends.28,38,39 However, this effect was able to be blocked, mainly by Akt signaling inhibition, indicating that Akt-Rac1/Cdc42 signaling is the underlying pathway of IGF-1-induced PPFs.
SRC-3 is a member of the SRC family and serves as a key “platform” coactivator that gathers multi-proteins to drive transcription. Consequently, SRC-3 regulates multiple physiological and pathophysiologic actions.40,41 Our study demonstrated that SRC-3 deficiency impaired IGF-1 signaling from several aspects, thereby affecting platelet production induced by IGF-1. Moreover, the expression of IGF-binding protein 3 is also downregulated when SRC-3 is abolished, resulting in decreased serum IGF-1.42 In summary, we infer that platelet production facilitated by IGF-1 in vivo may be systematically regulated by SRC-3, which affects not only IGF-1 signaling but also IGF-1 production (Figure 7J). However, we cannot exclude the possibility that other signaling pathways and transcription factors related to thrombopoiesis may be also regulated by SRC-3.
Previous studies have suggested that IGF-1 stimulates Akt phosphorylation and potentiates platelet activation,43,44 which are consistent with our data that IGF-1 modulates platelet activation primarily through Akt signaling. Considering this pathway also mediates platelet production promoted by IGF-1, we hypothesize that the increased risk of cardiovascular disease induced by altered levels of IGF-1 is not only due to platelet hyperactivity,45 but also the effect of IGF-1 on platelet production. Therefore, inhibition of target proteins along the IGF-1 pathway, especially Akt phosphorylation, may be a feasible approach to reduce the risk of arterial thrombosis in patients with cardiovascular disease, etc. Taken together, our data demonstrate that IGF-1 can facilitate thrombopoiesis by promoting CD34+ cell expansion, MK differentiation, PPF and platelet release mainly through activation of the AKT signal pathway. Furthermore, we also show that this promotion is independent of TPO signaling, and requires the help of SRC-3, which further enriches the recognition of thrombopoiesis.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Natural Science Fund of China (31500950, 81725019, 81573084, 81602790), the Program for Scientific and Technological Innovation Leader of Chongqing (CSTCKJCXLJRC06), and the Scientific Research Project of PLA (AWS16J014).
Authorship
Contribution: S.C. conceived and designed the study, performed the experiments, analyzed the data, and wrote the manuscript; M.H. performed the experiments, analyzed the data, and participated in manuscript preparation; M.S. and S.W. contributed to the animal experiments and data analysis; C.W. and F.C. performed the in vitro experiments and image scoring; Y.T., X.W., H.Z., and M.C. participated in the in vivo experiments; J.G., F.W., and Y.S. performed some of the in vitro experiments; and Y.X. and J.W. supervised the study, analyzed the data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Junping Wang, State Key Laboratory of Trauma, Burns and Combined Injury, Institute of Combined Injury, College of Preventive Medicine, Third Military Medical University, Gaotanyan St, 30 Chongqing 400038, China; e-mail: wangjunping@tmmu.edu.cn or wangjunp@yahoo.com; and Yang Xu, State Key Laboratory of Trauma, Burns and Combined Injury, Institute of Combined Injury, College of Preventive Medicine, Third Military Medical University, Gaotanyan St, 30 Chongqing 400038, China; e-mail: xyzq2023@163.com.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal