In this issue of Blood, Ceriani et al report that the metabolic heterogeneity estimated by the standardized uptake value (SUV) histogram of the intratumoral 18F-fluorodeoxyglucose (18FDG) uptake predicts the outcome in primary mediastinal B-cell lymphoma (PMBCL).1
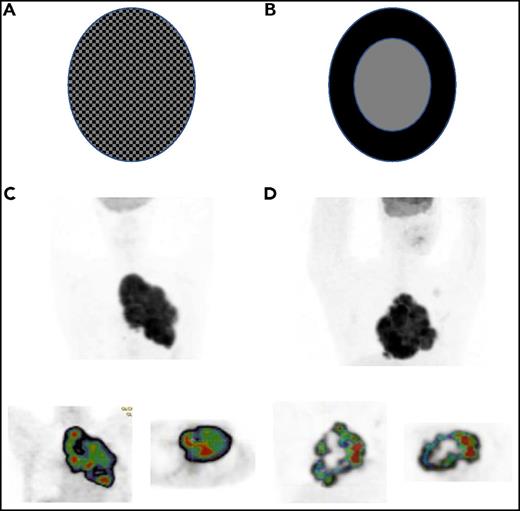
(A-B) Schematic distributions of SUV in 2 tumor volumes. Illustration courtesy of Irene Buvat, Unité d’Imagerie Moléculaire in Vivo, UMR1023 INSERM CEA, Orsay, France. (C-D) Two patients with PMBCL (NCT00498043 trial): (upper) maximum intensity projection image and (lower) cartography of the regional homogeneity indices (decreasing from red to blue) on (lower left) coronal and (lower right) transverse slice7 ; data from patient C: COV, 0.17; spatial homogeneity, 0.45, and patient D: COV, 0.17, spatial homogeneity, 0.28. Illustration processed with LIFEX software, www.lifexsoft.org/.
(A-B) Schematic distributions of SUV in 2 tumor volumes. Illustration courtesy of Irene Buvat, Unité d’Imagerie Moléculaire in Vivo, UMR1023 INSERM CEA, Orsay, France. (C-D) Two patients with PMBCL (NCT00498043 trial): (upper) maximum intensity projection image and (lower) cartography of the regional homogeneity indices (decreasing from red to blue) on (lower left) coronal and (lower right) transverse slice7 ; data from patient C: COV, 0.17; spatial homogeneity, 0.45, and patient D: COV, 0.17, spatial homogeneity, 0.28. Illustration processed with LIFEX software, www.lifexsoft.org/.
Three years ago, in Blood, the authors introduced a new prognostic index for PMBCL, the total lesion glycolysis (TLG), which uses quantitative analysis of baseline 18FDG/positron emission tomography (PET).2 The index was based on a series of 103 patients with PMBCL. Patients with high TLG had a reduced 5-year progression-free survival (PFS) and overall survival compared with patients with low TLG (99% and 100% vs 64% and 80%). TLG is derived from SUV metrics and is due to the high 18FDG uptake exhibited by both the malignant and environmental cells in PMBCL. The study was the first prospective study emphasizing the role of quantitative PET as a prognostic tool in PMBCL. Despite a good prognosis obtained with aggressive therapy in most patients (5-year survival rate >80%), it is critical to identify as early as possible both high-risk patients who require alternative approaches and those with low-risk disease who could potentially avoid consolidation radiotherapy.3 However, although the negative predictive value of TLG was excellent (92%), the positive predictive value was low (36%) and could not accurately select at baseline the group of high-risk patients.2
Although PMBCL is a subtype of diffuse large B-cell lymphoma, its clinical and molecular characteristics resemble those of nodular sclerosing Hodgkin lymphoma,4 and diagnosis can be challenging for the pathologist. Based on the variable architecture found in PMBCL tumors, a previous Blood commentary underlined that the risk stratification obtained with TLG could be improved by assessment of the heterogeneity of SUV distribution in the tumor.5 In line with this hypothesis, Ceriani et al now introduce a new parameter to their previous study. They characterize the homogeneity of the distribution of the SUV within the metabolic tumor volume by calculating the area under the curve of the cumulative SUV histogram (AUC-CSH). The lower is the area, the higher is the heterogeneity of the uptake values. AUC-CSH provides information such as the coefficient of variation (COV) of the SUV values (see the supplemental information in Ceriani et al). In this group of 103 PMBCL patients, AUC-CSH predicted outcome. Patients with a high heterogeneous distribution had an inferior 5-year PFS (73% vs 94%). Only AUC-CSH and TLG remained independent predictors of PFS in a Cox model with maximum SUV, bulky disease, International Prognostic Index, age-adjusted International Prognostic Index, and age.
Of greater value, a model combining TLG and AUC-CSH accurately identified a subset of high-risk patients (10% of the population) with both high TLG and high heterogeneity who had an 11% 5-year PFS who warrant study of more intensive therapy. On the other hand, there were no treatment failures in patients with low TLG and low heterogeneity; they may require less therapy than the current standard of care. This powerful model improves the accuracy of detecting high-risk (positive predictive value, 89%) and low-risk (negative predictive value, 100%) patients compared with TLG alone. However, the analysis of the SUV histogram with AUC-CSH does not encompass most of the information contained in the image in disregard of the spatial heterogeneity of the 18FDG uptake within a given metabolic volume, which can be characterized by textural indices. These indices likely more accurately describe the spatial arrangement of the 18FDG uptake of the total area, with different SUV located within the tumor.6,7
In the figure, panels A and B of the metabolic activity are coded with black (for a high SUV) or gray (for a low SUV) for each element and have the same tumor volume. Both have identical distribution of SUV values and thereby the same COV of this distribution. However, the SUV spatial distribution is clearly different. The spatial heterogeneity is greater in panel B, and this may affect the outcome. Panels C and D show PMBCL patients who have similar metabolic volumes and the same COV but very different textural (spatial) index of homogeneity; therefore, “metabolic heterogeneity” may be misleading terminology.
In this regard, the results presented in a recent study from Bouallègue et al are challenging.8 In 57 DLBCL and HL patients, they were not able to correlate the AUC-CSH of the largest tumor mass to the response at end-induction but showed that some textural spatial heterogeneity indices, such as the level of granularity of the tumor, were highly predictive. It will also be important to determine if the 8 patients who progressed in the high-risk group in the Ceriani et al study are those already identified in a previous study of this group with both high TLG and poor response at the end of induction immunochemotherapy.9 Although the study from Ceriani et al is a first important step in image analysis, the limitations of the method chosen to describe the heterogeneity and those that follow suggest that it is still too early to include AUC-CSH in the management of PMBCL. The risk of inclusion of necrotic regions because of partial volume effect in some patients with moderate SUV in the AUC-CSH cannot be excluded. The structural heterogeneity of PMBCL and the existence of transitional forms between PMBCL and nodular sclerosing Hodgkin lymphoma suggest that textural analysis would be more informative than AUC-CSH. Correlation with molecular data such as NF-κB gene expression in the high-risk group would further support the conclusions of the study.10 Finally, as noted by the authors, the small population size and the low number of PFS events (12) preclude any definitive conclusion. Nonetheless, this approach has merit and warrants further study to validate this important observation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal