In this issue of Blood, Hasan et al demonstrate that dedicator of cytokinesis 2 (DOCK2) can complex with receptor tyrosine kinase like orphan receptor 1 (ROR1) after Wnt5a treatment, leading to Rac1/2 activation and increased proliferation in chronic lymphocytic leukemia (CLL).1
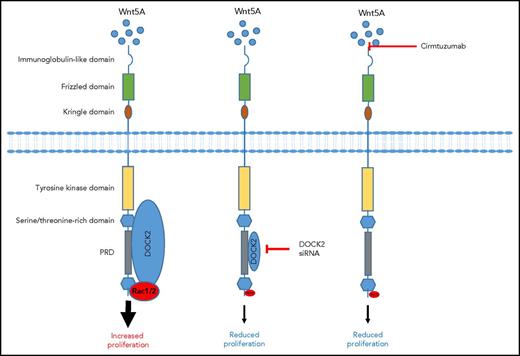
Wnt5a induces ROR1 signaling in CLL. Wnt5a engagement of ROR1 results in a signaling pathway that recruits DOCK2, leading to Rac1/2 signaling and increased proliferation. However, DOCK2 knockdown using siRNA reduces protein expression and consequently its binding to the PRD, leading to reduced Rac1/2 and proliferation. Similarly, blocking Wnt5a signaling with cirmtuzumab prevents DOCK2 recruitment to the PRD and prevents Rac1/2 activation and proliferation.
Wnt5a induces ROR1 signaling in CLL. Wnt5a engagement of ROR1 results in a signaling pathway that recruits DOCK2, leading to Rac1/2 signaling and increased proliferation. However, DOCK2 knockdown using siRNA reduces protein expression and consequently its binding to the PRD, leading to reduced Rac1/2 and proliferation. Similarly, blocking Wnt5a signaling with cirmtuzumab prevents DOCK2 recruitment to the PRD and prevents Rac1/2 activation and proliferation.
ROR1, also called neurotrophic tyrosine kinase receptor–related 1, is expressed primarily during embryogenesis, particularly in tissues associated with skeletal and neural development. However, ROR1 levels are much lower after birth. In contrast, patients with CLL are estimated to express between 1 × 104 and 7 × 104 ROR1 molecules per cell. Indeed, compared with CLL cells, adult tissues from the lungs, spleen, ovaries, and normal donor cells express very little ROR1,2 although 1 study did suggest that normal progenitor B-cell populations may express greater levels of ROR1 compared with mature B cells.3 The highest levels of ROR1 in adults are observed in adipose tissue and the pancreas, but expression is still ∼10- and 35-fold lower, respectively, compared with CLL cells,2 suggesting that this may be a drugable target that could distinguish between healthy and tumor tissues. Compared with the wild-type Eµ-Tcl-1 murine leukemia model cells, artificial overexpression of ROR1 in these leukemic cells resulted in a more aggressive disease.4 Similarly, a majority of tumor cells from patients with CLL express ROR1, and higher levels correlate with a poorer clinical outcome,5 suggesting a role for this receptor in tumor pathology. Indeed, ROR1 is not solely found in CLL; expression has also been documented in other B-cell malignancies,3,6 as well as in a number of solid tumors.7 These data support the idea that the receptor may play a more general role in tumor development. Wnt5a is a ligand for ROR1, and earlier studies showed that Wnt signaling could promote tumor cell proliferation and migration.8 This may at least in part be mediated by the interaction between ROR1 and hematopoietic lineage-specific protein 1 (HS-1), resulting in RhoA activation and subsequent downstream signaling.9
DOCK2 expression is restricted to hematopoietic cells and is involved in a number of intracellular signaling pathways. As a guanine nucleotide exchange factor, it functions to activate small G proteins such as Rac1/2. DOCK2 has a DOCK homology region 2, which stabilizes Rac1/2 in its nucleotide-free active state and subsequently activates a number of downstream pathways involved in survival, proliferation, and migration.10 These pathways include chemokine signaling such as CXCL12 and CXCL13 in T and B lymphocytes, respectively, and T-cell receptor and sphingosine-1 phosphate receptor signaling. Consequently, ROR1 may have a number of roles in CLL biology. However, our understanding of the role of DOCK2 in CLL pathology is limited.
In this current study, Hasan et al demonstrate that in primary CLL samples, ROR1 colocalizes with DOCK2 after Wnt5a treatment. Interestingly, although knockdown of DOCK2 with small interfering RNA (siRNA) prevented Wnt5A-induced proliferation of CLL cells, it was unable to fully inhibit Wnt5a-induced migration. This suggests that DOCK2 may only be partially responsible for Wnt5a-induced migration, that the variable and incomplete knockdown of the protein was still sufficient to enable some cell migration, or that an alternative receptor, which signals independently of DOCK2, may contribute to CLL cell migration. They further demonstrate in the MEC1 B-cell line that DOCK2 seemed to compete with cytosolic protein CrkL or Vav for binding to DOCK2. However, whether ROR1 sequestering DOCK2 contributes to changes in CrkL or Vav downstream signaling is unknown. It seems that ROR1/DOCK2 interactions result in the activation of a Rac1/2 signaling pathway but have no effect on RhoA. DOCK2 specifically interacted with proline-808 within the proline-rich domain (PRD) of ROR1, because replacing this amino acid with an alanine prevented Rac1/2 signaling and reduced migration. In contrast, replacing proline in the PRD of ROR1 at position 784, 826, or 841 bound DOCK2 as effectively as the wild-type protein and had no effect on Rac1/2 activation or migration. To further determine the role of Wnt5a in recruiting DOCK2 to ROR1, Hasan et al used cirmtuzumab, an anti-ROR1 antibody that inhibits Wnt5a signaling. They demonstrate that cirmtuzumab could prevent Wnt5a-induced colocalization of ROR1 with DOCK2, suggesting a role of this antibody in preventing CLL proliferation but with a reduced effect on migration (figure).
However, a number of questions remain unanswered. Why did DOCK2 knockdown only have a partial effect on migration in CLL cells, considering the key role of DOCK2 in chemotaxis in many other cell types? Is there any residual DOCK2 signaling, and consequently biological activity, because the protein is not completely inhibited by the small interfering RNA knockdown? Could this explain some of the differences observed between Wnt5a-induced proliferation and migration? How would a ROR1-expressing cell with knocked-out DOCK2 respond to Wnt5a? What is the contribution, if any, of the other prolines in the ROR1 PRD? Importantly, their report highlights a need to better understand the different roles of ROR1 in CLL biology and how the PRDs may regulate binding to different proteins associated with various signaling pathways (Rac1/2 and RhoA). While we await the outcome of cirmtuzumab in clinical trials either alone or in combination with other agents, a continued effort to understand its role in CLL is warranted given what seems to be an important role for ROR1 in a number of B-cell and solid tumor malignancies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal