In this issue of Blood, Stefanini et al untangle the specific roles of RAP1A and RAP1B in platelet production, signal transduction, and their individual contributions to hemostasis, thrombosis, and vascular integrity in vivo to find both redundant and isoform-specific functions.1
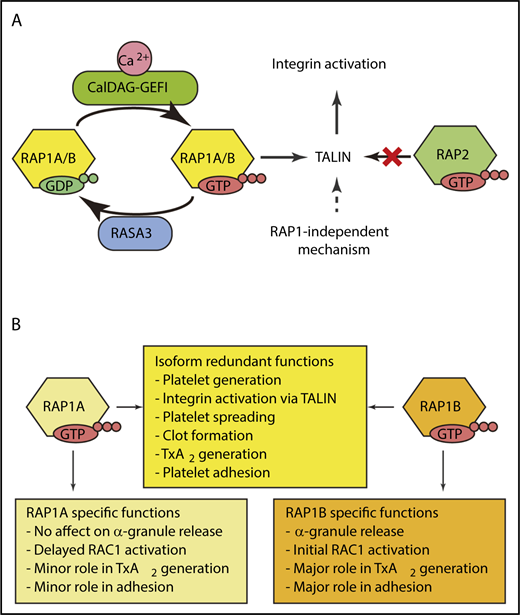
(A) RAP1A and RAP1B are GTPases that act as molecular switches to rapidly activate integrins. RAP1 is activated by the guanine exchange factor CalDAG-GEFI. The GTPase activating protein RASA3 serves as a counterbalance to keep RAP1 signaling quiescent in circulating platelets. Deleting both RAP1A and RAP1B identified RAP1-independent mechanisms for integrin activation that are not mediated by RAP2. (B) RAP1A and RAP1B have overlapping and redundant roles in platelets. TxA2, thromboxane A2. Illustration by Xu Han.
(A) RAP1A and RAP1B are GTPases that act as molecular switches to rapidly activate integrins. RAP1 is activated by the guanine exchange factor CalDAG-GEFI. The GTPase activating protein RASA3 serves as a counterbalance to keep RAP1 signaling quiescent in circulating platelets. Deleting both RAP1A and RAP1B identified RAP1-independent mechanisms for integrin activation that are not mediated by RAP2. (B) RAP1A and RAP1B have overlapping and redundant roles in platelets. TxA2, thromboxane A2. Illustration by Xu Han.
Platelets are critical for hemostasis. In this traditional role, platelets patrol the cardiovascular system as specialized cells that rapidly mount a response to stop bleeding via aggregation at the site of vascular damage.2 More recently, there is a growing appreciation for the critical role platelets have in maintaining vascular integrity during development and inflammation.3 In this context, individual platelets seal gaps in the vascular wall to mitigate leakage. Clearly, these physiological contexts have unique biophysical requirements that likely necessitate distinct kinetics of signal transduction and adhesion. Uncovering the fundamental mechanisms of how platelets respond as aggregates vs individual cells provides the foundation for understanding the many roles of platelets beyond hemostasis.
As mediators of primary hemostasis, platelets must respond by immediately adhering to breaches in the vasculature if they are to be effective at halting blood loss at the site of damage. Following initial adhesion, full platelet activation leads to a dramatic cytoskeletal rearrangement resulting in shape change, granule release, and activation of additional adhesion molecules.2 This highly coordinated series of events requires signaling machinery that is poised to trigger at a moment’s notice, yet has sufficient safeguards to prevent unwanted aggregation and its pathological consequences. Collagen initiates signaling through glycoprotein VI, whereas soluble agonists, such as thrombin, adenosine 5′-diphosphate, and thromboxane, initiate rapid signaling via their cognate G protein–coupled receptors (GPCRs). These pathways converge at the guanine exchange factor CALDAG-GEFI to activate the GTPase RAP1 (see figure panel A).4 RAP1 activation leads to rapid platelet adhesion and aggregation mediated by integrins. This pathway is held in check by the GTPase activating protein, RASA3. Platelets express 2 isoforms of RAP1, RAP1A and RAP1B.5 Until now, the relative contributions of each was not known.
Simultaneous global deletion of both Rap1a and Rap1b is lethal.6 However, deletion of individual RAP1 isoforms has provided some insights, albeit primarily through inference. For example, RAP1A has specific roles in immune cells in which RAP1B does not compensate. In contrast, Rap1b−/− mice have a mild platelet phenotype, suggesting RAP1A has functional redundancy in platelets, but the role of RAP1A in hemostasis has not been reported. These complexities are common in gene families where isoforms can compensate for one another with overlapping functions, making it difficult to assess the direct influence of each isoform. The elegant studies by Stefanini et al have taken advantage of megakaryocyte/platelet-specific knock-out mice for Rap1a (Rap1a-mKO), Rap1b (Rap1b-mKO), or both (Rap1a/b-mKO) to define the contribution of RAP1A and RAP1B in platelet production, platelet signaling, and their physiological relevance in vivo (see figure panel B).
Little is known regarding the role of RAP1 in megakaryocyte differentiation and proplatelet formation. Rap1a-mKO and Rap1b-mKO had normal platelet counts. In contrast, Rap1a/b-mKO mice had macrothrombocytopenia due to reduced proplatelet formation demonstrating that RAP1A and RAP1B have overlapping roles in megakaryocytes (see figure panel B). More importantly, this unexpected contribution of RAP1 signaling in megakaryocytes during platelet production opens several lines of investigation for future studies.
Experiments in heterologous systems have clearly defined the importance of RAP1 in integrin activation through talin.7 The complete loss of integrin activation in Rap1a/b-mKO platelets reiterates this point. The more interesting observation is the unequal contribution of RAP1A and RAP1B. Rap1b-mKO has a 50% reduction in integrin activation, which is the same as the global Rap1b knockout mice. In contrast, the Rap1a-mKO platelets had reduction of ∼15%. It is unclear if this is due to a functional difference between the isoforms, or if it is due to the ∼10-fold lower expression of RAP1A in mouse platelets. Further experiments comparing Rap1a/b-mKO to talin-mKO mice revealed a low level of integrin activation in the Rap1a/b-mKO platelets. Consistent with these observations, other integrin-dependent functions (aggregation, spreading, and clot retraction) were reduced, but not absent in Rap1a/b-mKO platelets. RAP2, a related protein, did not account for the RAP1 independent integrin activation. Collectively, these experiments point to a mechanism for talin-mediated integrin activation that is independent from RAP1A and RAP1B (see figure panel A).
The mechanistic ex vivo signaling experiments described above allowed for carefully designed in vivo studies to determine the relative contributions of each isoform in hemostasis, thrombosis, and vascular integrity. In both hemostasis and thrombosis assays, the Rap1a-mKO mice were not different from control mice. However, in line with the ex vivo studies, the Rap1b-mKO mice moderately prolonged bleeding and thrombosis. The residual platelet function in these mice was attributed to RAP1A because the Rap1a/b-mKO mice had dramatically prolonged times to achieve hemostasis or develop occlusive thrombi. In contrast, Rap1a/b-mKO mice had minimal blood lymphatic mixing, suggesting that RAP1 signaling in platelets has a minor role in maintaining vascular integrity during development. Rap1a/b-mKO mice also had minimal bleeding at sites of inflammation with the reverse passive Arthus reaction. These in vivo studies point to distinct pathways for platelet function depending on the physiological context. Collectively, the data also support the hypothesis that RAP1 signaling is essential when platelets need to respond rapidly.
The Bergmeier group has defined the role of ITAM-containing receptors for vascular integrity and that GPCR signaling is dispensable.8 The work presented in this issue of Blood extends these efforts and demonstrates another platelet signaling node that is essential for aggregation, but not for vascular development.1 Future studies that identify the relevant signaling networks for platelets within specific microenvironments and physiological contexts will provide mechanistic insights for the growing number of platelet functions, which has the potential for broad impact beyond hemostasis.9,10
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal