Key Points
Due to the higher affinity of current MCL-1 inhibitors, huMcl-1 mice have been established.
A therapeutic window for S63845 can be established in huMcl-1 mice transplanted with huMcl-1;Eµ-Myc lymphomas.
Abstract
Myeloid cell leukemia-1 (MCL-1) is a prosurvival B-cell lymphoma 2 (BCL-2) family member required for the sustained growth of many cancers. Recently, a highly specific MCL-1 inhibitor, S63845, showing sixfold higher affinity to human compared with mouse MCL-1, has been described. To accurately test efficacy and tolerability of this BH3-mimetic (BH3-only protein mimetic) drug in preclinical cancer models, we developed a humanized Mcl-1 (huMcl-1) mouse strain in which MCL-1 was replaced with its human homolog. huMcl-1 mice are phenotypically indistinguishable from wild-type mice but are more sensitive to the MCL-1 inhibitor S63845. Importantly, nontransformed cells and lymphomas from huMcl-1;Eµ-Myc mice are more sensitive to S63845 in vitro than their control counterparts. When huMcl-1;Eµ-Myc lymphoma cells were transplanted into huMcl-1 mice, treatment with S63845 alone or alongside cyclophosphamide led to long-term remission in ∼60% or almost 100% of mice, respectively. These results demonstrate the potential of our huMcl-1 mouse model for testing MCL-1 inhibitors, allowing precise predictions of efficacy and tolerability for clinical translation.
Introduction
Apoptosis is a form of programmed cell death that removes unwanted and potentially dangerous cells in multicellular organisms.1 Deregulation of apoptosis can result in accumulation of cells that should normally be deleted. The abnormal proliferation of mutated “undead” cells can lead to tumor development.2 Cell death hinges on the balance between the prosurvival and proapoptotic members of the B-cell lymphoma 2 (BCL-2) family of proteins. This family is divided into the prosurvival members (BCL-2, BCL–extra large [BCL-XL], BCL-WEHI [BCL-W], myeloid cell leukemia-1 [MCL-1], and BCL-2–related protein A1 [BCL2A1]/BFL-1), the BCL-2 homology domain 3 (BH3)-only proapoptotic proteins (eg, BCL-2 interacting mediator of cell death [BIM] and p53-upregulated modulator of apoptosis [PUMA]), and the multi-BH domain proapoptotic effectors (BCL-2 homologous antagonist/killer [BAK], BCL-2–associated X protein [BAX], and BCL-2–related ovarian killer [BOK]).3 Diverse stress stimuli induce expression of BH3-only proteins to initiate apoptosis, either indirectly by binding to the prosurvival BCL-2 proteins, thereby unleashing the proapoptotic effectors BAK and BAX, or by binding BAK and BAX directly. Following activation, BAK and BAX oligomerize on the outer mitochondrial membrane, perforating its surface, releasing cytochrome c and other apoptogenic factors. This unleashes the caspase cascade that causes demolition of the cell.3,4
Cancer cells can subvert the apoptotic machinery by upregulation of prosurvival, or downregulation of proapoptotic proteins.2 The MCL-1 locus is amplified in numerous human tumor types,5 and functional studies in mouse models showed that MCL-1 is essential for the sustained growth of hematopoietic6-11 cancers,6-11 and some solid tumor subtypes.12,13 Direct targeting of prosurvival BCL-2 family members with BH3 mimetics has proven success as cancer therapy. The BCL-2 inhibitor venetoclax is highly efficacious for the treatment of relapsed/refractory chronic lymphocytic leukemia (CLL).14,15 A clinically relevant specific and potent MCL-1 inhibitor, S63845,16 was shown to be efficacious as a monotherapy in several preclinical models of hematological malignancies and in combination with oncogenic kinase inhibitors in certain lung-, skin-, and breast cancer–derived cell lines and patient-derived xenograft models.16-18
The clinical application of MCL-1 inhibitors has been of substantial concern given the important prosurvival role of MCL-1 in many normal tissues. MCL-1 is widely expressed,19 and essential for embryonic development with homozygous Mcl-1 loss in mice resulting in failure to implant at the blastocyst stage.20 Conditional gene knockout studies showed that MCL-1 plays a vital role in the survival of cardiomyocytes,21,22 hematopoietic stem cells,23 developing and mature lymphocytes,24-28 and in the maintenance of oocytes in the ovarian reserve.29 Despite these reports, little toxicity was observed in mice treated with doses of the MCL-1 inhibitor S63845 that ablates mouse lymphoma cells.16 One caveat of these results is that S63845 has an approximately sixfold higher affinity for the human compared with the mouse MCL-1 protein and it is therefore possible that the mouse models used were not sensitive enough to reveal potential on-target toxicities. Thus, it was pivotal to more accurately test the MCL-1 inhibitor S63845 in a preclinical model in which the Mcl-1 locus was humanized (huMcl-1) by replacing the native coding region of the murine Mcl-1 locus with the huMCL-1 coding sequence. These huMcl-1 mice are healthy and fertile, and the intrinsic apoptotic pathway is intact in their cells, demonstrating that the huMCL-1 protein can functionally fully replace the murine protein. As predicted, cells from huMcl-1 mice were more sensitive to the MCL-1 inhibitor S63845 compared with wild-type mouse cells, but not to other cytotoxic agents that induce apoptosis. Accordingly, the maximum tolerated dose of S63845 was lower in the huMcl-1 mice compared with their wild-type counterparts. However, it was still possible to determine a therapeutic window for S63845 treatment, both alone and in combination with cyclophosphamide (CP), in a MYC-driven lymphoma model expressing huMCL-1. These findings demonstrate the utility of the huMcl-1 model to accurately determine the efficacy and tolerability of S63845 and potentially other MCL-1 inhibitory drugs in preclinical models of disease.
Materials and methods
Generation of huMcl-1 mice
Mice bearing the huMcl-1 allele were generated by Taconic Biosciences GmbH (Cologne, Germany). The targeting vector (Figure 1A) was transfected into the TaconicArtemis C57BL/6NTac embryonic stem (ES) cell line. Correctly targeted ES cells were injected into BALB/c blastocysts that were transferred into pseudopregnant NMRI females to generate chimeric offspring for further breeding. The flippase recognition target (FRT) site–flanked PuroR selection cassette was removed by crossing chimeric mice with the flippase (Flpe)-expressing C57BL/6-Tg(CAG-Flpe)2 Arte mice. Genotyping was performed by polymerase chain reaction (PCR) to confirm cassette removal, loss of the Flpe transgene, and presence of the wild-type or humanized Mcl-1 alleles (for PCR primers used, see supplemental Table 1, available on the Blood Web site).
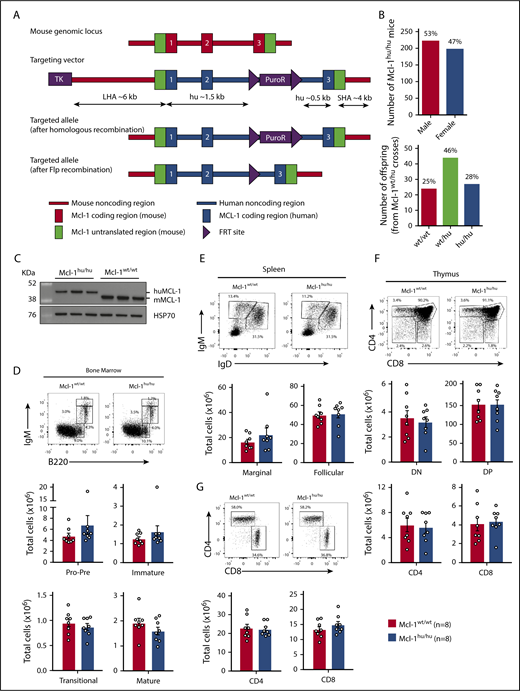
Humanized Mcl-1 mice show no defects under steady-state conditions. (A) Schematic representation of the murine Mcl-1 gene locus, the huMcl-1 targeting vector and the correctly targeted alleles, before and after Flpe-mediated recombination. (B) Offspring sex frequency of Mcl-1hu/hu mice and Mendelian ratios from intercrosses of Mcl-1wt/hu mice. (C) Thymocytes were isolated from Mcl-1hu/hu and Mcl-1wt/wt mice. MCL-1 protein (mouse and human) expression was detected by western blotting. Probing for heat shock protein 70 (HSP70) served as a loading control. (D-G) Single-cell suspensions were prepared from spleen, thymus and bone marrow of Mcl-1hu/hu and Mcl-1wt/wt mice and cell subsets were determined by immunostaining and FACS analysis. Data are presented as mean ± SEM, significance determined by the Student t test. (D) Representative FACS plot of B-cell development in the bone marrow (top) and total cell numbers per femur (bottom panels). Pro-B/pre-B (B220loIgM−), immature B (B220loIgMmid), transitional B (B220lo-hiIgMhi), and mature (B220hiIgMmid) B cells. (E) Representative FACS plot of peripheral B cells in the spleen (top) and total cell number (bottom). B cells defined as follicular (IgM+IgD+) and marginal zone (IgM+IgDlo) B cells. (F) Representative FACS plot analyzing T-cell development in the thymus (top) and total cell numbers calculated for each population (bottom panels). Immature double-negative thymocytes (DN; CD4−CD8−), double-positive thymocytes (DP; CD4+CD8+), and the mature CD4 and CD8 single-positive populations. (G) Representative FACS plot of peripheral T cell distribution (top) in the spleen as well as total cell numbers of CD4 and CD8 single-positive T cell populations (bottom panel). Ig, immunoglobulin.
Humanized Mcl-1 mice show no defects under steady-state conditions. (A) Schematic representation of the murine Mcl-1 gene locus, the huMcl-1 targeting vector and the correctly targeted alleles, before and after Flpe-mediated recombination. (B) Offspring sex frequency of Mcl-1hu/hu mice and Mendelian ratios from intercrosses of Mcl-1wt/hu mice. (C) Thymocytes were isolated from Mcl-1hu/hu and Mcl-1wt/wt mice. MCL-1 protein (mouse and human) expression was detected by western blotting. Probing for heat shock protein 70 (HSP70) served as a loading control. (D-G) Single-cell suspensions were prepared from spleen, thymus and bone marrow of Mcl-1hu/hu and Mcl-1wt/wt mice and cell subsets were determined by immunostaining and FACS analysis. Data are presented as mean ± SEM, significance determined by the Student t test. (D) Representative FACS plot of B-cell development in the bone marrow (top) and total cell numbers per femur (bottom panels). Pro-B/pre-B (B220loIgM−), immature B (B220loIgMmid), transitional B (B220lo-hiIgMhi), and mature (B220hiIgMmid) B cells. (E) Representative FACS plot of peripheral B cells in the spleen (top) and total cell number (bottom). B cells defined as follicular (IgM+IgD+) and marginal zone (IgM+IgDlo) B cells. (F) Representative FACS plot analyzing T-cell development in the thymus (top) and total cell numbers calculated for each population (bottom panels). Immature double-negative thymocytes (DN; CD4−CD8−), double-positive thymocytes (DP; CD4+CD8+), and the mature CD4 and CD8 single-positive populations. (G) Representative FACS plot of peripheral T cell distribution (top) in the spleen as well as total cell numbers of CD4 and CD8 single-positive T cell populations (bottom panel). Ig, immunoglobulin.
Coimmunoprecipitation, subcellular fractionation, and western blotting
Thymocytes were isolated from Mcl-1wt/wt and Mcl-1hu/hu mice; coimmunoprecipitation and subcellular fractionation was performed as described previously.30 For immunoprecipitation, 2.5 µg of monoclonal antibody (14C11-20) against MCL-131 and G sepharose beads were used before being analyzed by western blotting (antibodies listed in supplemental Table 2). For all other western blots, cell lysates were prepared in radioimmunoprecipitation assay (RIPA) buffer (supplemental Table 3) supplemented with cOmplete protease inhibitor (Roche). Protein concentration was determined by Bradford assay using the Protein Assay Dye Reagent Concentrate (Bio-Rad, Hercules, CA). Samples of 15 µg of protein were prepared in Laemmli buffer (supplemental Table 3), boiled for 5 minutes, and size-fractionated by gel electrophoresis on NuPAGE 10% Bis-Tris 1.5-mm gels (Life Technologies) in 2-(N-morpholino)ethanesulfonic acid (MES) buffer and then transferred onto nitrocellulose membranes (Life Technologies) using the iBlot membrane transfer system. Antibody dilution and blocking were performed in 5% skim milk, 0.1% Tween 20 in phosphate-buffered saline (PBS). For antibodies, refer to supplemental Table 3, in-house antibodies.31,32 Luminata Forte Western horseradish peroxidase (HRP) substrate (Millipore, Billerica, MA) was used for developing the signal, and membranes were imaged and analyzed using the ChemiDoc XRS+ machine with ImageLab software (Bio-Rad).
Immunostaining and flow cytometry
Thymus, spleen, and bone marrow were harvested and single-cell suspensions prepared in PBS (Gibco), 5 mM EDTA (Merck), supplemented with 5% fetal bovine serum (FBS; Sigma-Aldrich) for staining. Monoclonal antibodies (supplemental Table 4) were obtained from eBioscience, BioLegend, or generated in-house. Streptavidin-phycoerythrin (PE; BioLegend) was used to detect biotinylated antibodies. Propidium iodide (PI; 1 µg/mL) was used to exclude dead cells. Whole-organ cell counts were determined by the CASY counter (Schärfe System GmbH) (Figure 1; supplemental Figure 1) or by mixing a known concentration of APC Calibrite beads (Becton Dickinson) with each sample (Figure 3; supplemental Figure 3). Data were collected using LSR II, LSRFortessa, or LSRFortessa X-20 analyzers and examined using FlowJo 10 (Becton Dickson).
Tissue-culture and cell-viability assays
Eµ-Myc lymphoma cell lines were maintained as previously described.9 For all experiments, viability was determined by resuspending cells in Annexin V–binding buffer (0.1 M N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid [HEPES; pH 7.4], 1.4 M NaCl, 25 mM CaCl2) containing PI (1 µg/mL) and fluorescein isothiocyanate (FITC)- or Alexa Fluor 647–conjugated Annexin V (generated in-house). Spleen and thymi were harvested and single-cell suspensions prepared. To isolate B cells, splenocytes were pelleted and resuspended in 1 mL of red cell lysis buffer (156 mM ammonium chloride [BDH], 11.9 mM sodium bicarbonate [Merck], EDTA [Sigma-Aldrich]) for 5 minutes to deplete erythrocytes. Cells were then stained with biotinylated antibodies against CD4, CD8, MAC-1, and GR-1 (supplemental Table 4) to enrich for B cells by MagniSort Streptavidin Negative Selection Beads (Thermo Fisher), as per the manufacturer’s protocol. Isolated B cells and thymocytes, and Eµ-Myc lymphoma cell lines, were seeded at 5 × 104 cells per well in media,9 in triplicate per condition, in 96-well flat-bottomed plates and treated with indicated drugs (S63845 [Servier], dexamethasone, etoposide, phorbol myristate acetate [PMA], ionomycin [Sigma-Aldrich], ABT-199 [Active Biochem]) for the indicated times. The 50% inhibitory concentration (IC50) values were determined using nonlinear regression algorithms in Prism (GraphPad).
Animals and in vivo drug treatments
The care and use of mice for experimental purposes were carried out in accordance with the requirements set out by The Walter and Eliza Hall Institute (WEHI) Animal Ethics Committee. HuMcl-1 mice are described in “Generation of huMcl-1 mice”; Eµ-Myc33 and Mcl-1+/−25 have been described previously. All mice are kept on a C57BL/6-Ly5.2 background. The huMcl-1 allele was bred onto a C57BL/6-Ly5.1 background for use as recipient animals for all transplant experiments. Single-cell suspensions of 1 × 105Eµ-Myc lymphoma (Ly5.2) cells in PBS were injected into 8- to 12-week-old huMCL-1;Ly5.1 recipient mice by IV tail-vein injection. Recipient mice were sex matched to transplanted tumors. Working solutions of cyclophosphamide (CP) and 5-fluorouracil (5-FU; Sigma-Aldrich) were prepared in PBS. CP was administered by intraperitoneal injection, 5-FU by IV tail-vein injection. S63845 (Servier) was formulated extemporaneously and protected from light in 2% vitamin E/d-α-tocopheryl polyethylene glycol 1000 succinate (Sigma-Aldrich) in NaCl 0.9% (wt/vol) and delivered by IV tail-vein injection at the indicated doses and schedule. Mice were monitored for sickness and euthanized when deemed unwell by experienced mouse technicians.
Statistical analysis
Prism software (GraphPad) was used to generate survival curves and perform all statistical testing of data. All data are presented as mean plus or minus standard error of the mean (SEM) unless stated otherwise. P values of <.05 were considered significant.
Results
Generation of the huMcl-1 mouse model
The humanized Mcl-1 (huMcl-1) mouse model was generated by standard gene targeting in C57BL/6 ES cells. Expression of the huMCL-1 protein under the control of the murine Mcl-1 regulatory regions was achieved by substitution of the exons and interspersing introns of the mouse Mcl-1 locus with the huMCL-1 exon/intron sequences, while maintaining the flanking 5′ and 3′ untranslated regions (UTR) of the genomic locus of the mouse (Figure 1A). Homozygous loss of Mcl-1 causes embryonic lethality prior to embryonic day 4 in mice.20 Furthermore, changes in the 5′ UTR of the murine Mcl-1 locus resulted in infertility in homozygous male Mcl-1 floxed mice.31,34 Importantly, the humanization of MCL-1 in mice had no impact on embryonic development or fertility in both female and male Mcl-1hu/hu offspring, with the expected Mendelian ratios and sex distribution observed in intercrosses of Mcl-1wt/hu mice (Figure 1B). Western blot analysis of thymocytes from huMcl-1 mice showed efficient expression of the huMCL-1 protein, as evidenced by the predicted size difference between the mouse and huMCL-1 proteins (human, 38 kDa; mouse, 35 kDa; Figure 1C). A cohort of huMcl-1 mice was aged for >600 days with no obvious defects evident.
huMcl-1 mice have normal hematopoietic cell subset distribution
Fluorescence-activated cell sorter (FACS) analysis of the bone marrow revealed no differences in the frequencies and numbers of pro-B/pre-B (B220loIgM−), immature B (B220loIgM+), transitional B (B220midIgMhi), or mature B (B220hiIgMlo) cell subsets between huMcl-1 mice and wild-type controls (Figure 1D). There were also no abnormalities in IgM+ or IgD+ peripheral B lymphocytes in the huMcl-1 mice (Figure 1E). The frequencies and numbers of the stages of thymocyte development, as determined by staining for CD4 and CD8, were also comparable between huMcl-1 mice and wild-type controls (Figure 1F). Moreover, huMcl-1 mice had normal frequencies and numbers of mature CD4+ and CD8+ T lymphocytes in the spleen (Figure 1G), with normal distributions of naive (CD62L+CD44−), effector memory (CD62L−CD44+), and central memory (CD62L+CD44+) CD4+ T cells (supplemental Figure 1A). Finally, the huMcl-1 mice had no perturbations in macrophage/monocyte (MAC-1+GR-1lo) and neutrophil populations (MAC-1+GR-1hi) in the bone marrow and spleen (supplemental Figure 1B).
huMCL-1 protein can functionally replace mouse MCL-1 within the apoptotic machinery
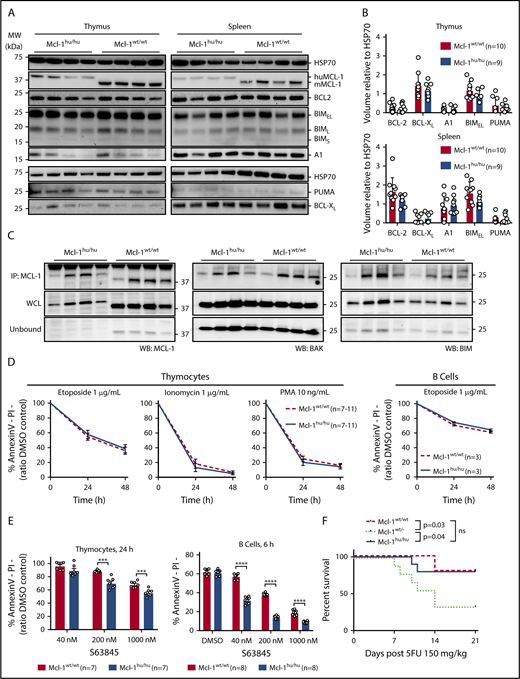
It is possible that other BCL-2 family proteins are differentially expressed to compensate for the expression of huMCL-1 instead of mouse MCL-1 protein in vivo. To investigate this, we carried out western blotting on protein extracted from thymocytes and splenocytes from huMcl-1 mice and wild-type controls. No differences in expression of proapoptotic BIM and PUMA or prosurvival BCL-2, BCL-XL, or A1 were observed (Figure 2A-B). The affinities of the antibodies used to detect the mouse MCL-1 vs huMCL-1 proteins is unknown, thus we could not compare the relative protein expression by western blot. To assess whether the huMCL-1 protein expressed in mouse cells can interact with the endogenous mouse proapoptotic BCL-2 relatives, coimmunoprecipitation assays were performed on thymocyte extracts. This revealed that in mouse cells, the huMCL-1 protein could bind mouse BIM and BAK in a similar manner as mouse MCL-1 (Figure 2C). Additionally, subcellular fractionation of thymocytes and splenocytes showed that huMCL-1 is mainly localized to the mitochondrial outer membrane, as is the case for mouse MCL-1 (supplemental Figure 2A). It is reported that the MCL-1 protein is stabilized upon binding to S63845.16 Stabilization of both huMCL-1 and mouse MCL-1 protein was evident in thymocytes isolated from Mcl-1wt/hu heterozygous mice that were treated ex vivo with S63845 (supplemental Figure 2B). To functionally assess the huMCL-1 protein, cells from the huMcl-1 mice and wild-type control mice were exposed to different cytotoxic stimuli in vitro. Importantly, survival of thymocytes and B cells from the huMcl-1 mice and control mice were comparable (Figure 2D). As predicted, thymocytes and B cells from the huMcl-1 mice were more sensitive to the MCL-1 inhibitor S63845 than those from wild-type mice (Figure 2E). The increased sensitivity to S63845 only became obvious at higher doses in thymocytes. This is likely due to the fact that CD4+CD8+ thymocytes rely on both MCL-1 and BCL-XL for survival.27 More striking differences were observed in B cells after 6 hours of treatment at lower doses of the MCL-1 inhibitor (Figure 2E; supplemental Figure 2C). In determining the IC50 of S63845, it became apparent that the huMCL-1–expressing B cells are about 7 or 4 times more sensitive to this compound compared with their wild-type counterparts at 24 h and 48 h, respectively (supplemental Figure 2D-E).
The apoptosis machinery remains intact in humanized Mcl-1 mice. (A) Thymocytes and splenocytes were isolated from Mcl-1hu/hu and Mcl-1wt/wt mice. Expression of MCL-1, BCL-2, BCL-XL, A1, BIM, and PUMA was detected by western blotting. Probing for HSP70 served as a loading control. Representative blots are shown. (B) Quantification of expression levels of BCL-2 family proteins in thymocytes and splenocytes from huMcl-1 (n = 9) and wild-type (n = 10) mice determined by western blotting. Chemiluminescent signals from each protein were normalized to the HSP70 loading control signal and plotted as arbitrary units; significance calculated by multiple Student t tests. (C) Lysates were prepared from thymocytes from huMcl-1 and wild-type mice and subjected to immunoprecipitation with an MCL-1 (binds both the mouse MCL-1 and huMCL-1 proteins) specific antibody. The immunoprecipitated material was immunoblotted for MCL-1 (left panel), BAK (middle panel), and BIM (right panel). Pulled-down fraction shown in top panels with whole-cell lysate (WCL) and unbound fractions shown below. (D) Thymocytes and splenic B cells were isolated from huMcl-1 and wild-type mice and exposed in culture to the indicated cytotoxic stimuli. Cell viability at 24 and 48 hours was assessed by Annexin V/PI staining and FACS analysis. (E) Thymocytes and splenic B cells were isolated from huMcl-1 and wild-type mice and treated with the MCL-1 inhibitor S63845 at the indicted concentrations. Cell viability at indicated time points was assessed by Annexin V/PI staining and FACS analysis. Data are presented as mean ± SEM; significance calculated by multiple Student t tests at each time point; *P < .05, **P < .01, ***P < .001, ***P < .0001. (F) Mice of the indicated genotypes were treated with a single dose of 150 mg/kg body weight 5-FU and survival monitored. Kaplan-Meier curves are shown; statistical analysis was performed by the log-rank (Mantel-Cox) test; *P < .05. DMSO, dimethyl sulfoxide; IP, immunoprecipitation; MW, molecular weight; ns, not significant; WB, western blot.
The apoptosis machinery remains intact in humanized Mcl-1 mice. (A) Thymocytes and splenocytes were isolated from Mcl-1hu/hu and Mcl-1wt/wt mice. Expression of MCL-1, BCL-2, BCL-XL, A1, BIM, and PUMA was detected by western blotting. Probing for HSP70 served as a loading control. Representative blots are shown. (B) Quantification of expression levels of BCL-2 family proteins in thymocytes and splenocytes from huMcl-1 (n = 9) and wild-type (n = 10) mice determined by western blotting. Chemiluminescent signals from each protein were normalized to the HSP70 loading control signal and plotted as arbitrary units; significance calculated by multiple Student t tests. (C) Lysates were prepared from thymocytes from huMcl-1 and wild-type mice and subjected to immunoprecipitation with an MCL-1 (binds both the mouse MCL-1 and huMCL-1 proteins) specific antibody. The immunoprecipitated material was immunoblotted for MCL-1 (left panel), BAK (middle panel), and BIM (right panel). Pulled-down fraction shown in top panels with whole-cell lysate (WCL) and unbound fractions shown below. (D) Thymocytes and splenic B cells were isolated from huMcl-1 and wild-type mice and exposed in culture to the indicated cytotoxic stimuli. Cell viability at 24 and 48 hours was assessed by Annexin V/PI staining and FACS analysis. (E) Thymocytes and splenic B cells were isolated from huMcl-1 and wild-type mice and treated with the MCL-1 inhibitor S63845 at the indicted concentrations. Cell viability at indicated time points was assessed by Annexin V/PI staining and FACS analysis. Data are presented as mean ± SEM; significance calculated by multiple Student t tests at each time point; *P < .05, **P < .01, ***P < .001, ***P < .0001. (F) Mice of the indicated genotypes were treated with a single dose of 150 mg/kg body weight 5-FU and survival monitored. Kaplan-Meier curves are shown; statistical analysis was performed by the log-rank (Mantel-Cox) test; *P < .05. DMSO, dimethyl sulfoxide; IP, immunoprecipitation; MW, molecular weight; ns, not significant; WB, western blot.
Next, the ability of huMCL-1 protein expressed in mouse cells to function within a whole animal was examined. MCL-1 is required for the maintenance of hematopoietic stem/progenitor cells23 and also for emergency hematopoiesis following myeloablative chemotherapy.35 The latter was demonstrated by the observation that Mcl-1wt/− mice were compromised in their recovery following 5-FU treatment. We exposed huMcl-1, Mcl-1wt/− (as a control), and wild-type mice to a single dose of 5-FU (150 mg/kg body weight) and examined hematopoietic recovery over a 21-day period. As reported,35 most Mcl-1wt/− mice did not recover from 5-FU treatment, whereas huMcl-1 mice recovered as well as the wild-type controls (Figure 2F). Collectively, these findings demonstrate that huMCL-1 is functional and the overall apoptotic machinery is intact in cells from the huMcl-1 mice, both in vitro and in vivo. Furthermore, as predicted, these cells show increased sensitivity to the MCL-1 inhibitor S63845.
Determining the maximum tolerated dose of S63845 in huMcl-1 mice
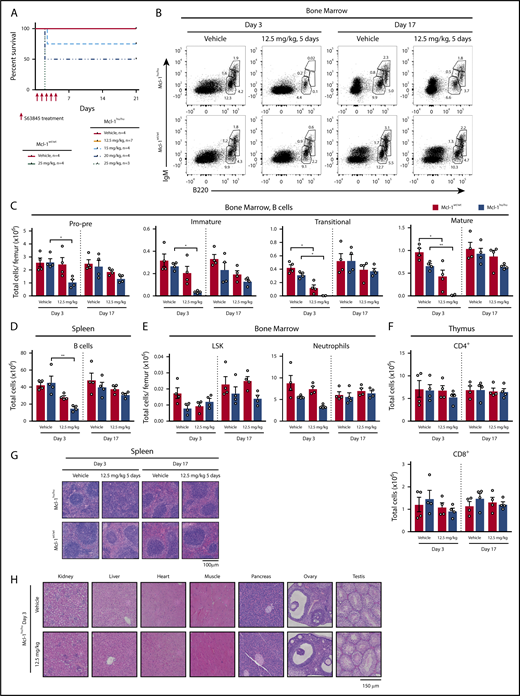
Given the binding affinity of S63845 is approximately sixfold higher for huMCL-1 compared with mouse MCL-1 protein,16 we determined the maximum tolerated dose (MTD) of S63845 in the huMcl-1 mice. Treatment of huMcl-1 mice on 5 consecutive days IV with doses ranging from 5 to 25 mg/kg body weight established an MTD of S63845 as high as 12.5 mg/kg (Figure 3A). All mice treated with 25 mg/kg S63845 needed to be euthanized due to increased respiration and inability to rise or ambulate. However, the exact cause of death could not be determined. Because the tolerability of the drug was considerably higher in wild-type mice, which all survived the 25 mg/kg dosing schedule (Figure 3A) and have previously been shown to have an MTD of 40 mg/kg,16 this demonstrates that the huMcl-1 mice are more sensitive to the MCL-1 inhibitor S63845.
S63845 exerts no enduring toxicity in the huMcl-1 mouse model at the MTD. (A) Kaplan-Meier survival curves of huMcl-1 or wild-type mice treated for 5 consecutive days with vehicle or increasing doses of S63845, as indicated. (B-H) The huMcl-1 and wild-type mice were treated with 5 consecutive doses of 12.5 mg/kg S63845 or vehicle. At 3 or 17 days posttreatment, organs were harvested for analysis. (B-F) Single-cell suspensions were prepared from spleen, thymus, and bone marrow of Mcl-1hu/hu and Mcl-1wt/wt mice and cell subsets were determined by immunostaining and FACS analysis. Data are presented as mean ± SEM, significance determined by the Student t test. *P < .05, **P < .01. (B) Representative FACS plot of B cells in the bone marrow and (C) total cell numbers per femur. B cells defined as pro-B/pre-B (B220loIgM−), immature B (B220loIgMmid), transitional B (B220lo-hiIgMhi), and mature B cells (B220hiIgMmid). (D) Total numbers of B cells in the spleen (B220+) (E) LSK cells (Lineage−SCA-1+c-KIT+) and macrophages/monocytes (MAC-1+GR-1lo) in the bone marrow (F) and mature T cells (CD4+ or CD8+) in the thymus at indicated time points. (G) Representative hematoxylin-and-eosin (H&E)-stained section of spleens from huMcl-1 or wild-type mice that had been treated with 12.5 mg/kg S63845 or vehicle and were harvested at either 3 or 17 days posttreatment. (H) Representative H&E-stained sections of various organs, as indicated, from huMcl-1 mice treated with 5 consecutive doses of 12.5 mg/kg S63845 or vehicle 3 days after treatment had ceased.
S63845 exerts no enduring toxicity in the huMcl-1 mouse model at the MTD. (A) Kaplan-Meier survival curves of huMcl-1 or wild-type mice treated for 5 consecutive days with vehicle or increasing doses of S63845, as indicated. (B-H) The huMcl-1 and wild-type mice were treated with 5 consecutive doses of 12.5 mg/kg S63845 or vehicle. At 3 or 17 days posttreatment, organs were harvested for analysis. (B-F) Single-cell suspensions were prepared from spleen, thymus, and bone marrow of Mcl-1hu/hu and Mcl-1wt/wt mice and cell subsets were determined by immunostaining and FACS analysis. Data are presented as mean ± SEM, significance determined by the Student t test. *P < .05, **P < .01. (B) Representative FACS plot of B cells in the bone marrow and (C) total cell numbers per femur. B cells defined as pro-B/pre-B (B220loIgM−), immature B (B220loIgMmid), transitional B (B220lo-hiIgMhi), and mature B cells (B220hiIgMmid). (D) Total numbers of B cells in the spleen (B220+) (E) LSK cells (Lineage−SCA-1+c-KIT+) and macrophages/monocytes (MAC-1+GR-1lo) in the bone marrow (F) and mature T cells (CD4+ or CD8+) in the thymus at indicated time points. (G) Representative hematoxylin-and-eosin (H&E)-stained section of spleens from huMcl-1 or wild-type mice that had been treated with 12.5 mg/kg S63845 or vehicle and were harvested at either 3 or 17 days posttreatment. (H) Representative H&E-stained sections of various organs, as indicated, from huMcl-1 mice treated with 5 consecutive doses of 12.5 mg/kg S63845 or vehicle 3 days after treatment had ceased.
S63845 exerts no enduring toxicity in the huMcl-1 mice at the MTD
There have been many reports on the importance of MCL-1 in several vital tissues21-29 raising the question of whether an MCL-1 inhibitor would find its therapeutic use in the clinic. Previously and unexpectedly, it was shown that S63845 exerts very little toxic effects at the MTD in mice expressing mouse MCL-1.16 However, given the higher affinity of S63845 for huMCL-1, we revisited this finding by treating huMcl-1 and wild-type control mice for 5 consecutive days with either vehicle or 12.5 mg/kg S63845 and monitored the acute impact and recovery 3 or 17 days posttreatment, respectively. In the huMcl-1 mice, the pro-B/pre-B (B220loIgM−), immature B (B220loIgM+), transitional B (B220midIgMhi), and mature B (B220hiIgMlo) B cells in the bone marrow were significantly reduced at 3 days but mostly recovered at 17 days posttreatment (Figure 3B-C). Similar observations were made for B cells in the blood (supplemental Figure 3A) and spleen (Figure 3D). Despite the reduction in splenic B cells, an increase in cellularity was noted in the spleens of all huMcl-1 mice treated with S63845 3 days following treatment, but these values normalized at 17 days posttreatment (supplemental Figure 3B). FACS analysis revealed that the increased splenic cellularity was due to an increase in EryA (Lineage−Ter119hiCD71+FSC-Ahi) and EryB (Lineage−Ter119hiCD71+FSC-Alo) erythrocyte progenitor populations, with the more mature EryC (Lineage−Ter119hiCD71−FSC-Alo) remaining stable throughout the experiment (supplemental Figure 3D). Moreover, the red blood cell counts in the blood showed a modest reduction at day 3 posttreatment, but again these numbers completely recovered at day 17 (supplemental Figure 3E). No significant changes were observed in the Lineage−SCA-1+c-KIT+ (LSK) population in the bone marrow (Figure 3E) of the S63845-treated huMcl-1 mice. A trend toward neutrophil reduction in the bone marrow was seen at day 3, however, this did not reach statistical significance (Figure 3E). No reductions in neutrophils were seen in the spleen (supplemental Figure 3F). Moreover, there were no changes in the numbers of mature CD4+ and CD8+ T cells (Figure 3F), immature double-negative (DN1-4; Lineage−TCRb−CD4−CD8−), and double-positive (DP; CD4+CD8+) thymocytes or mature T cells (T-cell receptor β–positive) in the spleen of S63845-treated huMcl-1 mice (supplemental Figure 3G). Histological analysis revealed increased size and loss of normal architecture of the spleen, which complements the FACS data (Figure 3G). Histological analysis of major organs revealed no damage in response to S63845 (Figure 3H). These data indicate that S63845 treatment can be tolerated in huMcl-1 mice, with only a transient reduction of certain hematopoietic cell subsets.
Eµ-Myc cell lines expressing huMCL-1 are more sensitive to S63845
To test whether malignant cells also demonstrated an increased sensitivity toward MCL-1 inhibition with S63845, huMcl-1 mice were crossed with the Eµ-Myc–transgenic animals. Although the latency and tumor phenotype did not differ between mouse or huMCL-1–expressing Eµ-Myc mice (Figure 4A-B), the in vitro sensitivity of lymphoma cell lines generated from sick Eµ-Myc;huMcl-1 mice toward S63845 was approximately sixfold higher compared with control Eµ-Myc lymphoma cells expressing mouse MCL-1 (25 nM vs 160 nM, respectively; Figure 4C-D). Conversely, there was no significant difference in sensitivity when these cells were treated with etoposide or the BCL-2–specific BH3 mimetic ABT-199/venetoclax (supplemental Figure 4A-B), providing evidence that the increased sensitivity is due to the higher affinity of S63845 to the huMCL-1 protein.
HuMcl-1;Eµ-Myc lymphoma cells are more sensitive to S63845 compared with control Eu-Myc lymphoma cells expressing mouse MCL-1. (A) Eµ-Myc–transgenic mice that were Mcl-1wt/wt, Mcl-1wt/hu, or Mcl-1hu/hu were aged and monitored for tumor-free survival. Kaplan-Meier survival curve shown with median latency for each genotype. (B) Primary wild-type or huMcl-1 Eµ-Myc lymphoma cells were isolated and immunostained for surface Ig (sIg) expression (IgD and/or IgM) and analyzed by flow cytometry. p53 and p19 expression were detected by western blotting. Proportions of p53 wild-type (p53 low-negative, p19 low-negative), p53 knockout (p53 negative, p19 high), and p53 mutant (p53 high, p19 high) are shown. (C) Viability of Eµ-Myc lymphoma cells that are wild-type for Mcl-1 (n = 3, red bars) or homozygous for the huMcl-1 allele (n = 7, blue bars) after treatment with increasing concentrations of S63845. Cell viability was determined by Annexin V/PI staining and FACS analysis. Data points are IC50 values calculated from a range of concentrations (1.6 nM to 1 µM) analyzed in triplicate. (D) Cell viability data generated and shown in panel C were pooled to compare the overall IC50 values of control Eµ-Myc and huMcl-1;Eµ-Myc lymphoma cell lines. Combined IC50 curves for cell lines of each genotype are shown, with IC50 values depicted in the legend. (E-G) Survival curves of huMcl-1;Ly5.1 mice transplanted with huMcl-1;Eµ-Myc lymphomas and treated with vehicle, S63845 alone for 5 consecutive days (E), or S63845 in combination with CP (F-G) (doses as indicated). n indicates the number of recipient mice with number of cell lines tested indicated in parentheses. Significance relative to vehicle shown in graph; significance relative to combination treatment shown on the right. Calculated by Mantel-Cox test. ***P < .001, ****P < .0001. ko, knockout; mut, mutant; ns, not significant; wt, wild-type.
HuMcl-1;Eµ-Myc lymphoma cells are more sensitive to S63845 compared with control Eu-Myc lymphoma cells expressing mouse MCL-1. (A) Eµ-Myc–transgenic mice that were Mcl-1wt/wt, Mcl-1wt/hu, or Mcl-1hu/hu were aged and monitored for tumor-free survival. Kaplan-Meier survival curve shown with median latency for each genotype. (B) Primary wild-type or huMcl-1 Eµ-Myc lymphoma cells were isolated and immunostained for surface Ig (sIg) expression (IgD and/or IgM) and analyzed by flow cytometry. p53 and p19 expression were detected by western blotting. Proportions of p53 wild-type (p53 low-negative, p19 low-negative), p53 knockout (p53 negative, p19 high), and p53 mutant (p53 high, p19 high) are shown. (C) Viability of Eµ-Myc lymphoma cells that are wild-type for Mcl-1 (n = 3, red bars) or homozygous for the huMcl-1 allele (n = 7, blue bars) after treatment with increasing concentrations of S63845. Cell viability was determined by Annexin V/PI staining and FACS analysis. Data points are IC50 values calculated from a range of concentrations (1.6 nM to 1 µM) analyzed in triplicate. (D) Cell viability data generated and shown in panel C were pooled to compare the overall IC50 values of control Eµ-Myc and huMcl-1;Eµ-Myc lymphoma cell lines. Combined IC50 curves for cell lines of each genotype are shown, with IC50 values depicted in the legend. (E-G) Survival curves of huMcl-1;Ly5.1 mice transplanted with huMcl-1;Eµ-Myc lymphomas and treated with vehicle, S63845 alone for 5 consecutive days (E), or S63845 in combination with CP (F-G) (doses as indicated). n indicates the number of recipient mice with number of cell lines tested indicated in parentheses. Significance relative to vehicle shown in graph; significance relative to combination treatment shown on the right. Calculated by Mantel-Cox test. ***P < .001, ****P < .0001. ko, knockout; mut, mutant; ns, not significant; wt, wild-type.
Regression of huMcl-1;Eµ-Myc lymphomas in vivo after treatment with S36845, either alone or in combination with cyclophosphamide
To test the sensitivity of Eµ-Myc lymphomas expressing huMCL-1 to S63845 in vivo, we transplanted huMcl-1;Eµ-Myc lymphoma cell lines into huMcl-1;Ly5.1 recipient mice (ie, both lymphoma and normal cells expressed huMCL-1). After 3 days, mice were treated for 5 consecutive days with vehicle or 12.5 mg/kg S63845 and monitored for signs of sickness. Sixty percent of mice were cured at this dose (Figure 4E; supplemental Figure 4C). Next, we aimed to improve tumor-free survival in mice transplanted with huMcl-1;Eµ-Myc lymphoma cell lines. To this end, huMcl-1;Eµ-Myc lymphoma cell lines were transplanted into huMcl-1;Ly5.1 recipient mice and 2 days later treated with vehicle or a low dose of CP (50 mg/kg). Three days later, mice were treated for 5 consecutive days with a low dose of S63845 (7.5 mg/kg). Treatment with 50 mg/kg CP or 7.5 mg/kg S63845 by themselves resulted in ∼50% or ∼25% long-term tumor-free survival, respectively. Excitingly though, only 1 mouse became sick with lymphoma in the CP plus S63845 combination treatment, thereby increasing the tumor-free survival to almost 100% (Figure 4F; supplemental Figure 4D). For Eµ-Myc lymphoma lines that previously showed regression at 12.5 mg/kg body weight S63845 (hME160, hME184, hME273; supplemental Figure 4C), tumor-free survival could be achieved with as little as 3.75 mg/kg body weight S63845 in combination with CP (Figure 4G). These findings, in a highly relevant mouse model in which both the lymphoma cells and the normal cells express huMCL-1, clearly demonstrate that there is a therapeutic window for S63845, and that the efficacy can be enhanced by combining treatment with cyclophosphamide.
Discussion
Here, we describe a novel humanized Mcl-1 mouse model, in which huMCL-1 protein is expressed instead of mouse MCL-1 under the control of the mouse Mcl-1 regulatory regions. Normal embryonic development and fertility of the Mcl-1hu/hu mice is noteworthy given that even small changes to the Mcl-1 locus or loss of only 1 allele have consequences in adult mice,31,34 with homozygous loss of Mcl-1 being very early embryonic lethal.20 Notably, the intrinsic apoptotic pathway remains intact in cells from the huMcl-1 mice with no detectable compensatory changes in the levels of other antiapoptotic or proapoptotic BCL-2 family proteins (Figure 2). This is critical because the expression of certain BCL-2 family member(s) could be expected to change to compensate for loss of another. For example, tumor cells can acquire resistance to inhibitors of BCL-2, BCL-XL, and BCL-W (eg, ABT-737) by upregulating MCL-1.36 Because the huMcl-1 mice are normal and their cells have normally functioning apoptotic machinery, they represent an ideal model to predict tolerability and efficacy of MCL-1 inhibitors.
MCL-1 is regarded as an exciting therapeutic target due to its requirement for the sustained growth of many cancers, and S63845 has been developed as a highly selective and potent MCL-1 inhibitor. A so-far unique feature for BH3 mimetics is that S63845 binds more tightly to human compared with mouse MCL-1, and one could imagine that any newly generated compounds targeting MCL-1 would likely bind in the same region, and therefore, will likely have similar properties. Because previous in vivo experiments have all been conducted with mouse MCL-1 as the target, it was difficult to ascertain whether the results are truly reflective of the therapeutic potential of S63845 and, perhaps more importantly, its safety. Our studies using the huMcl-1 mouse model support the notion that a therapeutic window for MCL-1 inhibitors might be established, with a transient loss of B cells being the most significant side effect observed at doses of S63845 that could halt lymphoma growth in a substantial fraction of mice. These data may be viewed as conflicting with previous data showing the importance of MCL-1 in many essential normal cell types, such as cardiomyocytes. We believe that the lack of significant side effects is due to the transient action and hence inhibition of MCL-1 by S63845, in contrast to the irreversible loss of MCL-1 elicited by genetic deletion. Additionally, it is not yet known whether administered S63845 is available in all organs throughout the mouse, which may further explain the differences seen between genetic deletion vs drug-mediated inhibition of MCL-1. Further pharmacokinetic studies must be performed to address this question.
Although S63845 appears to be relatively well tolerated, as previously shown in mice expressing mouse MCL-1, we have shown that S63845’s higher affinity for huMCL-1 vs mouse MCL-1 is relevant in vivo with the MTD of S63845 in huMcl-1 mice being ∼3 times lower than that seen in wild-type mice. Although the MTD of S63845 was determined within a 5-day treatment window only, it would also be desirable to determine the MTD after a much longer treatment period to mimic potential clinical use as applied for other inhibitors of prosurvival BCL-2 proteins, such as ABT-199/venetoclax.14 However, we are unable to carry out these experiments due to animal ethics restrictions that regulate the number of daily IV injections that a mouse can receive, particularly if a curative regime can be achieved with fewer injections as demonstrated in this study (Figure 4). We anticipate that the the huMcl-1 mouse model will be invaluable for future preclinical work using S63845 (and other MCL-1 inhibitors) for determining a therapeutic window for treatment of cancers and possibly other diseases in which MCL-1 is important. As a proof of principle, we generated huMcl-1;Eµ-Myc lymphomas and showed that they are more sensitive to S63845, both in vitro and in vivo, than Eµ-Myc lymphomas expressing mouse MCL-1. The observation that huMcl-1 mice could tolerate doses of S63845 that could prevent growth of huMcl-1;Eµ-Myc lymphomas reinforces the notion that a therapeutic window of MCL-1 inhibitors may be established in the clinic. Although phase 1 clinical trials with MCL-1 inhibitors have recently commenced, it is important to generate relevant preclinical data in different cancer models using huMcl-1 mice. This will help reach rational decisions on which malignancies will benefit the most and to determine which other anticancer agents can cooperate with MCL-1 inhibitors in tumor cell killing and still be tolerable as a combination therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Herold and Strasser laboratories, and Andrew W. Roberts, Philippe Bouillet, and David C. S. Huang, for reagents and advice regarding the project; Giovanni Siciliano, Krystal Hughes, Dan Fayle, Hannah Johnson, and Cassandra D’Alessandro for technical assistance with in vivo experiments and animal husbandry; and Simon Monard and team in the WEHI flow cytometry facility.
This work was supported by the Australian National Health and Medical Research Council (project grant 1145728 [M.J.H.], 1143105 [M.J.H. and A.S.], 1086291 [G.L.K.], program grant 1016701 [A.S.], and fellowship 1020363 [A.S.]), the Leukemia & Lymphoma Society of America (LLS SCOR 7001-13 [A.S. and M.J.H.]), the Cancer Council of Victoria (1086157 and 1147328 [G.L.K.], 1052309 [A.S.], and Venture Grant [M.J.H. and A.S.]), a Victorian Cancer Agency fellowship (MCRF17028 [G.L.K.]), a Leukaemia Foundation Postgraduate Award (M.S.B.), and a Grant in Aid (A.S. and G.L.K.), sponsored research funding from Servier, bequests from the Estate of Antony Redstone and the Craig Perkins Cancer Research Foundation (A.S. and G.L.K.) as well as by operational infrastructure grants through the Australian Government Independent Research Institute Infrastructure Support Scheme (9000220) and the Victorian State Government Operational Infrastructure Support Program.
Authorship
Contribution: M.S.B., A.S., G.L.K., and M.J.H. conceived and designed the experiments; M.S.B., C.C., G.D., L.T., and G.L.K. performed experiments; G.L. contributed expertise and provided reagents; and M.S.B., A.S., G.L.K., and M.J.H. wrote the paper with help from the other authors.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marco J. Herold, The Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC 3052, Australia; e-mail: herold@wehi.edu.au.
REFERENCES
Author notes
G.L.K. and M.J.H. contributed equally to this study.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal