Abstract
Epstein-Barr virus (EBV)–related and EBV-unrelated classical Hodgkin lymphomas (cHLs) are morphologically and phenotypically indistinguishable. However, the tumor microenvironment of EBV-related cHLs contains higher numbers of macrophages and higher expression levels of PD-L1 than that of EBV-unrelated cHLs. Moreover, viral oncoprotein LMP1 may sustain an immunosuppressive microenvironment by inducing/enhancing production of immunosuppressive cytokines and the expression of PD-1. The presence of enhanced immunosuppressive features in EBV-related cHL should make EBV-related cHL patients more susceptible to checkpoint blockade.
Introduction
Classical Hodgkin lymphoma (cHL), the most common cancer among young people, is a relatively rare disease, accounting for 10% of all lymphomas.1 A considerable body of evidence indicates that cHL is a neoplastic disease with heterogeneous clinical and pathologic features.1,2 Histologically, cHL includes different patterns traditionally grouped in the following subtypes: nodular sclerosis (NS), mixed cellularity (MC), lymphocyte-rich, and lymphocyte-depletion subtypes.1 However, as shown by gene expression profiling studies, Hodgkin and Reed-Sternberg (HRS) cells, the characteristic cHL tumor cells, have consistently lost the expression of most B-cell genes, irrespective of histologic subtypes.3 Downregulation of B-cell transcription factors (OCT2, BOB1, and PU1)4 leads to loss of the B-cell phenotype in HRS cells, which in turn usually express CD30, CD15, CD40, and IRF4/MUM1. Recurrent genetic alterations detected in HRS cells frequently affect the NF-κB, Janus kinase/signal transducer and activator of transcription (JAK/STAT), and phosphatidylinositol 3-kinase/AKT (PI3K/AKT) signaling pathways.5
Twenty-five percent to 30% of patients with advanced-stage disease are not cured by conventional first-line chemotherapy and show either primary refractoriness to chemotherapy or early disease relapse.6 Only a minority of primary refractory and early relapsing patients affected by cHL are cured by second-line salvage chemotherapy. Recently, the treatment strategy for relapsed and refractory/relapsed HL patients, who still represent an unmet clinical need, included immunotherapy. Remarkable therapeutic advances in refractory/relapsed HL patients emerged through the use of checkpoint inhibitors. This topic is of particular relevance in light of the presence of enhanced immunosuppressive features in EBV-related cHL that should make EBV-related cHL patients more susceptible to checkpoint blockade. Hopefully, novel agents will increase the efficacy and reduce toxicity in patients with both newly diagnosed and relapsed and refractory disease.6-8
Tumor microenvironment
The inflammatory/immune cell infiltrate of the tumor microenvironment shows striking interactions with HRS cells and stimulates the production of molecules that support their growth and survival (such as CD30L or CD40L), in addition to immunosuppressive factors including programmed death 1 (PD-1).9,10 This latter finding indicates that HRS cells escape immunosurveillance and interact with immune cells in the tumor microenvironment for survival and growth.11 Moreover, the cross talk between tumor cells and microenvironmental components is thought to play a lymphomagenic role through activation of critical intracellular signaling.12,13
EBV-related and EBV-unrelated cHL
Association with Epstein-Barr virus (EBV) is less frequent in NS (10% to 40%) than in MC (∼75% of cases) cHL.1,14,15 The varying frequency of association of cHL with EBV infection is probably related to different levels of patient immunosuppression.16 One of the main features characterizing HIV-associated cHL is its strong association with EBV infection.17 In fact, virtually all these cases show HRS cells harboring the EBV genome and intensely expressing the LMP1 viral oncoprotein, whereas only a fraction of HIV-unrelated cases carry EBV infection (Table 1). In most tumors that arise in severely immunocompromised individuals, EBV is the main driver of tumor growth. In cHL, EBV contributes to the chronic inflammatory microenvironment that surrounds and supports tumor cells.
EBV-related cHL according to the immunocompetence status of the host
| Host/Hodgkin lymphoma . | EBV infection . |
|---|---|
| Without known immunosuppression | |
| cHL, NS | Variably present |
| cHL, MC | Usually present |
| Rare subtypes | Variably present |
| With acquired immunodeficiency | |
| HIV-associated cHL | Present |
| Posttransplant, cHL type PTLD | Present |
| Iatrogenic, methotrexate | Variably present |
| Host/Hodgkin lymphoma . | EBV infection . |
|---|---|
| Without known immunosuppression | |
| cHL, NS | Variably present |
| cHL, MC | Usually present |
| Rare subtypes | Variably present |
| With acquired immunodeficiency | |
| HIV-associated cHL | Present |
| Posttransplant, cHL type PTLD | Present |
| Iatrogenic, methotrexate | Variably present |
Pathogenetic and microenvironmental features of EBV-related and EBV-unrelated cHL
Elevated levels of anti-EBV antibodies have been associated with an increased risk of cHL. Growing evidence indicates that genetic factors control the levels of antibodies against EBV antigens.18 HLA genotyping and genome-wide association studies provided evidence for associations between HLA alleles and cHL.19-22 A specific association of the class I region with EBV-related cHL cases has been shown.23 Therefore, the HLA background of cHL patients may have a role in defining the inherent risk to develop an EBV-positive cHL.22,24-26 In this regard, the HLA-A*02 allele is protective against EBV-related cHL.
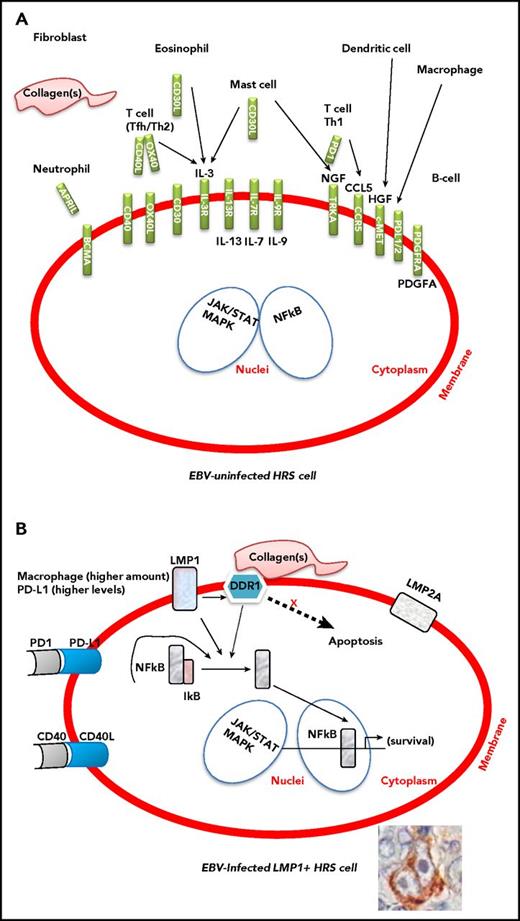
HRS cells in EBV-related and EBV-unrelated cHL are morphologically and phenotypically indistinguishable, but they leverage preferential pathogenic pathways to escape immunosurveillance.11 In fact, EBV infection enhances genetic mechanisms crucial for HL development, namely, 9p24.1 amplification.27-29 EBV-related and EBV-unrelated cHL are also indistinguishable based on the cellular composition of the tumor microenvironment (Figure 1). Notably, however, in EBV-related cHL, the cellular infiltrate of the tumor microenvironment is composed of immune cells, including high numbers of natural CD4+CD25+ regulatory T cells (Tregs) that functionally contribute to evading an immune attack.30 The cellular infiltrate is also composed of inflammatory cells that conversely provide support for the growth and survival of tumor cells. Specifically, compared with the tumor microenvironment of EBV-unrelated cHLs, the tumor microenvironment of EBV-related cHLs contains higher numbers of macrophages.13,31
Interactions between the tumor microenvironment and HRS cells in EBV-uninfected and EBV-infected cHL. The presence of enhanced immunosuppressive features, such as higher numbers of M2 macrophages and elevated expression levels of PD-L1, in EBV-related cHL should make EBV-related cHL more susceptible to checkpoint blockade. (A) The inflammatory/immune cell infiltrate of the tumor microenvironment and the molecules that support the growth and survival of HRS cells. (B) The contribution of LMP1, LMP2a, DDR1, PD-L1, and CD40 to the survival of HRS cells. Constitutive activation of NF-κB and JAK/STAT signaling pathway is the molecular hallmark of HRS cells. The expression of EBV-LMP1 is thought to contribute to activation of the NF-κB and JAK/STAT pathway.
Interactions between the tumor microenvironment and HRS cells in EBV-uninfected and EBV-infected cHL. The presence of enhanced immunosuppressive features, such as higher numbers of M2 macrophages and elevated expression levels of PD-L1, in EBV-related cHL should make EBV-related cHL more susceptible to checkpoint blockade. (A) The inflammatory/immune cell infiltrate of the tumor microenvironment and the molecules that support the growth and survival of HRS cells. (B) The contribution of LMP1, LMP2a, DDR1, PD-L1, and CD40 to the survival of HRS cells. Constitutive activation of NF-κB and JAK/STAT signaling pathway is the molecular hallmark of HRS cells. The expression of EBV-LMP1 is thought to contribute to activation of the NF-κB and JAK/STAT pathway.
EBV, especially through LMP1, contributes to the peculiar microenvironment by stimulating the production of many cytokines and chemokines, including CCL5 (RANTES), CCL17 (TARC), and interleukin-8, by HRS cells. HRS cells also produce immunosuppressive factors including interleukin-10, galectin 1 (Gal-1), transforming growth factor β, and PD-L1, which could all inhibit cytotoxic T-cell responses against EBV-infected HRS cells.7,30 In cHL, PD-L1 expression is genetically regulated by 9p24.1 amplification. Similar frequencies of 9p24.1 gains/amplifications have been recently shown in EBV-related and EBV-unrelated cHL cases.28 However, EBV-positive cases had higher PD-L1 expression levels, suggesting further upregulation of PD-L1 by viral infection. The topography of PD-L1+ and PD-1+ cells in the tumor microenvironment has been examined. PD-L1+ macrophages colocalize with PD-L1+ HRS cells that are in contact with PD-1+ T cells.32,33
In EBV-unrelated cHL, the microenvironment provides survival signals to HRS cells by various ligand–receptor interactions, including CD40–CD40L, CD30–CD30L, APRIL–BCMA, and NGF–TRKA.9,34 In EBV-related cHL, LMP1 itself may contribute to the survival of HRS cell precursors by constitutively activating several pathways, including NF-κB, JAK/STAT, and PI3K/AKT.17,30
LMP1 can induce expression of the collagen receptor, discoidin domain receptor 1 (DDR1), a receptor tyrosine kinase (RTK), in B cells.35 Following binding to collagen, DDR1 phosphorylation triggers the activation of downstream signaling pathways, including NF-κB.35 Activation of these pathways by DDR1 is implicated in oncogenesis and development of inflammatory infiltrate. In EBV-related cHL, LMP1 upregulates DDR1 and modulates NF-κB in association with activated DDR1 together with CD40 signaling in HRS cells.36 Notably, activation of DDR1 and CD40 is mediated by external ligands expressed on environmental collagen and T cells, respectively. Therefore, the DDR1 pathway is considered an additional or alternative pathway to known signaling pathways in cHL pathogenesis (Figure 1).
Recent evidence indicates that EBV can influence the tumor microenvironment through the secretion of viral and cellular components into exosomes, small vesicles that are released from cells. Exosomes produced by tumor cells in the EBV-infected nasopharyngeal carcinoma model contain LMP1. LMP1 can activate critical signaling pathways in uninfected neighboring cells, suggesting messenger functions of virus-modified exosomes.37,38
Strategies for therapeutic targeting based on the tumor microenvironment and the immunocompetence status of the host
Therapeutic targeting of the tumor microenvironment is an attractive strategy to reshape the immunosuppressive features of cHL, and several strategies are currently being investigated at the preclinical and clinical levels to address this issue. These strategies include but are not limited to small molecules capable of targeting macrophages and monoclonal antibodies targeting Tregs as well as PD-1 receptor. In preclinical models, repolarizing tumor-associated macrophages into proinflammatory macrophages using the novel PI3Kδ/γ inhibitor RP6530 results in significant therapeutic activity39 (C.C.-S., unpublished observations). In a recently reported phase 1 study, ADCT-301, a novel pyrrolobenzodiazepine-based antibody drug conjugate, exerted relevant antitumor activity in relapsed/refractory Hodgkin/non-Hodgkin lymphoma by targeting CD25-expressing Tregs.40 Both of these strategies are likely to be more effective in EBV-related cHL because of the higher content of macrophages and CD25-regulatory T cells reported in the microenvironment of EBV-related cHL. However, checkpoint blockade therapy represents a therapeutic breakthrough in cHL and may have particular relevance in EBV-related cHL.13,30,31
The increased PD-L1 and PD-L2 expression detected in the majority of cHL patients relies on 9p24.1 copy gain; its major targets are PD-1 ligands and almost always JAK2 as well.41 Interestingly, JAK2 copy gain results in increased JAK2 protein expression and JAK/STAT signaling, which further enhances PD-1 ligand expression.41 In EBV-related cHL, PD-L1 upregulation additionally relies on virus infection through LMP1 that acts via the AP-1 and JAK/STAT pathways.27 The relative contribution of genetic alterations and EBV infection to PD-1 ligand overexpression is difficult to quantify. Although the distribution of genetic alterations in EBV-negative and EBV-positive cHL was similar in a recently reported series, EBV-positive cHLs were more likely to have high PD-L1 expression, suggesting that viral infection might further induce PD-L1 expression.28 Genetic alterations of PD-L1 and PD-L2 loci are associated with clinical outcome.28 A recent study involving 108 patients with newly diagnosed cHL showed that 97% of patients had alterations of the PD-L1/PD-L2 loci through either copy gain (56% of the patients) or amplification (36% of the patients), which were associated with overexpression of the PD-L1 protein. Progression-free survival was significantly shorter for patients with a 9p24.1 amplification.
The physical colocalization of PD-L1–expressing HRS cells with PD-L1–expressing tumor-associated macrophages further enhances PD-1 signaling in cHL, resulting in an immunoprotected microenvironmental niche.32 The overexpression of PD-1 ligands, predominantly on HRS cells, but also on tumor-associated macrophages, as well as the expression of PD-1 receptors on intratumoral T cells, suggests significant suppression of T-cell function mediated by the PD-1/PD-L1 axis. This evidence provides a strong rationale for the use of PD-1 blockade to reverse T-cell inhibition and allow for a more effective antitumor immune response in cHL. Even though clinical studies with checkpoint inhibitors reported thus far have failed to show significant differences in responses in EBV-related vs EBV-unrelated cHL, the lack of differences may also be related to the limited number of EBV-positive patients enrolled in these studies.
Based on this genetic, morphologic, and phenotypic background, it is unsurprising that checkpoint blockade therapy has dramatically emerged as the most attractive strategy for the therapeutic targeting of relapsed/refractory cHL. Despite the complexity of PD-1 signaling and the partial understanding of its regulation, PD-1 receptor blockade using nivolumab and pembrolizumab has emerged as a better treatment strategy than inhibition of PD-L1. Phase 1 studies and subsequent registration trials using anti–PD-1 receptor antibodies in relapsed/refractory cHL patients who have failed brentuximab vedotin and/or autologous stem cell transplant showed unprecedented response rates with average values of 22% complete remission and 65% partial remission, as well as a long-lasting duration of responses detected after either nivolumab42,43 or pembrolizumab.44,45
Although results with PD-1 blockade hint at a potential paradigm shift in cHL, several compelling issues need to be addressed. Among these issues, response assessment is probably the most urgent issue to be addressed. Notably, clinical benefit is associated with PD-1 blockade in 30% to 40% of patients with positron emission tomography scans suggestive of progressive disease. A new provisional category termed “indeterminate response” (IR) was recently introduced to better classify this scenario.46 This new terminology more appropriately describes these lesions/conditions, with additional tests (biopsy or subsequent imaging) needed to define flare/pseudoprogression vs true progressive disease.46,47 Indeed, considering tumor flare or pseudoprogression as progressive disease may lead to the premature discontinuation of PD-1 blockade therapy, which, if prolonged, could lead to late responses (both partial or complete) and sustained clinical benefits in a significant proportion of patients with IR.
Upon continuation of PD-1 blockade therapy, a subset of IR patients will eventually experience true progressive disease with clinical worsening, likely because of the emergence of a refractory/resistant phenotype to checkpoint inhibitors. Although the mechanisms of resistance/refractoriness to checkpoint inhibitors have in part been identified in solid tumors,48,49 they remain unknown in lymphoid malignancies. Recent evidence in solid tumors suggests that heavy infiltration of immune-suppressive myeloid cells can correlate with resistance to checkpoint blockade.50 Therefore, the characterization of the cHL microenvironment with a specific focus on inflammatory and immune cells might provide important information on the mechanisms of response to checkpoint blockade therapy. In this clinical scenario, mutational analysis of circulating cell-free tumor DNA may play a key role in identifying the genetic determinants of resistance/refractoriness to checkpoint blockade therapy and may significantly contribute to the design of innovative strategies for disease outcome prediction and monitoring, as well as identification of new therapeutic strategies to overcome resistance to checkpoint inhibitors.47,51,52
Summary
Immune dysregulation and EBV viral infection may provide alternative survival signals that make neoplastic cells in EBV-related cHL less dependent on the presence of genetic lesions otherwise important for lymphomagenesis in immunocompetent host and EBV-unrelated cHL patients. Different pathogenic pathways are variably triggered by interactions of HRS cells with critical microenvironmental components and concomitant EBV viral infection.
LMP1 viral oncoprotein may directly contribute to generation of an immunosuppressive microenvironment through its ability to induce/enhance production of immunosuppressive cytokines and the expression of Gal-1 and PD-L1. Immunomodulatory molecules such as PD-1 and its ligands PD-L1 and PD-L2 can enable tumor cells to evade the host immune system by usurping immune checkpoint pathways. The presence of these enhanced immunosuppressive features, especially including high numbers of M2 macrophages and elevated expression levels of PD-L1 (Figure 1), in EBV-related cHL should make EBV-related cHL patients more susceptible to checkpoint blockade.
Acknowledgments
This work was supported in part by grants from Centro di Riferimento Oncologico, Aviano, Italy (intramural project “Infectious agents and cancer”) (A.C.), and from the Italian Association for Cancer Research, Milan, Italy (project no. 16722) (C.C.-S).
Authorship
Contribution: A.C. designed the review; and A.C., A.G., and C.C.-S. contributed to the writing and proofreading of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Antonino Carbone, Centro di Riferimento Oncologico Aviano, Istituto Nazionale Tumori, IRCCS, Via F. Gallini 2, 33081 Aviano, Italy; e-mail: acarbone@cro.it.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal