Key Points
We found no evidence of somatic NLRP3 mosaicism in the pathogenesis of Schnitzler syndrome.
Pathogenic inflammasome activation is supported by increased ASC, IL-18, IL-6, and anakinra response.
Abstract
To date, the pathogenic mechanisms underlying Schnitzler syndrome remain obscure, in particular, the interplay between the monoclonal protein and increased interleukin-1β (IL-1β) production, although interest in the contribution of genetic factors has been fueled by detection of somatic NLRP3 mosaicism in 2 patients with the variant-type Schnitzler syndrome. At 2 specialist UK centers, we have identified 21 patients who fulfilled diagnostic criteria for Schnitzler syndrome with urticarial rash, fever, arthralgia, and bone pain; 47% reported weight loss, 40% fatigue, and 21% lymphadenopathy. An immunoglobulin M (IgM) κ paraprotein was detected in 86%; the remainder had IgM λ or IgG κ. Patients underwent searches for germ line and somatic mutations using next-generation sequencing technology. Moreover, we designed a panel consisting of 32 autoinflammatory genes to explore genetic susceptibility factor(s) to Schnitzler syndrome. Genetic analysis revealed neither germ line nor somatic NLRP3, TNFRSF1A, NLRC4, or NOD2 mutations, apart from 1 patient with a germ line NLRP3 p.V198M substitution. The proinflammatory cytokines and extracellular apoptosis-associated speck-like protein with caspase recruitment domain (ASC) measured in the serum of Schnitzler syndrome patients during active disease were significantly higher than healthy controls. Ninety-five percent of our cohort achieved a complete response to recombinant IL-1 receptor antagonist (anakinra). Our findings do not support a role for somatic NLRP3 mosaicism in disease pathogenesis; although elevated levels of ASC, IL-6, and IL-18 in patients’ serum, and the response to anakinra, suggest that Schnitzler syndrome is associated with upregulated inflammasome activation. Despite its rarity, Schnitzler syndrome is an important diagnosis as treatment with IL-1 antagonists dramatically improves quality of life for patients.
Introduction
Schnitzler syndrome is a very rare, adult-onset, apparently acquired autoinflammatory disease. It was first described in 1972 by French dermatologist Liliane Schnitzler, with just over 300 cases reported to date. A required hallmark of the disease is the presence of a monoclonal protein, which is immunoglobulin M-κ (IgM-κ) in the vast majority of reported cases (classical type), although monoclonal IgG has been identified in a minority (variant type).1 The clinical phenotype varies to some extent between patients, but the presence of a chronic urticarial-looking rash and a monoclonal IgM or IgG paraprotein are the obligate Strasbourg criteria for diagnosis.2 Other less frequent symptoms, constituting minor diagnostic criteria, include recurrent fever, bone pain, lymphadenopathy, headache, myalgia, arthralgia, fatigue, weight loss, peripheral neuropathy, neutrophilic dermal infiltrate on skin biopsy, leukocytosis, and/or elevated plasma C-reactive protein (CRP). Evolution of the usually quite subtle and asymptomatic underlying clonal disorder to lymphoplasmacytic lymphoma (LPL), Waldenström macroglobulinemia (WM), or IgM myeloma has been reported to occur in 15% to 20% of patients over median follow-up of 13 years from diagnosis,3 which appears broadly comparable with that reported for IgM monoclonal gammopathy of unknown significance (MGUS) (18% at 10 years).4
Clinically, with the exception of adult onset, Schnitzler syndrome bears a striking resemblance to cryopyrin-associated periodic syndrome (CAPS). This autosomal-dominant disorder is caused by gain-of-function mutations in the NLRP3 gene, which encodes a key component of the NLRP3-inflammasome and disease-associated mutations resulting in marked upregulation of inflammasome activity and substantially increased production of interleukin-1β (IL-1β). CAPS is characterized by an urticaria-like rash from early infancy, fever, and inflammation involving many organ systems, and is associated with significant risk of development of amyloid A (AA) amyloidosis.
A further similarity between Schnitzler syndrome and CAPS is their dramatic response to IL-1 antagonists, implying a pivotal role of excess IL-1 production in their pathogenesis.5-7 No IL-1–blocking agents have yet been specifically licensed for the extremely rare indication of Schnitzler syndrome. To date, anakinra (recombinant IL-1 receptor antagonist) has been used most often for the treatment of patients with Schnitzler syndrome but canakinumab, monoclonal antibodies against IL-1β, and rilonacept (an IL-1 Trap) have also been reported to induce complete disease responses.8-10 Despite substantial clinical similarities between CAPS and Schnitzler syndrome, the pathogenic mechanisms underlying the latter syndrome remain obscure, in particular, the interplay between the monoclonal protein and increased IL-1β production. Thus far, no genetic influence has been identified in Schnitzler syndrome, although speculation about the contribution of genetic factors has been fueled by the finding of the common NLRP3 p.V198M variant in 2 patients with the classical-type Schnitzler syndrome,11,12 and the detection of somatic NLRP3 mosaicism in 2 patients with the variant-type Schnitzler syndrome.13
We report here a study of the clinical features and response to treatment with IL-1 blockade in 21 patients with Schnitzler syndrome identified at 2 specialist UK centers. The recognition that adult-onset CAPS may be caused by somatic NLRP3 mutations and the close clinical similarity to Schnitzler syndrome raises the possibility that somatic NLRP3 mosaicism could contribute to the disease pathogenicity, as de Koning et al previously reported.13 To address this hypothesis, we used next-generation sequencing (NGS) technology to search for somatic NLRP3 mutations in our cohort of patients. Moreover, we have looked for a common susceptibility factor by targeting 32 genes associated with inherited autoinflammatory diseases. We have measured the levels of proinflammatory cytokines and the extracellular apoptosis-associated speck-like protein with caspase recruitment domain (ASC) aggregates during an active disease state in the serum of Schnitzler syndrome patients.
Patients and methods
Between 2000 and 2015, 18 patients referred to the Autoinflammatory Disorders Clinic at the National Amyloidosis Centre (NAC) in London have been diagnosed with Schnitzler syndrome according to the Strasbourg criteria. Three further patients were identified at the Specialist Unit for Autoinflammatory Disorders at Leeds Teaching Hospitals NHS Trust.
All patients underwent comprehensive clinical and laboratory investigations including routine blood and urine biochemistry, a search for a monoclonal protein by immunofixation, electrophoresis of serum and urine, and serum free light chains, serial measurements of inflammatory markers serum AA protein and CRP, and skin and bone marrow biopsies.
Informed consent was provided by all subjects, and the ethical approval for the study was obtained from the Royal Free Hospital and University College Medical School Research Ethics Committee for this retrospective study (reference number 06/Q0501/42) and from the Leeds (East) Research Ethics Committee (04/Q1206/107), in accordance with the Declaration of Helsinki.
Genetic studies
All genetic analyses were performed on DNA samples isolated from whole blood. The NLRP3 gene, exons 3, 4, and 6 were initially analyzed by Sanger sequencing using methods previously described.12 Subsequently, all samples underwent NGS analysis using amplicon-based deep sequencing (ADS) and were sequenced on either IonTorrent or Illumina MiSeq platforms. The mean depth of cover for each amplicon was 3500X, which was sufficient to detect somatic mosaicism down to 3%.14 Sorted bam files aligned to GRCh37 with Burrows-Wheeler Aligner (BWA)-maximal exact matches were assessed using Genome Analysis Toolkit (GATK) DepthOfCoverage targeted at the genomic interval 1:247579200-247612650.
The Agilent eArray online tool (https://earray.chem.agilent.com/suredesign/) was used to design a NGS gene panel that targeted 32 genes associated with monogenic autoinflammatory diseases (supplemental Table 1, available on the Blood Web site).15 Captured and indexed libraries (QXT Target Enrichment System) were sequenced as a multiplex of 16 samples on an Illumina MiSeq sequencer in paired-end mode. The mean depth of cover for each amplicon was 250X, which was sufficient to detect somatic mosaicism down to 5%.15
Read alignment, variant calling, and annotation were performed using Agilent Sure Call v3.0 software.
Proinflammatory cytokines and the extracellular ASC protein aggregate detection
Serum was separated from whole blood collected into tubes containing sodium polyanethol sulfonate and stored at −30°C. Our selection criteria for this study were samples collected from patients before commencing therapy with an IL-1–blocking agent. Seventeen Schnitzler syndrome patients were included as well as 7 CAPS patients who had a germ line mutation in the NLRP3 gene; in addition, serum was obtained from healthy controls (HCs). In the remaining 4 Schnitzler syndrome patients, pretreatment serum was not available.
Cytokine measurements
The Meso Scale Discovery (MSD) platform was used to detect the following cytokines: IL-1α, IL-1β, tumor necrosis factor-α, interferon-γ, IL-6, IL-8, IL-10, IL-12p40, IL-12p70, and IL-17α. The MSD platform allows for the detection of 10 separate assays within a single well of a 96-well plate, combining both electrochemiluminescence (ECL) and spatial-gridding technology. Subsequent detection of cytokines was achieved by the addition of an MSD ECL molecule (Sulfo-Tag), which requires electrical stimulation for the activation of its chemiluminescence properties. Imaging of the resulting light signal was measured using the MSD Sector Imager instrument. The reference ranges are based on the upper and lower limits detected in the serum of 20 HCs.
IL-18 levels were measured using an enzyme-linked immunosorbent assay and the Human IL-18 Matched Antibody Kit (Thermo Fisher Scientific). The 96-well plates were precoated with human monoclonal IL-18 antibody and incubated overnight. Serum samples and standards were added to the wells, followed by the addition of IL-18 antibodies labeled with biotin, in combination with streptavidin–horseradish peroxidase to form immune complexes. Unbound material was washed away, with chromogen solution added until the transformation of the colorless solution to blue. The 96-well plate was then measured using the Multiskan microplate reader (Thermo Fisher Scientific) and results are expressed as picograms per milliliter.
Detection of ASC protein aggregates (specks) in serum
Two hundred microliters of HC or patient serum were incubated with 5 µL of phycoerythrin anti-ASC (TMS-1) antibody (Biolegend) for 1 hour, and subsequently analyzed on the LSRII flow cytometer instrument (BD Biosciences). Nonfluorescent 1-µm microspheres (Thermo Fisher Scientific) were used as a guide to gate around ASC specks. ASC speck events were reported as total events in the set gate divided by 200 (ASC speck per microliter).
Quality-of-life assessments
The QualityMetric SF36v2 Health Survey is designed to measure functional health and well-being from the patient’s perspective. There are 8 health domains (physical functioning, role physical, bodily pain, general health, social function, role emotional, mental health, and vitality), which are scored individually out of 100 points; the result is expressed in comparison with American norms. Scores closer to 100 represent a better quality-of-life (QoL), and a change of 10 points or more in a domain is considered clinically significant. Patients were asked to complete the surveys before starting treatment and on treatment.
Statistics
The Kruskal-Wallis 1-way analysis of variance (ANOVA) test was used to determine statistical significance for cytokine and ASC speck measurements.
Results
Patients
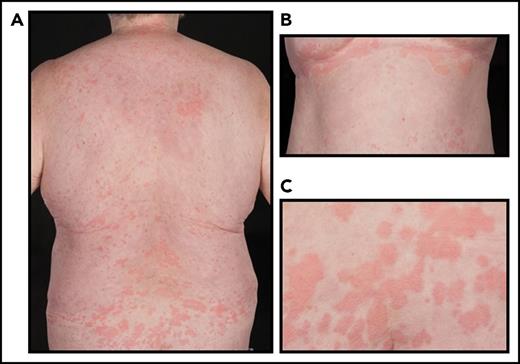
Clinical and laboratory findings in our cohort are presented in Table 1. The median age at disease onset was 54.7 years (range, 37-79 years). All patients were of British Caucasian ancestry and two-thirds were male. All patients fulfilled the Strasbourg diagnostic criteria for Schnitzler syndrome; they presented with urticarial rash, constitutional upset, fever accompanied by fatigue, arthralgia, myalgia, and bone pain. Six patients presented with rash and severe fatigue, which preceded other symptoms for up to 2 years, but eventually all subjects suffered febrile episodes with rigors and night sweats. The rash typically covered the trunk and limbs, sparing the face, palms, and soles (Figure 1). Symptoms were initially intermittent, lasting from 1 to 5 days, but over time the disease evolved with both more frequent and severe episodes. Eventually, all had almost daily symptoms with a profound effect on their QoL (Figure 2B). Additional findings in our cohort included significant weight loss (47%), fatigue (40%), and lymphadenopathy (21%). Two patients complained of headaches during the febrile episodes and 1 suffered with hearing loss. Median baseline CRP level in our cohort was 67.6 mg/L (range, 18-257 mg/L). Sixteen patients had skin biopsies, which were reviewed by a single international expert, Dan Lipsker, and all were consistent for Schnitzler syndrome. A low-level paraprotein was identified in each case: 18 patients had IgM κ type, 2 had IgM λ, and 1 IgG κ. The most feared complications of Schnitzler syndrome include AA amyloidosis and evolution to a lymphoplasmacytic malignancy. To date, over a median follow-up of 64 months (range, 19-113 months), clonal disease progression requiring chemotherapy has not occurred in any patient, and doubling of the paraprotein concentration has occurred in only 1 case.
Clinical and laboratory findings in Schnitzler syndrome cohort
| Pt no. . | Sex . | Age at symptom onset, y . | Rash . | Constitutional symptoms, fatigue/fever . | Bone pain . | Lymphadenopathy . | Hearing loss . | Baseline CRP, mg/L . | Hb, g/dL . | Characteristic skin biopsy . | M protein . | κ:λ ratio . | Bone marrow histology . | Response to anakinra . | Duration of anakinra to date, mo . | Clonal progression requiring chemotherapy . | Genes/variants identified by ADS and autoinflammatory gene panel . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 36.8 | + | + | + | 0 | 0 | 40 | 130 | + | 3 g IgM κ | 1.3 | No overt LPL | Partial | 96 | 0 | NLRP7 p.R156Q |
| 2 | F | 37.9 | + | + | + | 0 | 0 | 257 | 100 | + | 3 g IgM κ | 0.9 | No overt LPL | Complete | 82 | 0 | PLCG2 p.M28L |
| 3 | M | 41.9 | + | + | + | 0 | + | 18 | 135 | + | 12 g IgM κ | 4.1 | No overt LPL | Complete | 57 | 0 | NLRP6 p.L801F |
| 4 | M | 43.9 | + | + | + | 0 | 0 | 60 | 154 | + | 7 g IgG κ | 0.8 | 15% PC | Complete | 38 | 0 | CARD14 p.R682W/p.E422K; TRAP1 p.Y444N |
| 5 | F | 44.8 | + | + | + | 0 | 0 | 120 | 139 | + | 1 g IgM κ | 1.1 | No overt LPL | Complete | 85 | 0 | NOD2 p.R684W; NLRP7 p.E507V |
| 6 | F | 49.6 | + | + | + | 0 | 0 | 89 | 133 | + | 4 g IgM κ | 1.4 | ND | Complete | 24 | 0 | CARD14 p.E422K |
| 7 | M | 49.9 | + | + | + | 0 | 0 | 19 | 128 | ND | 9 g IgM κ | 2.5 | No overt LPL | Complete | 92 | 0 | IL10 p.P1379S |
| 8 | M | 52.8 | + | + | + | + | 0 | 45 | 143 | + | 4 g IgM λ | 0.9 | No overt LPL | Complete | 115 | 0 | None |
| 9 | M | 57.1 | + | + | + | 0 | 0 | 18 | 133 | + | 7 g IgM κ | 1.8 | LPL | Complete | 93 | 0 | NLRP3 p.V198M; PLCG2 p.M28L; SH3BP2 p.G313R |
| 10 | M | 58.1 | + | + | + | 0 | 0 | 49 | 121 | + | 3 g IgM κ | 2.2 | LPL | Complete | 41 | 0 | NOD2 p.T189M |
| 11 | M | 59.6 | + | + | + | + | 0 | 79 | 105 | + | 7 g IgM λ | 0.6 | No overt LPL | Died of AA amyloid pretreatment | ND | 0 | CARD14 p.S200N; NLRP7 p.V518A/p.E507V |
| 12 | M | 61.7 | + | + | + | 0 | 0 | 40 | 138 | + | 5 g IgM κ | 8.0 | No overt LPL | Complete | 60 | 0 | NLRP7 p.L29V |
| 13 | F | 60.7 | + | + | + | 0 | 0 | 112 | 128 | + | 9 g IgM κ | 15.7 | Low-grade MZL | Complete | 25 | 0 | PLCG2 p.M28L |
| 14 | F | 68.4 | + | + | + | 0 | 0 | 140 | 100 | + | 8 g IgM κ | 9.5 | No overt LPL | Complete | 83 | 0 | NOD2 p.M863V/p.A1006fs |
| 15 | M | 78.9 | + | + | + | 0 | 0 | 143 | 138 | ND | 7 g IgM κ | 3.6 | No overt LPL | Complete | 91 | 0 | ND |
| 16 | F | 39.7 | + | + | + | + | 0 | 26 | 121 | + | 16 g IgM κ | 1.9 | No overt LPL | Complete | 17 | 0 | PLCG2 p.M28L |
| 17 | M | 40.7 | + | + | + | + | 0 | 20 | 131 | + | 4 g IgM κ | 5.1 | No overt LPL | Complete | 15 | 0 | None |
| 18 | M | 61.2 | + | + | + | 0 | 0 | 80 | 95 | + | 8 g IgM κ | 3.1 | No overt LPL | Complete | 15 | 0 | None |
| 19 | F | 59 | + | + | + | 0 | 0 | 75.2 | 119 | ND | 5 g IgM κ | ND | CD+ B- cell LPD, MZL | Complete | 45 | 0 | None* |
| 20 | M | 43.6 | + | + | + | + | 0 | 110 | 100 | + | 6 g IgM κ | 0.591 | MGUS | Complete | 27 | 0 | None* |
| 21 | F | 51 | + | + | + | 0 | 0 | ND | ND | ND | 14 g IgM κ | ND | No overt LPL | Complete | 60 | 0 | None* |
| Pt no. . | Sex . | Age at symptom onset, y . | Rash . | Constitutional symptoms, fatigue/fever . | Bone pain . | Lymphadenopathy . | Hearing loss . | Baseline CRP, mg/L . | Hb, g/dL . | Characteristic skin biopsy . | M protein . | κ:λ ratio . | Bone marrow histology . | Response to anakinra . | Duration of anakinra to date, mo . | Clonal progression requiring chemotherapy . | Genes/variants identified by ADS and autoinflammatory gene panel . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | M | 36.8 | + | + | + | 0 | 0 | 40 | 130 | + | 3 g IgM κ | 1.3 | No overt LPL | Partial | 96 | 0 | NLRP7 p.R156Q |
| 2 | F | 37.9 | + | + | + | 0 | 0 | 257 | 100 | + | 3 g IgM κ | 0.9 | No overt LPL | Complete | 82 | 0 | PLCG2 p.M28L |
| 3 | M | 41.9 | + | + | + | 0 | + | 18 | 135 | + | 12 g IgM κ | 4.1 | No overt LPL | Complete | 57 | 0 | NLRP6 p.L801F |
| 4 | M | 43.9 | + | + | + | 0 | 0 | 60 | 154 | + | 7 g IgG κ | 0.8 | 15% PC | Complete | 38 | 0 | CARD14 p.R682W/p.E422K; TRAP1 p.Y444N |
| 5 | F | 44.8 | + | + | + | 0 | 0 | 120 | 139 | + | 1 g IgM κ | 1.1 | No overt LPL | Complete | 85 | 0 | NOD2 p.R684W; NLRP7 p.E507V |
| 6 | F | 49.6 | + | + | + | 0 | 0 | 89 | 133 | + | 4 g IgM κ | 1.4 | ND | Complete | 24 | 0 | CARD14 p.E422K |
| 7 | M | 49.9 | + | + | + | 0 | 0 | 19 | 128 | ND | 9 g IgM κ | 2.5 | No overt LPL | Complete | 92 | 0 | IL10 p.P1379S |
| 8 | M | 52.8 | + | + | + | + | 0 | 45 | 143 | + | 4 g IgM λ | 0.9 | No overt LPL | Complete | 115 | 0 | None |
| 9 | M | 57.1 | + | + | + | 0 | 0 | 18 | 133 | + | 7 g IgM κ | 1.8 | LPL | Complete | 93 | 0 | NLRP3 p.V198M; PLCG2 p.M28L; SH3BP2 p.G313R |
| 10 | M | 58.1 | + | + | + | 0 | 0 | 49 | 121 | + | 3 g IgM κ | 2.2 | LPL | Complete | 41 | 0 | NOD2 p.T189M |
| 11 | M | 59.6 | + | + | + | + | 0 | 79 | 105 | + | 7 g IgM λ | 0.6 | No overt LPL | Died of AA amyloid pretreatment | ND | 0 | CARD14 p.S200N; NLRP7 p.V518A/p.E507V |
| 12 | M | 61.7 | + | + | + | 0 | 0 | 40 | 138 | + | 5 g IgM κ | 8.0 | No overt LPL | Complete | 60 | 0 | NLRP7 p.L29V |
| 13 | F | 60.7 | + | + | + | 0 | 0 | 112 | 128 | + | 9 g IgM κ | 15.7 | Low-grade MZL | Complete | 25 | 0 | PLCG2 p.M28L |
| 14 | F | 68.4 | + | + | + | 0 | 0 | 140 | 100 | + | 8 g IgM κ | 9.5 | No overt LPL | Complete | 83 | 0 | NOD2 p.M863V/p.A1006fs |
| 15 | M | 78.9 | + | + | + | 0 | 0 | 143 | 138 | ND | 7 g IgM κ | 3.6 | No overt LPL | Complete | 91 | 0 | ND |
| 16 | F | 39.7 | + | + | + | + | 0 | 26 | 121 | + | 16 g IgM κ | 1.9 | No overt LPL | Complete | 17 | 0 | PLCG2 p.M28L |
| 17 | M | 40.7 | + | + | + | + | 0 | 20 | 131 | + | 4 g IgM κ | 5.1 | No overt LPL | Complete | 15 | 0 | None |
| 18 | M | 61.2 | + | + | + | 0 | 0 | 80 | 95 | + | 8 g IgM κ | 3.1 | No overt LPL | Complete | 15 | 0 | None |
| 19 | F | 59 | + | + | + | 0 | 0 | 75.2 | 119 | ND | 5 g IgM κ | ND | CD+ B- cell LPD, MZL | Complete | 45 | 0 | None* |
| 20 | M | 43.6 | + | + | + | + | 0 | 110 | 100 | + | 6 g IgM κ | 0.591 | MGUS | Complete | 27 | 0 | None* |
| 21 | F | 51 | + | + | + | 0 | 0 | ND | ND | ND | 14 g IgM κ | ND | No overt LPL | Complete | 60 | 0 | None* |
ADS, amplicon-based deep sequencing; F, female; Hb, hemoglobin; LPD, lymphoproliferative disorder; M, male; MZL, marginal zone lymphoma; ND, not done; PC, plasma cells; Pt, patient.
Testing was limited to ADS for the NLRP3 gene.
Characteristic urticarial rash in a patient diagnosed with Schnitzler syndrome. (A) Posterior and (B) anterior trunk, and (C) closeup view of the skin lesions.
Characteristic urticarial rash in a patient diagnosed with Schnitzler syndrome. (A) Posterior and (B) anterior trunk, and (C) closeup view of the skin lesions.
Acute-phase protein levels and QoL by SF36 before and during treatment with anakinra. (A) Acute-phase protein CRP levels measured before administration of treatment (median baseline, 67 mg/L [range, 18-257 mg/L]) and while on treatment (median baseline, 5 mg/L [range, 3-10 mg/L]) showed a significant reduction (P < .0001). (B) Patients were surveyed before starting anakinra, and while on treatment of 3 to 4 months. A comparison of the mean scores in each domain before and on treatment was statistically significant (Mann Whitney P = .0003); clinically meaningful improvements, to well above that of healthy age-matched US controls (normalized to 50), were seen in all domains (a change of 10 points or more is considered clinically significant). BP, bodily pain; GH, general health; MH, mental health; PF, physical function; RE, role emotional; RP, role physical; SF, social function; VT, vitality.
Acute-phase protein levels and QoL by SF36 before and during treatment with anakinra. (A) Acute-phase protein CRP levels measured before administration of treatment (median baseline, 67 mg/L [range, 18-257 mg/L]) and while on treatment (median baseline, 5 mg/L [range, 3-10 mg/L]) showed a significant reduction (P < .0001). (B) Patients were surveyed before starting anakinra, and while on treatment of 3 to 4 months. A comparison of the mean scores in each domain before and on treatment was statistically significant (Mann Whitney P = .0003); clinically meaningful improvements, to well above that of healthy age-matched US controls (normalized to 50), were seen in all domains (a change of 10 points or more is considered clinically significant). BP, bodily pain; GH, general health; MH, mental health; PF, physical function; RE, role emotional; RP, role physical; SF, social function; VT, vitality.
One patient was diagnosed with AA amyloidosis and died of complications of renal failure before treatment with anakinra. The median time from onset of symptoms to initiation of treatment with anakinra in the remaining 20 cases was 62 months (range, 21-276 months); 95% reported disappearance of all symptoms accompanied by normalization of plasma CRP concentration (<10 mg/L) (Figure 2A). Responses have been maintained for a median treatment duration of 60 months (range, 15-115 months). No patients have discontinued treatment and adverse events have been confined to minor infections, none requiring secondary care intervention and none directly attributed to anakinra therapy. Responses were accompanied by improvements in QoL (Figure 2B).
Genetic studies
Sanger sequencing of the NLRP3 gene confirmed the heterozygous, germ line p.V198M variant in our previously reported patient.12 NGS analysis confirmed the p.V198M substitution; no additional nucleotide alternations, including somatic NLRP3 variants, have been identified, despite applying a very sensitive method for detection of somatic mutations. Analysis of the targeted 32 gene panel associated with inherited autoinflammatory diseases showed no somatic mutations in the TNFRSF1A, NLRC4, or NOD2 genes (associated with dominant autoinflammatory diseases characterized by rash) and did not identify any genetic factor predisposing to Schnitzler syndrome. The various rare variants identified in our cohort with the minor allele frequency < 0.01 (allele frequency was obtained from the 1000 Genome Project and Exome Aggregation Consortium [ExAC] public databases) are listed in Table 1. Notably, some of these variants, including NLRP7 p.R156Q in patient 1, NOD2 p.R684W in patient 4, CARD14 p.S200N in patient 11, and a compound NOD2 p.M863V and p.A1006fs in patient 14, have previously been reported on Infevers database (https://fmf.igh.cnrs.fr/ISSAID/infevers/) but their significance is currently unknown.
Detection of ASC protein aggregates and proinflammatory cytokines the serum
Extracellular ASC protein aggregates and cytokine levels were measured in the serum collected before administration of IL-1–blocking therapy in 17 Schnitzler syndrome patients, 7 CAPS patients who had a germ line mutation in the NLRP3 gene, and in HCs. The Kruskal-Wallis 1-way ANOVA test was used to derive significances between the nonparametric data obtained.
Median levels of extracellular ASC specks per microliter of serum (ASC speck per microliter) in Schnitzler syndrome subjects, CAPS patients, and HCs were 385/µL (range, 100-897/µL), 334/µL (range, 102-546/µL), and 106/µL (range, 23-157/µL), respectively (Figure 3A). Median concentrations of IL-18 in these 3 groups were 750 pg/mL (range, 371-1852 pg/mL), 688 pg/mL (range, 411-1141 pg/mL), and 119 pg/mL (range, 23-278 pg/mL), respectively (Figure 3B); median concentrations of IL-6 in these 3 groups were 4 pg/mL (range, 1-97 pg/mL), 3.7 pg/mL (range, 1.8-52.6 pg/mL), and 0.3 pg/mL (range, 0.2-0.8 pg/mL), respectively (Figure 3C). The levels of ASC specks, IL-6, and IL-18 were similar between Schnitzler syndrome and CAPS patients, and both groups had significantly higher levels compared with HCs (Figure 3).
ASC protein aggregates and proinflammatory cytokines measured in the serum obtained from 2 control groups and the Schnitzler syndrome patients prior to their treatment with anakinra. (A) Extracellular ASC specks per microliter in the serum from HCs (n = 11), patients with Schnitzler syndrome (n = 17), and CAPS (n = 7). Significant difference was observed between HCs and Schnitzler syndrome (**P = .0151) and between HC and CAPS (*P = .0304). (B) IL-18 levels in HCs (n = 21), patients with Schnitzler syndrome (n = 17), and CAPS (n = 7). Significant difference was observed between HCs and Schnitzler syndrome (****P < .0001) and between HC and CAPS patients (***P = .0003). (C) IL-6 levels in HC (n = 12), patients with Schnitzler syndrome (n = 17), and CAPS (n = 7). Significant difference was observed between HCs and Schnitzler syndrome (***P = .0005) and between HCs and CAPS (**P = .0016) patients. No significant difference of ASC protein aggregates, IL-18, and IL-6 was observed between the Schnitzler syndrome and CAPS cohorts. The Kruskal-Wallis 1-way ANOVA test was used to derive significances between the nonparametric data obtained. P values ≤ .05 were regarded as significant. SchS, Schnitzler syndrome.
ASC protein aggregates and proinflammatory cytokines measured in the serum obtained from 2 control groups and the Schnitzler syndrome patients prior to their treatment with anakinra. (A) Extracellular ASC specks per microliter in the serum from HCs (n = 11), patients with Schnitzler syndrome (n = 17), and CAPS (n = 7). Significant difference was observed between HCs and Schnitzler syndrome (**P = .0151) and between HC and CAPS (*P = .0304). (B) IL-18 levels in HCs (n = 21), patients with Schnitzler syndrome (n = 17), and CAPS (n = 7). Significant difference was observed between HCs and Schnitzler syndrome (****P < .0001) and between HC and CAPS patients (***P = .0003). (C) IL-6 levels in HC (n = 12), patients with Schnitzler syndrome (n = 17), and CAPS (n = 7). Significant difference was observed between HCs and Schnitzler syndrome (***P = .0005) and between HCs and CAPS (**P = .0016) patients. No significant difference of ASC protein aggregates, IL-18, and IL-6 was observed between the Schnitzler syndrome and CAPS cohorts. The Kruskal-Wallis 1-way ANOVA test was used to derive significances between the nonparametric data obtained. P values ≤ .05 were regarded as significant. SchS, Schnitzler syndrome.
Discussion
Schnitzler syndrome is an extremely rare clinical entity whose definition includes the presence of a monoclonal gammopathy, usually but not exclusively of IgM isotype. Although monoclonal gammopathy is regarded as central to the diagnosis of Schnitzler syndrome, the nature of the association remains unclear. A recent study identified somatic NLRP3 mosaicism in 2 cases with variant-type Schnitzler syndrome using NGS analysis on DNA samples isolated from whole blood.13 The mutation in each case was subsequently found to be restricted to cells of myeloid lineage. The authors postulated that a population of myeloid cells with an acquired NLRP3 mutation produces abnormally high quantities of IL-1β, inducing chronic stimulation and clonal expansion of local B cells expressing IgM or, less commonly, IgG, implying that the M-protein might be a byproduct of inflammation rather than a pathogenic trigger.13 Our results reported in this study do not support this hypothesis. We performed a highly sensitive NGS analysis, able to detect very low levels of somatic mosaicism, on DNA samples isolated from 21 Schnitzler syndrome patients, and have not identified either germ line or somatic NLRP3 mutations in any of our cases, other than the presence of p.V198M in the NLRP3 gene, a common variant of uncertain significance, in 1 case. Recently, Zhou et al and Mensa-Vilaro et al reported 2 unrelated adult patients with late-onset but otherwise typical CAPS caused by myeloid-restricted somatic NLRP3 mutations, with no monoclonal gammopathy.14,16 Similarly, at the NAC, we have identified 8 such patients with mosaic NLRP3 mutations.17 There are striking similarities between the 2 reported Schnitzler syndrome cases and the patients with late-onset CAPS caused by somatic NLRP3 mutations, although neither IgM nor IgG paraprotein were found in the latter group. The phenomenon of transient paraproteinemia in the serum of patients with acute and chronic inflammatory illnesses is well recognized although relatively poorly reported and not well understood.18,19 None of our 42 patients presenting after the age of 40 years with genetically confirmed CAPS and longstanding untreated IL-1–mediated inflammation had a MGUS of any isotype at presentation nor on follow-up. These data are supported by a similar absence of paraprotein development in 54 adult patients presenting to our center with confirmed untreated tumor necrosis factor receptor–associated periodic syndrome or familial Mediterranean fever. Consistent with the phenomenon of transient paraproteinemia, the 2 reported variant-type Schnitzler syndrome patients with somatic NLRP3 mosaicism had very low concentrations of IgG paraproteinemia, which was undetectable at follow-up. The persistence of IgM paraproteins in Schnitzler syndrome and occasional progression to WM or LPL suggests a very different pathogenesis. A pathogenic role for the monoclonal protein is supported by a single case report of remission of Schnitzler syndrome after chemotherapy with rituximab-cyclophosphamide-dexamethasone induced a complete clonal response in a patient with WM.20 Thus, we suggest that the 2 reported cases could reasonably be diagnosed with late-onset CAPS due to somatic mosaicism rather than variant-type Schnitzler syndrome. Consequently, we urge searching for mosaic NLRP3 mutations in patients thought to have Schnitzler syndrome who do not completely fulfill the Strasbourg criteria, given the possibility that these patients may indeed have late-onset CAPS. Such a diagnosis may facilitate access to treatment because the anti–IL-1 agents anakinra, canakinumab, and rilonacept are all licensed for CAPS.
Furthermore, our data do not support somatic mosaicism in NLRP3, TNFRSF1A, NLRC4, or NOD2 genes underlying the pathogenesis of this intriguing disease. NGS analysis of 32 genes associated with inherited autoinflammatory diseases failed to reveal any common susceptibility factor for this disorder. A number of novel and/or previously reported variants were found, but whether they are incidental or relevant to the pathogenesis of Schnitzler syndrome needs to be further investigated.
Although we did not find any evidence for somatic mosaicism in the NLRP3 gene, we obtained indirect evidence that Schnitzler syndrome is associated with upregulated inflammasome activation and cytokine release. High levels of ASC aggregates have previously been identified in the serum of untreated CAPS patients who had either germ line or somatic NLRP3 mutation.17,21 ASC speck levels in these patients were directly linked to their disease severity. During pyroptosis (programmed cell death mediated by caspase-1), ASC specks are released by dying cells and can propagate inflammation in the neighboring cells by activating the NLRP3 inflammasome and caspase-1, leading to release of active IL-1β.21 We assessed levels of the circulating ASC protein aggregates in our cohort before starting therapy with an IL-1–blocking agent and detected significantly higher levels of ASC aggregates in the sera from Schnitzler syndrome patients when compared with HCs, but similar to that in CAPS patients. Our results suggest that both diseases are associated with inflammasome activation, but this is a relatively novel assay, not yet offered routinely as a diagnostic test and our use of it here is exploratory. We have a number of caveats including the absence of data on long-term stability of the ASC speck aggregates in frozen stored human sera; moreover, formation of ASC specks has not been systematically studied in situations where NLRP3-inflammasome activation might occur as a result of physiological processes, such as following invasive bacterial infection. Consequently, we do not yet know whether elevated ASC speck aggregates is either specific or discriminatory for Schnitzler syndrome and related autoinflammatory disorders.
Quantifying circulating IL-1β is not practical: it is virtually undetectable in human plasma even in patients with active CAPS22 ; but elevated levels of both IL-6 and IL-18 have been observed in patients with rheumatoid arthritis,23,24 Schnitzler syndrome,20,25,26 and autoinflammatory diseases27 and thus can be used as a sensitive marker of disease severity. IL-18, similar to IL-1β, requires processing by caspase-1 for its biological activation, whereas the production IL-6 is under tight control of IL-1β. We found that both IL-6 and IL-18 levels were elevated in the sera from Schnitzler syndrome patients compared with HCs and similar to that in CAPS patients.
Despite its rarity, Schnitzler syndrome is an important diagnosis to consider as spontaneous remission of symptoms is unlikely and the impact on patient life is profound. Treatment with IL-1–blocking agents effectively ameliorates the symptoms related to the autoinflammatory component of Schnitzler syndrome and abolishes the biochemical inflammatory response, thereby dramatically improving patients’ QoL (Figure 2B). The treatment is well tolerated and abrogates the risk of developing AA amyloidosis, which is a known complication of any inflammatory disorder associated with sustained overproduction of serum AA protein. To date, AA amyloidosis has been reported in 7 of the 292 patients with Schnitzler syndrome (2%). In our cohort, 1 patient died of AA amyloidosis before treatment with anakinra could be administered. So far, there is no evidence that treatment with any specific blockade of IL-1 adversely affects the behavior of the associated B-cell clone. Indeed, there is a single report suggesting the opposite, with the monoclonal protein concentration having fallen in 1 Schnitzler syndrome patient following anakinra therapy28 ; benefits of anakinra have also been reported in patients with smoldering myeloma evidenced by reduction of the proliferative index.29
To date, clinically significant progression of the clonal B-cell disorder has not occurred in any of our patients, and only 1 has seen a doubling of their low-level paraprotein; none have yet required chemotherapy. Follow-up is currently too short to draw any firm conclusions as to whether anakinra treatment might beneficially affect clonal proliferation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank all patients and the physicians and nurses from the National Amyloidosis Centre and the Leeds Teaching Hospitals for participation in this study.
This work was supported by grant SAF2015-68472-C2-1-R (Ministry of Economy and Competitiveness/European Fund for Regional Development) from the Spanish Ministry of Economy and Competitiveness, by Rosetrees, and in part by Swedish Orphan Biovitrum AB, who also provided grant funding for the NGS experiments reported in this manuscript.
Authorship
Contribution: D.M.R. designed and performed research, analyzed data, and wrote the manuscript; J.I.A. analyzed data and finalized the paper; S.P., A.M.-V., E.O., T.S., R.O., H.T., and A.B. performed experiments and analyzed the data; D.L. and P.B. helped with the research/diagnosis and with finalizing the paper; J.D.G., A.D.W., T.L., R.W., T.Y., P.N.H., S.S., and H.J.L. saw the patients included in this study and helped with this study; and S.S. and H.J.L. designed the research study and composed and finalized the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dorota M. Rowczenio, National Amyloidosis Centre, University College London Medical School, Rowland Hill St, London, NW3 2PF, United Kingdom; e-mail: d.rowczenio@ucl.ac.uk.

![Figure 2. Acute-phase protein levels and QoL by SF36 before and during treatment with anakinra. (A) Acute-phase protein CRP levels measured before administration of treatment (median baseline, 67 mg/L [range, 18-257 mg/L]) and while on treatment (median baseline, 5 mg/L [range, 3-10 mg/L]) showed a significant reduction (P < .0001). (B) Patients were surveyed before starting anakinra, and while on treatment of 3 to 4 months. A comparison of the mean scores in each domain before and on treatment was statistically significant (Mann Whitney P = .0003); clinically meaningful improvements, to well above that of healthy age-matched US controls (normalized to 50), were seen in all domains (a change of 10 points or more is considered clinically significant). BP, bodily pain; GH, general health; MH, mental health; PF, physical function; RE, role emotional; RP, role physical; SF, social function; VT, vitality.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/9/10.1182_blood-2017-10-810366/4/m_blood810366f2.jpeg?Expires=1767697710&Signature=eGCVYgT2phNMaBR3gxGm4qxlL5FMr6C2nfmxBxnT-RI2z6d77vdXt1bi4mwe9WDvYcRQHbJRmYCV3c9udIlo-hquVLbtaAyIS8ImWkPa~qt8p94dKog8ra-S9qBcCQ4vldGnpt6hFGvHbwkhIu~JQFek1VsF7R8FtMUBmq8SwuyH1~nurH4t7BKyAD8v45EjdpfF-lOJ1HxdTgGmDJkrfgm7Q70y5K2jCCGPc8pbdrNixjr~BrlhKR5Y3XErMWQvAIqzxXD72ea0MulG2yhkUpBNfquw1c9sJs0G-m-rBz8Xw1xPOHPtP-7AwTYqWm4AoGeZym7bSpZ74kkaPRoVVw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal