Key Points
AAV5 liver-directed wild-type hFIX gene transfer was well tolerated and clinically effective in severe and moderate-severe hemophilia B.
No cellular immune responses to the AAV5 vector were detected, and FIX expression levels were stable for the entire observation period.
Abstract
Gene therapy for hemophilia B aims to ameliorate bleeding risk and provide endogenous factor IX (FIX) activity/synthesis through a single treatment, eliminating the requirement for FIX concentrate. AMT-060 combines an adeno-associated virus-5 (AAV5) vector with a liver-specific promoter driving expression of a codon-optimized wild-type human FIX gene. This multinational, open-label study included 10 adults with hemophilia B (FIX ≤2% of normal) and severe-bleeding phenotype. No participants tested positive for AAV5-neutralizing antibodies using a green-fluorescent protein-based assay, and all 10 were enrolled. A single dose of 5 × 1012 or 2 × 1013 genome copies of AMT-060/kilogram was administered to 5 participants each. In the low-dose cohort, mean endogenous FIX activity increased to 4.4 IU/dL. Annualized FIX use was reduced by 81%, and mean annualized spontaneous bleeding rate (ASBR) decreased from 9.8% to 4.6% (53%). In the higher-dose cohort, mean FIX activity increased to 6.9 IU/dL. Annualized FIX use decreased by 73%, and mean ASBR declined from 3.0 to 0.9 (70%). There was no reduction in traumatic bleeds. FIX activity was stable in both cohorts, and 8 of 9 participants receiving FIX at study entry stopped prophylaxis. Limited, asymptomatic, and transient alanine aminotransferase elevations in the low-dose (n = 1) and higher-dose (n = 2) cohorts were treated with prednisolone. No decrease in FIX activity or capsid-specific T-cell responses were detected during transaminase elevations. A single infusion of AMT-060 had a positive safety profile and resulted in stable and clinically important increases in FIX activity, a marked reduction in spontaneous bleeds and FIX concentrate use, without detectable cellular immune responses against capsids. This trial was registered at www.clinicaltrials.gov as #NCT02396342; EudraCT #2013-005579-42.
Introduction
Hemophilia B is a monogenic X-linked recessive coagulation disorder causing a deficiency of coagulation factor IX (FIX) and affecting ∼25 000 individuals globally.1-3 The natural course of individuals with the severe phenotype of hemophilia B (FIX <1 U/dL) is characterized by lifelong spontaneous hemorrhages into joints, soft tissues, and muscles2 ; these hemorrhages typically lead to disabling synovitis, crippling arthropathy, and muscle atrophy.4 Treatment consists of intravenous factor replacement by injection of purified plasma-derived or recombinant FIX,2 which is inherently expensive, requires complex infrastructure, and is not available in some countries.
Factor IX concentrate can be administered on demand or prophylactically. On-demand therapy at the time of a bleed is effective at stopping individual hemorrhages but does not prevent occurrence of bleeds. Thus, by comparison, prophylaxis, which consists of regular infusions of FIX concentrate given up to 3 times weekly to prevent (spontaneous) bleeding, is associated with better joint health outcomes.2,5 However, low trough plasma levels may not optimally protect against bleeds, sometimes subclinical, in the hours just before subsequent factor infusion.6 Prophylaxis is expensive, with 1 study estimating individual annual costs of more than €135 000,7 and burdensome, requiring lifelong multiple weekly intravenous infusions from early childhood.8
Gene transfer potentially offers constant and sustained endogenous production of functional FIX. Gene transfer is particularly attractive for the treatment of hemophilia because even a modest rise in clotting factor activity can substantially attenuate the bleeding risk.9 Durable FIX expression was recently demonstrated in 10 participants who received a single infusion of adeno-associated virus (AAV) serotype 8 vectors containing a wild-type FIX gene with a liver-specific promoter (LP1).10,11 A dose-dependent increase in circulating FIX was established, with a mean FIX activity of 5.1% lasting for at least 4 years. In the highest-dose cohort, the annual use of FIX replacement therapy and bleeding rates were both reduced by 90%.11 However, treatment efficacy was limited by apparent activation of T cells in response to the viral capsid of 4 of 6 participants in the high-dose group (2 × 1012 genome copies [gc] per kg), resulting in clearance of transduced hepatocytes and consequential loss of 50% to 70% of FIX activity. This activation accompanied an otherwise asymptomatic, transient elevation of alanine aminotransferase (ALT) levels, which normalized after a short course of prednisolone.11 A similar loss of transgene activity has been reported after gene transfer with AAV2.12
These data provided the rationale for development of the novel gene therapy vector tested in this trial. AMT-060 combines the previously tested human wild-type FIX gene cassette with an AAV5 capsid.10,11 AAV5 is capable of transducing liver tissue13 and may offer a preferential immune profile. The prevalence of neutralizing antibodies (NAbs) was lower for AAV5 vs AAV2 (3.2% vs 59% in one study and 18% vs 30% in another)14,15 and for AAV5 vs AAV8 (3.2% and 19%, respectively),14 although it should be noted that prevalence rates vary by population and region. In addition, AAV5 does not appear to elicit cellular immune responses against the capsid,16 which may distinguish it from other AAV serotypes. Here, we report the planned interim analysis of a 5-year phase 1/2 study. These data reflect the safety and efficacy of AMT-060 in adults with hemophilia B with FIX activity <2% with a follow-up of up to 1 year.
Materials and methods
Study design and participants
This multinational, open-label, phase 1/2, dose-escalation study included 10 adults with hemophilia B (FIX activity ≤2% of normal) receiving either prophylactic FIX or on-demand FIX with ≥4 bleeds per year or hemophilic arthropathy (clinicaltrials.gov #NCT02396342; EudraCT #2013-005579-42). Participants were men, ≥18 years old, with either (a) severe FIX deficiency (FIX <1%) and a severe bleeding phenotype or (b) moderately severe FIX deficiency (FIX ≥1% and ≤2%) and a severe bleeding phenotype. Individuals who tested positive for preexisting NAbs to AAV5 were excluded. A sample was considered positive at 29% inhibition of transduction compared with the negative control (pooled NAb-negative human sera). Full inclusion/exclusion criteria are listed in supplemental Table 1. The study was approved by the institutional review board/institutional ethics committee at each center. All participants provided written informed consent. The trial was performed according to the Declaration of Helsinki and the principles of Good Clinical Practice.
AMT-060
AMT-060 is a novel gene transfer product that consists of an AAV5 vector incorporating a small gene cassette containing codon-optimized wild-type hFIX under the control of an LP1,10,11 which allows formation of self-complementary vectors. The vector was manufactured in insect cells using a baculovirus expression system in accordance with Good Manufacturing Practices.17 Vector titer was measured using a conventional quantitative polymerase chain reaction–based approach with plasmid DNA as the primary reference. The method is similar to those described for the characterization of internationally accepted AAV reference standards.18,19
Procedures
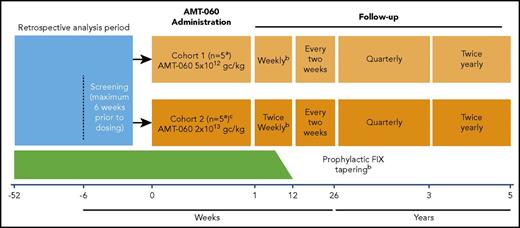
AMT-060 was administered as a single 250-mL peripheral intravenous infusion for 30 minutes. Because this was a first-in-human study, participants were monitored at the clinical trial site for 24 hours after administration of AMT-060. Participants were treated in 2 consecutive, escalating dose cohorts: cohort 1 (n = 5) 5 × 1012 gc/kg (low dose) and cohort 2 (n = 5) 2 × 1013 gc/kg (high dose) (Figure 1). After administration of AMT-060 to the first two participants in each cohort, the Data Monitoring Committee evaluated available safety data before the next participant was treated. The decision to progress to cohort 2 was made after the completion of cohort 1 dosing and a Data Monitoring Committee safety review. Participants continued with their prestudy regimen of FIX prophylaxis after AMT-060 administration until 6 to 12 weeks after treatment. At that time, participants who achieved FIX activity ≥2.0 IU/dL (trough levels) for at least 2 consecutive measurements tapered their FIX replacement therapy during a 2-week period. Continued withholding of prophylactic FIX replacement therapy was encouraged if FIX activity ≥2.0 IU/dL was maintained after tapering for at least 2 consecutive measurements. Guidance was provided to investigators about when to perform another measure of FIX based on FIX activity (1-2 days for 2.0-2.9 IU/dL up to 4-5 days for ≥8.0 IU/dL). A tapering course of corticosteroids was recommended if ALT levels increased to >1.5- to 2-fold of baseline levels, in the absence of alternative etiology.
Study design.aAfter the administration of AMT-060 to each of the first 2 participants in each cohort, the Data Monitoring Committee evaluated available safety data during 24 hours before dosing of the next participant could be initiated. bProphylactic FIX replacement therapy was generally tapered between weeks 6 and 12 if FIX activity was ≥2.0 IU/dL in at least 2 consecutive visits. Investigators could taper prophylaxis later than 12 weeks at their discretion. The decision to continue tapering/withholding of prophylactic FIX replacement therapy was based on the individual assessment by the investigator but included the requirement to document that the participant could maintain a FIX activity level ≥2.0 IU/dL. cCohort 2 dosing was initiated after the completion of cohort 1 dosing and review of initial safety data by the Data Monitoring Committee.
Study design.aAfter the administration of AMT-060 to each of the first 2 participants in each cohort, the Data Monitoring Committee evaluated available safety data during 24 hours before dosing of the next participant could be initiated. bProphylactic FIX replacement therapy was generally tapered between weeks 6 and 12 if FIX activity was ≥2.0 IU/dL in at least 2 consecutive visits. Investigators could taper prophylaxis later than 12 weeks at their discretion. The decision to continue tapering/withholding of prophylactic FIX replacement therapy was based on the individual assessment by the investigator but included the requirement to document that the participant could maintain a FIX activity level ≥2.0 IU/dL. cCohort 2 dosing was initiated after the completion of cohort 1 dosing and review of initial safety data by the Data Monitoring Committee.
Outcome measures
Safety outcomes included treatment-related adverse events (TRAEs), NAbs to AAV5, total immunoglobulin M (IgM) and IgG antibodies against AAV5, AAV5 capsid-specific T cells, antibodies to FIX (including inhibitors), shedding of AMT-060 vector, and inflammatory markers (interleukin-1β [IL-1β], IL-2, IL-6, interferon-γ [IFN-γ], and monocyte chemotactic protein-1) (supplemental Table 2). Serious adverse events were defined as events that resulted in death, were life threatening; required hospitalization or prolongation of existing hospitalization; resulted in persistent or significant disability or incapacity, congenital anomaly, or birth defect; or were judged medically important by the investigator. Adverse events were categorized as mild (awareness of symptoms, sign, illness, or event that is easily tolerated), moderate (discomfort sufficient to cause interference with usual activity), or severe (incapacitating with inability to work or undertake further normal activities).
Efficacy outcome measures included use of FIX concentrate as well as endogenous FIX plasma activity and protein levels measured ≥10 days after FIX concentrate use (supplemental Table 2). Details of biochemical assessments are given in supplemental Methods. Bleed details including circumstances, location, severity, and treatment were assessed. Retrospective data on bleeds and FIX use were collected from participant diaries and hospital records for the year before study entry. Bleed data and FIX consumption after gene transfer were recorded prospectively by the participants with use of an electronic diary, and they were reviewed by the physician at study visits. Study visits occurred weekly (cohort 1) or twice weekly (cohort 2), up to week 12; every 2 weeks from week 12 to week 26 and quarterly between 6 months and 1 year. The study will continue with quarterly visits until year 3 and twice-annual visits from years 3 to 5 after treatment. Joint status was assessed with the Hemophilia Joint Health Score version 2.1.20
Immunological analyses
Details of immunological analyses are provided in supplemental Methods.
Data analysis
This report represents a planned interim analysis with data current to 8 November 2016; further analyses of this same population are planned to provide a total of 5-year follow-up. Categorical data are presented with number of cases and percentage of the total number of cases. Continuous data are shown as a mean with corresponding 95% confidence interval (CI) or standard deviation or as a median with minimum and maximum as appropriate. Because of the sample size, no formal statistical analysis was performed. Other details of the data analyses are given in supplemental Methods.
Results
Demographic and baseline characteristics
Ten participants from The Netherlands (n = 6), Germany (n = 3), and Denmark (n = 1) were enrolled. There were no screening failures because of preexisting AAV5 NAbs. Nine participants were classified as having severe hemophilia B (<1.0 IU/dL FIX activity), and 1 participant in cohort 1 had moderate hemophilia B (FIX activity of 1.5 IU/dL). Nine participants were receiving FIX prophylaxis before AMT-060 treatment, and 1 participant with severe hemophilia (cohort 2) was receiving on-demand therapy as needed. Participants who were enrolled in cohort 1 were generally older; had more severe arthropathy; and, despite prophylactic FIX (average 4000 IU weekly), experienced more bleeds in the year before study entry compared with participants in cohort 2 (Table 1). The genotype of each participant is listed in supplemental Table 3.
Demographics and baseline characteristics
| Variable . | Cohort 1 (n = 5) . | Cohort 2 (n = 5) . |
|---|---|---|
| Age, y | 69 (35-72) | 35 (33-46) |
| Weight, kg | 84.5 (71.2-89.1) | 84.0 (71.4-96.0) |
| FIX use | ||
| Prophylaxis, IU/week* | 4000 (2000-8000) | 4000 (4000-10 500) |
| Annualized mean, IU/y | 354 800 | 173 200 |
| Mean bleeds in the year before enrollment†, n | ||
| Total | 14.4 | 4.0 |
| Spontaneous | 9.8 | 3.0 |
| Traumatic | 2.8 | 1.0 |
| Unknown | 1.8 | 0.0 |
| Hemophilia Joint Health Scores‡ | 27 (2-49) | 6 (0-17) |
| HIV-positive status, n | 1 | 0 |
| Prior hepatitis C infection, n | 4 | 2 |
| Variable . | Cohort 1 (n = 5) . | Cohort 2 (n = 5) . |
|---|---|---|
| Age, y | 69 (35-72) | 35 (33-46) |
| Weight, kg | 84.5 (71.2-89.1) | 84.0 (71.4-96.0) |
| FIX use | ||
| Prophylaxis, IU/week* | 4000 (2000-8000) | 4000 (4000-10 500) |
| Annualized mean, IU/y | 354 800 | 173 200 |
| Mean bleeds in the year before enrollment†, n | ||
| Total | 14.4 | 4.0 |
| Spontaneous | 9.8 | 3.0 |
| Traumatic | 2.8 | 1.0 |
| Unknown | 1.8 | 0.0 |
| Hemophilia Joint Health Scores‡ | 27 (2-49) | 6 (0-17) |
| HIV-positive status, n | 1 | 0 |
| Prior hepatitis C infection, n | 4 | 2 |
Values are median (minimum-maximum) unless otherwise stated.
One participant in cohort 2 received on-demand treatment and was therefore not included.
Historical bleed data was missing for 1 participant in cohort 2, who was therefore not included.
Joint status was assessed using the Hemophilia Joint Health Score version 2.1.20
Safety
Adverse events.
Three participants in each cohort (participants 1, 3, and 5 [cohort 1] and participants 6, 7, and 10 [cohort 2]) experienced a total of 14 TRAEs (4 TRAEs in cohort 1 and 10 TRAEs in cohort 2). Most related events were classified as mild, some as moderate (Table 2). Seven of the TRAEs were experienced by participant 6 (increase in liver enzyme levels [n = 2], pyrexia, anxiety, palpitations, headache, and rash). The increase in liver enzyme levels may reflect a single event, as the original ALT elevation of 55 U/L (normal range, 10-50 U/L) was recorded as recovered on day 7 (51 U/L); however, on the following day, ALT levels were 66 U/L and prednisolone treatment was initiated. Three protocol-defined serious adverse events were classified as possibly or probably related to treatment: mild, asymptomatic elevations in liver enzymes (participant 1); short, self-limiting fever in the first 24 hours after AMT-060 (participant 3); and elevation in ALT levels (participant 6).
TRAEs classified as possibly/probably related to treatment by the reporting investigator
| TRAE . | Number of events (number of participants) . | Number of events (number of participants) . |
|---|---|---|
| Cohort 1 (n = 5) . | Cohort 2 (n = 5) . | |
| Any TRAEs | 4 (3) | 10 (3) |
| Liver enzymes increased | 1 (1) | 3 (2) |
| Pyrexia | 1 (1) | 2 (2) |
| Anxiety | 1 (1) | 1 (1) |
| Drug ineffective* | 1 (1) | 0 |
| Palpitations | 0 | 1 (1) |
| Headache | 0 | 1 (1) |
| Prostatitis | 0 | 1 (1) |
| Rash | 0 | 1 (1) |
| TRAE . | Number of events (number of participants) . | Number of events (number of participants) . |
|---|---|---|
| Cohort 1 (n = 5) . | Cohort 2 (n = 5) . | |
| Any TRAEs | 4 (3) | 10 (3) |
| Liver enzymes increased | 1 (1) | 3 (2) |
| Pyrexia | 1 (1) | 2 (2) |
| Anxiety | 1 (1) | 1 (1) |
| Drug ineffective* | 1 (1) | 0 |
| Palpitations | 0 | 1 (1) |
| Headache | 0 | 1 (1) |
| Prostatitis | 0 | 1 (1) |
| Rash | 0 | 1 (1) |
TRAEs were defined as adverse events that were assessed by the investigator as possibly or probably related to AMT-060 administration.
Because this was an early trial of gene therapy, lack of efficacy was included as an adverse event of special notification and therefore was automatically reported. Participant 6 in cohort 2 experienced 7 TRAEs (2 instances of increases in liver enzymes, pyrexia, anxiety, palpitations, headache, and rash).
Liver biomarker abnormalities (elevated levels of transaminases).
Three participants experienced mild asymptomatic elevations in ALT levels not associated with changes in FIX activity (supplemental Figure 1). Participant 1 (cohort 1) had an ALT elevation with a peak of 61 U/L at week 10 (upper limit of normal, 40 U/L). Participant 7 (cohort 2) experienced an ALT elevation, with peaks of 54 U/L (week 6) and 81 U/L (week 9). The first peak in ALT levels coincided with concomitant ciprofloxacin treatment, whereas the second peak coincided with alcohol consumption. In participants 1 and 7, ALT values returned to normal within 2 and 6 weeks, respectively, of starting a course of tapering prednisolone. Participant 6 (cohort 2) had a mild asymptomatic elevation of ALT levels between week 4 and week 22 that peaked at 85 U/L at week 16 and was treated with a tapering regimen of prednisolone. The participant was weaned from prednisolone between week 18 and week 26. ALT values reached the normal reference range by week 26. FIX activity remained stable throughout the duration of ALT elevations in all 3 patients.
Immune and inflammatory biomarkers.
No participants had preexisting NAbs to AAV5 based on a functional inhibition assay. In a separate assay to determine Ig protein levels, participants 3 and 4 had low titers of preexisting IgG antibodies against AAV5. Participants 1, 7, 8, 9, and 10 had low titers of preexisting anti-AAV5 IgM antibodies. As expected, all participants experienced a humoral immune response to AAV5 within 1 week of gene transfer (supplemental Figure 2). No participants developed inhibitors to FIX. There were no detectable signs of sustained AAV5 capsid-specific T-cell activation. A single T-cell measurement of 33 spot-forming units per 1 million peripheral blood mononuclear cells in response to AAV5-capsid peptide pools, which was marginally above the threshold for a positivity of 17, was detected in participant 3 at week 9 (supplemental Figure 3). The increased ALT levels (weeks 7-26) in participant 6 coincided with the increased levels of an inflammatory marker (IFN-γ, weeks 3-17; supplemental Figure 1 and supplemental Figure 4); however, in the absence of cytotoxic immunity against transduced hepatocytes, or overt clinical signs and symptoms, these increases did not appear to be clinically relevant. No other clinically relevant changes in inflammatory biomarkers were observed in either cohort.
Detection of vector DNA.
In cohort 1, shedding of vector DNA was detected in nasal secretions (to week 18), saliva (to week 20), feces (to week 16), urine (to week 11), semen (to week 48), and whole blood (through last assessment) (supplemental Figure 5). In cohort 2, shedding of vector DNA was detected in nasal secretions (to week 12), saliva (to week 16), feces (to week 20), urine (week 22), semen (to week 22), and whole blood (through last assessment) (supplemental Figure 5).
Endogenous FIX activity after gene transfer
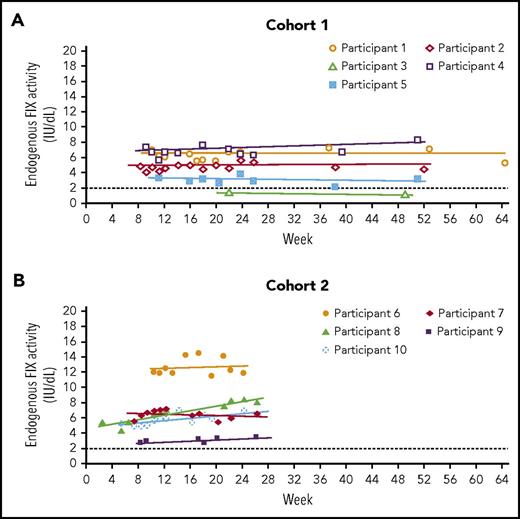
In cohort 1, residual endogenous FIX activity increased from <1.0 IU/dL (n = 4) or 1.5 IU/dL (n = 1) to a mean of 4.4 IU/dL (95% CI, 1.5-7.3 IU/dL; n = 5) at 52 weeks, with 4 of 5 participants achieving mean FIX levels ≥2.0 IU/dL (range, 3.0-6.8 IU/dL) (Table 3). In cohort 2, residual endogenous FIX activity increased from <1.0 IU/dL to a mean of 6.9 IU/dL (95% CI, 2.6-11.3 IU/dL; n = 5) at 26 weeks, with 4 of 5 participants achieving mean FIX levels >5.0 IU/dL (Table 3). FIX activity levels remained stable for the duration of follow-up in both cohorts (Figure 2). Consistent with activity, mean FIX protein concentrations varied between 1.3% and 12.3% in 8 of the 10 participants; 2 participants in cohort 1 and cohort 2 had mean FIX protein concentrations higher than 50% during the 1-year and 26-week follow-up periods, presumably because of genetic mutations that resulted in full production of a nonfunctional protein (supplemental Table 4). Disease severity was lowered in all participants: severe to mild (n = 5), severe to moderate (n = 4), and moderate to mild (n = 1) (Table 3).
Mean steady-state FIX levels and prophylaxis status by participant
| Participant . | Pre-AMT-060 gene transfer . | Post-AMT-060 gene transfer . | ||||||
|---|---|---|---|---|---|---|---|---|
| FIX activity at diagnosis, IU/dL . | Hemophilia B phenotype . | FIX prophylaxis . | Presumed CRM status* (activity/antigen ratio) . | Mean steady-state FIX, IU/dL activity (95% CI) . | FIX prophylaxis . | Hemophilia B phenotype . | Lessening of severity . | |
| 1 | <1 | Severe | Yes | +(0.1) | 6.2 (5.8-6.6) | No | Mild | Yes |
| 2 | <1 | Severe | Yes | +(0.4) | 4.7 (4.5-5.0) | No | Moderate | Yes |
| 3 | <1 | Severe | Yes | –(NC)† | 1.3 (–0.7 to 3.2) | Yes | Moderate | Yes |
| 4 | 1.5 | Moderate | Yes | –(1.4) | 6.8 (6.3-7.3) | No | Mild | Yes |
| 5 | <1 | Severe | Yes | –(1.2) | 3.0 (2.6-3.4) | No | Moderate | Yes |
| 6 | <1 | Severe | Yes | –(1.1) | 12.7 (11.9-13.5) | No | Mild | Yes |
| 7 | <1 | Severe | Yes | +/−(0.7) | 6.4 (6.0-6.7) | No | Mild | Yes |
| 8 | <1 | Severe | No‡ | –(1.6) | 6.8 (5.8-7.7) | No | Mild | Yes |
| 9 | <1 | Severe | Yes | +(0.0) | 3.1 (2.8-3.3) | No | Moderate | Yes |
| 10 | <1 | Severe | Yes | –(1.4) | 5.8 (5.4-6.2) | No | Mild | Yes |
| Participant . | Pre-AMT-060 gene transfer . | Post-AMT-060 gene transfer . | ||||||
|---|---|---|---|---|---|---|---|---|
| FIX activity at diagnosis, IU/dL . | Hemophilia B phenotype . | FIX prophylaxis . | Presumed CRM status* (activity/antigen ratio) . | Mean steady-state FIX, IU/dL activity (95% CI) . | FIX prophylaxis . | Hemophilia B phenotype . | Lessening of severity . | |
| 1 | <1 | Severe | Yes | +(0.1) | 6.2 (5.8-6.6) | No | Mild | Yes |
| 2 | <1 | Severe | Yes | +(0.4) | 4.7 (4.5-5.0) | No | Moderate | Yes |
| 3 | <1 | Severe | Yes | –(NC)† | 1.3 (–0.7 to 3.2) | Yes | Moderate | Yes |
| 4 | 1.5 | Moderate | Yes | –(1.4) | 6.8 (6.3-7.3) | No | Mild | Yes |
| 5 | <1 | Severe | Yes | –(1.2) | 3.0 (2.6-3.4) | No | Moderate | Yes |
| 6 | <1 | Severe | Yes | –(1.1) | 12.7 (11.9-13.5) | No | Mild | Yes |
| 7 | <1 | Severe | Yes | +/−(0.7) | 6.4 (6.0-6.7) | No | Mild | Yes |
| 8 | <1 | Severe | No‡ | –(1.6) | 6.8 (5.8-7.7) | No | Mild | Yes |
| 9 | <1 | Severe | Yes | +(0.0) | 3.1 (2.8-3.3) | No | Moderate | Yes |
| 10 | <1 | Severe | Yes | –(1.4) | 5.8 (5.4-6.2) | No | Mild | Yes |
Only values at least 10 d after the preceding FIX administration are included.
CRM, cross-reactive material.
Baseline measurements for CRM were not collected.
The majority of individuals showed post-AMT-060 activity/antigen ratios of ∼1:1. Ratios lower than 1:1 are suggestive of CRM positivity.
Used on-demand FIX replacement before study entry.
FIX activity across time. (A) Cohort 1; (B) cohort 2. Only values at least 10 days after the preceding FIX concentrate administration, so that they are uncontaminated by exogenous FIX, are included. Participant 3 continued with prophylaxis after AMT-060 treatment so that only limited samples uncontaminated by exogenous FIX were available. The dotted line at FIX activity of 2 IU/dL indicates the threshold required for ceasing prophylaxis per protocol. FIX prophylaxis was continued after AMT-060 and tapered between week 6 and week 12. *Participant 4 had a moderate hemophilia B phenotype at baseline (FIX activity 1.5 IU/dL).
FIX activity across time. (A) Cohort 1; (B) cohort 2. Only values at least 10 days after the preceding FIX concentrate administration, so that they are uncontaminated by exogenous FIX, are included. Participant 3 continued with prophylaxis after AMT-060 treatment so that only limited samples uncontaminated by exogenous FIX were available. The dotted line at FIX activity of 2 IU/dL indicates the threshold required for ceasing prophylaxis per protocol. FIX prophylaxis was continued after AMT-060 and tapered between week 6 and week 12. *Participant 4 had a moderate hemophilia B phenotype at baseline (FIX activity 1.5 IU/dL).
Use of exogenous FIX concentrate and bleeding
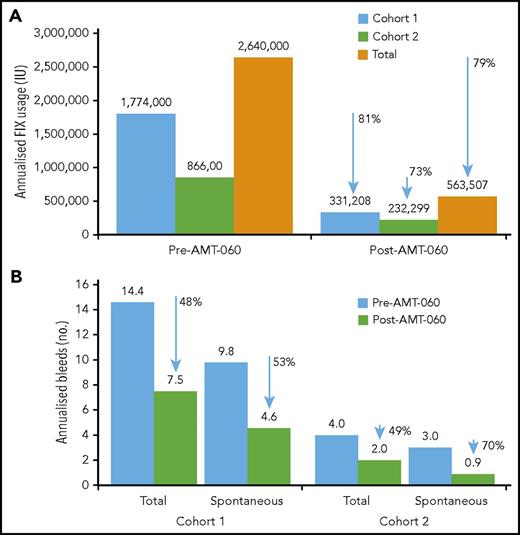
After AMT-060 treatment, 8 of the 9 participants who had been receiving FIX prophylaxis at the time of study entry stopped FIX prophylaxis. Participant 3 in cohort 1 continued taking FIX prophylaxis per protocol with endogenous (trough) FIX activity levels <2 IU/dL (Table 3). The total annualized reduction of exogenous FIX use after AMT-060 treatment was 79% overall (81% in cohort 1 and 73% in cohort 2) (Figure 3).
Annualized FIX usage and bleeds. (A) Annualized FIX usage; (B) annualized bleeds. Cumulative annualized FIX activity excludes use of factors related to surgery. The total use of FIX was collected retrospectively from patient diaries and hospital record data at the year before screening and prospectively after AMT-060 (excluding the tapering/prophylaxis period) as part of the statistical analysis. Follow-up after discontinuation of prophylaxis ranged from 39 to 65 weeks for the participants in cohort 1 and from 11 to 34 weeks for the participants in cohort 2. One participant in cohort 1 continued FIX prophylaxis after AMT-060 infusion. One participant in cohort 2 used on-demand FIX therapy before study entry. One participant in cohort 2 was missing historical bleed data.
Annualized FIX usage and bleeds. (A) Annualized FIX usage; (B) annualized bleeds. Cumulative annualized FIX activity excludes use of factors related to surgery. The total use of FIX was collected retrospectively from patient diaries and hospital record data at the year before screening and prospectively after AMT-060 (excluding the tapering/prophylaxis period) as part of the statistical analysis. Follow-up after discontinuation of prophylaxis ranged from 39 to 65 weeks for the participants in cohort 1 and from 11 to 34 weeks for the participants in cohort 2. One participant in cohort 1 continued FIX prophylaxis after AMT-060 infusion. One participant in cohort 2 used on-demand FIX therapy before study entry. One participant in cohort 2 was missing historical bleed data.
Annualized spontaneous bleeds were reduced from a mean of 9.8 in the year before treatment with AMT-060 to 4.6 in the year after treatment of participants in cohort 1, corresponding to a 53% reduction, and total bleeds were reduced by 48% (Figure 3). In 4 participants from cohort 2, annualized spontaneous bleeds were reduced from a mean of 3.0 to 0.9 (70% reduction) and total bleeds were reduced by 49% (Figure 3). Mean annualized traumatic bleeds remained stable in both cohorts (2.8 vs 2.9 and 1.0 vs 1.1 before and after AMT-060 in cohorts 1 and 2, respectively). Participant 10 was not included in the calculation because historical bleed data were not available. This participant, however, did not experience any bleeds after the intervention. All postintervention bleeds were classified as mild to moderate severity by the treating physician.
Discussion
In this successful study of liver-directed gene therapy for hemophilia, 10 patients with moderate (n = 1) or severe (n = 9) hemophilia B underwent gene transfer with AMT-060: 9 participants receiving FIX prophylaxis and 1 participant receiving on-demand FIX. Gene transfer was well tolerated with no severe TRAEs. After a single administration, FIX activity increased to levels classified as mild hemophilia in 6 participants and moderate hemophilia in 4 participants, and remained stable for the duration of follow-up. Routine prophylaxis was discontinued in 8 of 9 participants who required prophylaxis before gene therapy, resulting in large reductions in annualized factor consumption from ∼2.64 million to 563 507 IU, a total saving of ∼2.1 million units across all 10 participants. These data are underscored by self-reported data indicating that participants experienced fewer spontaneous bleeds after receiving AMT-060 than they had previously while receiving FIX prophylaxis. Cohort 1 consisted of an older population (median age, 69 years) with severe arthropathy before gene transfer (median Hemophilia Joint Health Score, 27). In this group, it is likely that the annualized bleeding rate (ABR) was still relatively high after treatment (4.6), due at least in part to preexisting joint damage. It will be of interest in this population whether continuing reductions in arthropathy and decrease of ABR will be observed with longer-term follow-up. In contrast, cohort 2 consisted of a much younger population (median age, 35 years) with lower preexisting joint damage (median Hemophilia Joint Health Score, 6). In this population, spontaneous bleeds were much lower after treatment and ABR was reduced to 0.9 posttreatment. Mild elevations in liver transaminases occurred in 3 participants and were treated with prednisolone, but these elevations resolved without loss of FIX activity or other detectable indication of ongoing immune response, toxicity, or other response against AMT-060 or transduced hepatocytes.
It is important to realize that no participants failed screening because of preexisting NAbs against AAV5, although 2 participants (participants 3 and 4) and 5 participants (participants 1, 7, 8, 9, and 10) had low titers of anti-AAV5 IgG and anti-AAV5 IgM at baseline. AMT-060 was well tolerated and effective in all participants including those with low-titer antibodies, which suggests no likely impact of low-titer IgG or IgM on transduction, clinical effectiveness, or cellular immune response after treatment. This initial observation will need to be confirmed in further studies. After treatment, 2 or more consecutive observations of elevated inflammatory cytokine levels were reported in participant 1 (IL-2 and IFN-γ) and participant 6 (IFN-γ). In both of these participants, the elevations were transient and did not affect FIX activity. Vector DNA shedding was detected in whole blood until the last assessment, and to week 48 in semen. This trend is longer than that reported in the trial of scAAV2/8-LP1-hFIXco, in which clearance was reported by day 60.12 In a likewise manner, in a trial using a similar AAV5 vector at similar doses used in this study, shedding became undetectable by day 30.16 The AAV vector is nonpathogenic, cannot replicate and shed DNA, and is noninfectious, so the risk for third parties such as family and health care personnel via prolonged shedding from the treated individual is considered to be marginal. For most bodily fluids, a trend toward dose-dependency was observed, with higher mean levels of shedding in the higher-dose group. This trend was least clear in semen, in which mean shedding was numerically lower in the higher-dose group compared with the lower-dose group at 8/12 time points, possibly related to the difference in age between the 2 cohorts. This finding is consistent with that of an earlier study by Manno et al, which reported that younger trial participants cleared vector DNA from semen more rapidly than older participants.12
The absence of FIX activity loss and detectable AAV5 capsid-specific T-cell responses associated with the ALT elevations contrast with previous reports of gene transfer using AAV8 and other novel capsid constructs.10,21,22 On the basis of these past reports, the prevailing hypothesis is that elevated ALT levels reflect cellular immune responses against transduced hepatocytes, resulting in cell destruction and loss of transgene expression.23 Although we cannot exclude the possibility that the elevated levels observed in the present study may reflect immune responses to the vector capsid that we were unable to detect despite using the current "gold standard" assay for T-cell activation, it is notable that no decreases in FIX activity occurred during transaminase elevations, raising the possibility of a different etiology for this phenomenon with AAV5. This lack of detectable capsid-specific T-cell responses is compatible with a previous study of AAV5 vector gene transfer of porphobilinogen deaminase for acute intermittent porphyria, which reported no anti-AAV5 cellular immune responses.16 In a similar manner, increases in ALT levels without T-cell activation or loss of transgene expression were preliminarily reported in a study that used an AAV5-FVIII construct in participants with hemophilia A.24 The sole similarity among the 3 participants with ALT elevations in our study was a stronger increase in FIX protein levels after gene transfer. This trend may indicate a possible mechanistic cause such as leakage resulting from the endoplasmic reticulum or other cellular stress,25-27 particularly as recombinant AAV vectors have been reported to activate unfolded protein response–signaling pathways, suggesting endoplasmic reticulum stress, during the course of intracellular trafficking.25 It is likely that only a fraction of all hepatocytes are transduced by the vector, so it is possible that the subset of transduced hepatocytes could experience “cellular stress” even when circulating levels of FIX do not appear to be excessively high. Continued evaluation of data emerging from multiple ongoing clinical studies of liver-directed gene transfer will help illuminate this issue.
AMT-060 uses the same wild-type FIX gene cassette and LP1 promoter previously described by Nathwani et al in a trial of AAV8-mediated gene transfer.10,11 Similar levels of steady-state FIX activity were achieved in this study, in which we used an AAV5 vector (mean, 4.4 IU/dL in cohort 1; mean, 6.9 IU/dL in cohort 2) compared with the previous study, in which Nathwani et al used an AAV8 vector (mean, 5.1%), although the highest dose of AAV5 vector used (2 × 1013 gc/kg) was greater than the highest dose of the AAV8 vector used (2 × 1012 gc/kg).11 Studies have shown that a gain-of-function FIX transgene (FIX Padua) increased the efficacy of liver-directed gene therapy with AAV at least 6- to 10-fold in murine28 and canine29 hemophilia models. Preliminary reports of two AAV vectors containing FIX Padua in early clinical trials have indicated increases in group mean FIX values from <2% to ∼29% in one, and from <2% to up to 45% in another.30,31 Interestingly, because the FIX Padua variant is known to increase specific activity by 5- to 10-fold, this pattern suggests that these vectors achieved similar transgene expression levels as observed in the present study.32,33 In the one study that provided available long-term results, sustained transgene expression at 1 year was only observed in 2 of 8 participants, and only 1 participant had sustained expression at 2.5 years.31 As of 1 year (cohort 1) and 6 months (cohort 2), FIX activity levels have been stable in our participants. Nathwani et al recently reported sustained FIX expression from this gene cassette at 5 years and later.34 In order to follow long-term efficacy and safety, we will continue monitoring for 5 years in our study as well.
We observed a modest dose response with our participants in cohort 2, who received a higher vector dose (2 × 1013 gc/kg). As a result, FIX activity of higher than 5 IU/dL was achieved more consistently in these participants (4 participants in cohort 2 vs 2 participants in cohort 1). Despite the fourfold increase in dose, mean FIX activity increased by ∼1.6-fold in the higher-dose group. The modest dose response observed in this study corresponds with the study by Nathwani et al, in which large increases of the dose from 2 × 1011 to 6 × 1011 and 2 × 1012 gc/kg did not result in correspondingly large increases in FIX activity (1.8%, 2.5%, and 5.1%, respectively).11 The gene therapy vector and associated transgenic protein are dependent on complex interactions with multiple endogenous pathways and functions to realize the therapeutic effect. Furthermore, an understanding of how individual variations in native physiology may affect treatment outcome is lacking. It is possible that certain factors are limiting transgene production that dose increases do not fully overcome (eg, biodistribution of the vector away from the vessels in order to transduce a higher proportion of hepatocytes).
Participants in cohort 1 were notably older and had poorer bleed control despite prophylaxis, had more extensive joint damage at study entry, and were more likely to have medically significant comorbidities than were participants in cohort 2. This setting makes direct comparison of clinical outcomes between the 2 dose cohorts challenging but offers the valuable opportunity to observe the impact of gene transfer in this heterogeneous population. The reduction in spontaneous bleeds was lower in cohort 1 (53%) compared with cohort 2 (70%). It will be of interest whether this difference is maintained as the follow-up period extends. It is interesting to note that during the course of the year after gene transfer in cohort 1, a decrease in bleeds occurred, culminating in cessation of spontaneous bleeds. This pattern has not been observed in cohort 2 at 6-month follow-up; rather, spontaneous bleeds decreased immediately with only a single reported spontaneous bleed after the end of prophylaxis. Bleed data were self-reported by the participants, and it is possible that bleeds in cohort 1 may have been overreported and self-treated due to caution on the part of the participants, which waned as participants adjusted to a life without FIX prophylaxis. We also noticed increased levels of physical activity shortly after AMT-060 treatment, causing traumatic bleeds in some patients and possibly underlying the lack of treatment impact on annualized rates of traumatic bleeds at up to 1-year follow-up. In addition, given the degree of joint disease at study entry in cohort 1, it is possible that the decrease in bleeds across time represents gradual resolution of joint damage or decreased vessel fragility with long-term hemorrhagic control. However, although these data indicate reduced bleeding across time, many potential confounding factors exist, including potential lifestyle and activity changes after the gene transfer. Therefore, these findings require further confirmation during long-term follow-up.
The strengths of this study include strict adherence to the protocol, as AMT-060 is administered as a single infusion and all participants remain in the study; and rigorous follow-up with an extensive panel of safety measures and central laboratory testing. As with other studies of rare disorders, there were restrictions on the number of participants that could be enrolled, which limited both group sizes and the range of AMT-060 doses that were examined. As with other similar trials, we collected pretreatment data retrospectively, based on patient diaries that were filled out prospectively as part of routine care in these patients.11 Also, individuals with hemophilia may inaccurately identify bleeds based on joint or muscle pain,35 especially when chronic arthropathy and associated bone pain are present, which may have affected prospective bleed reporting.
In conclusion, a single infusion of AMT-060 is well tolerated, safe, and results in stable expression of endogenous FIX for up to 1 year of follow-up. Improvement of disease severity was observed in all participants and allowed the majority (8 of 9 participants) to discontinue FIX prophylaxis. The lack of detectable T-cell response and consequential loss of FIX activity after AAV5 gene transfer have not been observed with other AAV serotypes and is deserving of further investigation. These encouraging results support further clinical investigation of AAV5-based gene transfer for hemophilia B in a phase 3 trial.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the study participants and their families. The authors would also like to thank the following individuals for their assistance with the conduct of the study: L. Landman (Academic Medical Centre Amsterdam); M. J. H. A. Kruip, G. Mulders, P. van der Graaf, R. Bouamar, and C. Bakker (Erasmus University Medical Centre); F. Peyvandi (Fondazione IRCCS Cà Granda Ospedale Maggiore Milan); E. Funding, R. Duus Müller, R. Svensgaard, C. Nielsen, and Mette Nordahl Rahbek (Rigshospitalet Copenhagen); S. Gundermann, K. Scholz, and S. Krekeler (University Hospital Frankfurt am Main); F. Yspeerd, K. Thedinga, M. Voskuilen, B. Molmans, M. Segers, and B. Waarts (University Medical Center Groningen); P. van der Valk, E. J. van Beers, D. Dekker, M. van Haaften-Spoor, S. Oortwijn-De Loo, A. Braem-Enneman, M. Timmer, and H. Aanstoot (University Medical Centre Utrecht); C. Kulick-Hofmann, A. Orlovic, and Y. Limberg (Vivantes Klinikum Berlin); Carolin Poppe (German Red Cross Blood Service, Frankfurt); and Ilse Tuinhof, Corine Baljé-Volkers, and Tatyana De Bruyne (uniQure biopharma B.V.).
This work was supported by uniQure, which provided funding for writing support, provided by Mike Lappin of GK Pharmacomm Ltd. Eileen Sawyer, a uniQure employee, provided critical feedback on the manuscript.
The authors had full responsibility for development of this work.
Authorship
Contribution: W.M., K.M., M.C., P.K., R.K., R.S., G.C., and F.W.G.L. were study investigators; W.M., K.M., M.C., P.K., R.K., R.S., and F.W.G.L. enrolled and treated participants and conducted clinical follow-up; W.M., K.M., M.C., P.K., R.K., R.S., M.T., G.C., J.S., H.B., E.S., F.C., C.M., and F.W.G.L. were involved in the design of the research, interpretation of the data, and the development of the paper; the study sponsor, uniQure biopharma B.V., collaborated with the authors on the study design, data collection, data analysis, and data interpretation; and all authors provided critical feedback on the manuscript and approved the final version of the paper prior to submission.
Conflict-of-interest disclosure: W.M. reports receiving consultant fees from uniQure B.V. during the conduct of the study; grants and personal fees from Novo Nordisk; and personal fees from Bayer, Shire, Biotest, Pfizer, Octapharma, LFB, CSL Behring, SOBI, Biogen, and BPL outside of the submitted work. K.M. reports receiving consulting fees from uniQure B.V. during the conduct of the study and has received travel support from Baxter and Pfizer; travel support and speaker fees from Bayer and Sanquin; and speaker fees from Boehringer Ingelheim, BMS, and Aspen outside of the submitted work. M.C. reports receiving consultant fees from uniQure B.V. during the conduct of the study and has received grants, personal fees, and nonfinancial support from CSL Behring and Bayer outside of the submitted work. P.K. reports receiving a trial-related fee from uniQure B.V. during the conduct of the study. R.K. reports receiving grants and personal fees from Shire/Baxalta, Bayer, CSL Behring, and Pfizer outside of the submitted work; and personal fees from SOBI, Biotest, Chiesi, Octapharma, and Novo Nordisk outside of the submitted work. R.S. reports receiving financial support from uniQure B.V. paid to his institution during the conduct of the study. At the time of contributing, M.T. was a uniQure employee. G.C. reports receiving a trial-related fee from uniQure B.V. during the conduct of the study, and personal fees from Novo Nordisk, Shire, Sobi, CSL Behring, Pfizer, and Bayer outside of the submitted work. F.C. is a Chiesi Farmaceutici S.p.A. employee. At the time of contributing, C.M. was a uniQure employee. F.W.G.L. reports receiving fees paid to his institution from uniQure B.V. during the conduct of the study, has been a consultant for Shire and Novo Nordisk outside of the submitted work, and has received research grants from CSL Behring and Baxalta/Shire outside of the submitted work. The remaining authors declare no competing conflicts of interest.
The current affiliation for M.T. is Medpace, Cincinnati, OH.
The current affiliation for C.M. is Therachon AG, Basel, Switzerland.
Correspondence: Frank W. G. Leebeek, Erasmus University Medical Centre, P.O. Box 2040, 3000 CA Rotterdam, The Netherlands; e-mail: f.leebeek@erasmusmc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal