Key Points
Exchange transfusions lower global CBF and OEF in SCA, suggesting transfusions reduce infarct risk by relieving cerebral metabolic stress.
In SCA, OEF is highest in the deep white matter, where infarct risk is high; transfusions reduce the volume of tissue with elevated OEF.
Abstract
Blood transfusions are the mainstay of stroke prevention in pediatric sickle cell anemia (SCA), but the physiology conferring this benefit is unclear. Cerebral blood flow (CBF) and oxygen extraction fraction (OEF) are elevated in SCA, likely compensating for reduced arterial oxygen content (CaO2). We hypothesized that exchange transfusions would decrease CBF and OEF by increasing CaO2, thereby relieving cerebral oxygen metabolic stress. Twenty-one children with SCA receiving chronic transfusion therapy (CTT) underwent magnetic resonance imaging before and after exchange transfusions. Arterial spin labeling and asymmetric spin echo sequences measured CBF and OEF, respectively, which were compared pre- and posttransfusion. Volumes of tissue with OEF above successive thresholds (36%, 38%, and 40%), as a metric of regional metabolic stress, were compared pre- and posttransfusion. Transfusions increased hemoglobin (Hb; from 9.1 to 10.3 g/dL; P < .001) and decreased Hb S (from 39.7% to 24.3%; P < .001). Transfusions reduced CBF (from 88 to 82.4 mL/100 g per minute; P = .004) and OEF (from 34.4% to 31.2%; P < .001). At all thresholds, transfusions reduced the volume of peak OEF found in the deep white matter, a location at high infarct risk in SCA (P < .001). Reduction of elevated CBF and OEF, both globally and regionally, suggests that CTT mitigates infarct risk in pediatric SCA by relieving cerebral metabolic stress at patient- and tissue-specific levels.
Introduction
Children with sickle cell anemia (SCA) are >100 times more likely to experience a stroke compared with healthy children.1 Historically, overt strokes, defined by the presence of a localizing neurologic symptom, occurred in 11% of children with SCA.2 Without treatment, a majority experienced a recurrent stroke within 5 years.3,4 Silent cerebral infarcts, which do not cause focal neurologic deficits but are associated with neurocognitive impairment, occur in up to 39% of children with SCA.5-8 Chronic transfusion therapy (CTT) reduces primary and recurrent overt stroke risks4,9 and recurrent silent cerebral infarct risk.7 Despite the benefits, transfusions impose long-term medical complications (ie, alloimmunization, iron overload, transfusion reactions, and need for IV access). At present, physiologic mechanisms by which transfusions mitigate ischemic risk at the tissue level are not well understood, preventing the development of stroke-prevention therapies that mimic the effects of CTT.
The severe anemia in SCA results in decreased arterial oxygen content (CaO2). To maintain cerebral oxygen delivery in the setting of low CaO2, cerebral autoregulatory mechanisms dilate arterioles to increase cerebral blood flow (CBF). Indeed, CBF is elevated in both children and adults with SCA.10-14 Cerebral oxygen delivery (CBF × CaO2) and the fraction of oxygen extracted (OEF) comprise the total cerebral metabolic rate of oxygen utilization (CMRO2 = CaO2 × CBF × OEF), providing a metric of the oxygen metabolic demand of the brain. In addition to a compensatory increase in CBF, OEF can increase to meet cerebral metabolic demand, which has recently been demonstrated in SCA.14,15 Elevated OEF can be highly predictive of future ischemic stroke, as has been demonstrated in patients with non-SCA carotid occlusion.16,17
Clarifying and quantifying the influence of CTT on cerebral oxygen metabolism may enable personalized, goal-directed (rather than empiric) CTT as well as development of other cerebral infarct prevention strategies. CTT increases total hemoglobin (Hb) and decreases the percentage of Hb S, thereby increasing CaO2. In small series of adults with SCA (N < 12), transfusions lowered CBF,11,13 but the effects of transfusions on oxygen metabolism have not been studied. Until recently, positron emission tomography (PET) imaging was the exclusive modality to measure OEF and CMRO2, limiting pediatric studies because of the radiation exposure associated with PET. We employed novel magnetic resonance imaging (MRI) methods to quantify voxel-wise OEF and CMRO2 based on the paramagnetic properties of deoxyhemoglobin.18,19 We hypothesized that exchange transfusions would lower both CBF and OEF while maintaining CMRO2, thereby reducing cerebral metabolic stress. To test this hypothesis, we prospectively enrolled children with SCA undergoing CTT, comparing global and regional CBF, OEF, and CMRO2 before and after exchange transfusions. Furthermore, we hypothesized that CTT may chronically reduce, but not normalize, cerebral metabolic stress, which we evaluated by comparing CBF and OEF in children with SCA receiving CTT to those in nontransfused children with SCA and healthy children.
Patients and methods
The institutional review board at Washington University in St. Louis approved this study. All participants or their parents/guardians provided informed consent in accordance with the Declaration of Helsinki.
Patients
We recruited 3 cohorts of children from the pediatric sickle cell disease clinic at Washington University in St. Louis: children with Hb SS or S-β0 thalassemia receiving at least 12 months of CTT for stroke prevention, age-matched children with Hb SS or Hb S-β0 thalassemia not receiving CTT, and age-matched healthy siblings without sickle-cell trait. Exclusion criteria were: sickle-cell trait as confirmed by laboratory or newborn screen results, pregnancy, contraindications to MRI, inability to tolerate MRI without sedation, stem-cell transplantation, history of neurologic illness other than stroke in the SCA population, and history of any neurologic illness in the healthy control population. Per standard practice at our institution, CTT was defined as manual exchange transfusion or erythracytapheresis performed every 4 to 6 weeks, excluding simple transfusion. Our manual exchange transfusion protocol is to phlebotomize 10 mL/kg and transfuse 5 to 20 mL/kg (up to 2 units) packed red blood cells, based on pretransfusion Hb. Erythracytapheresis volume is calculated to target posttransfusion hematocrit of 36% and Hb S < 30%.20
Laboratory investigation
Venous Hb, hematocrit, and Hb analyses were obtained within 48 hours before transfusion and repeated immediately after transfusion. Capillary electrophoresis quantified variant and normal Hbs. Venous cooximetry measured carboxyhemoglobin and methemoglobin (dyshemoglobins) for research purposes to improve arterial oxygen estimations. In control participants for whom measured Hb was not available (n = 7), Hb was imputed using a general linear model accounting for age and sex derived from a larger cohort including those not part of this prospective analysis.21 Dyshemoglobins were subtracted from total Hb measurements, because they do not participate in oxygen exchange.22,23 All Hb measurements were adjusted according to the following formula: Hb = [total measured Hb] − (total Hb × % carboxyhemoglobin) − (total Hb × % methemoglobin). If dyshemoglobin measurements were not available at a given time point, we applied the average value from the respective cohort (ie, pretransfusion SCA, posttransfusion SCA, nontransfused SCA, or control). Pulse oximetry–measured oxygen saturation (SpO2) was obtained before MRI start. Estimated arterial oxygen content was calculated according to the equation CaO2 = 1.36 × [Hb] × [SpO2].
Image protocol and processing
Patients underwent MRI within 24 hours before and after transfusion on a Siemens 3T Tim Trio or 3T Biograph mMR scanner (Erlangen, Germany) with 12-channel head coil. Sequences acquired included: T1-weighted magnetization-prepared rapid acquisition gradient echo (MPRAGE; echo time (TE)/repetition time (TR), 2.94/1800 milliseconds; inversion time (TI), 1000 milliseconds; flip angle, 8°; 1 × 1 × 1 mm voxel resolution) to perform segmentation analysis, diffusion-weighted imaging to evaluate for acute infarcts; T2-weighted fluid-attenuated inversion recovery (FLAIR) in axial and coronal planes (TE/TR, 93/9000 milliseconds; TI, 2500 milliseconds; flip angle, 150°; 0.86 × 0.86 mm in-plane resolution; 5-mm slice thickness) to delineate prior infarcts, and 3-dimensional time-of-flight magnetic resonance angiography to assess for vasculopathy. Pseudocontinuous arterial spin labeling (ASL) measured CBF with multiple-slice 2-dimensional echo planar imaging24 (TE/TR, 12/3840 milliseconds; in-plane voxel resolution, 3 × 3 mm; slice thickness, 5 mm; 18 slices; number of measurements, 80; label duration, 1.5 seconds; postlabel delay, 1.0 second). A single-compartment model was used for CBF quantification.25 An inversion recovery sequence measured blood T1 within the superior sagittal sinus, required for individual CBF quantification because T1 varies with age and hematocrit.26 An asymmetric spin echo sequence measured tissue deoxyhemoglobin, permitting OEF quantification as described previously.27,28 Only CBF and OEF sequences were required to be repeated after transfusion. The tissue model for OEF requires the knowledge of the volume susceptibility difference between fully-oxygenated and fully-deoxygenated blood (Δχ0), which has not previously been reported for Hb S. In a separate study, we measured Δχ0 of Hb S and Hb A in ex vivo blood with varying oxygenation states at a controlled temperature of 37°C and did not find statistically different Δχ0 between Hb S and Hb A.29 Therefore, we used the published Δχ0 value of 0.18 ppm (cgs) for all OEF calculations.30 CMRO2 within each voxel was calculated as CMRO2 = CaO2 × CBF × OEF.31,32
We used the FMRIB Automated Segmentation Tool on the MPRAGE maps to segment gray and white matter.33 To minimize partial volume effects, we performed a 1-voxel morphologic erosion of gray-white boundaries on segmented CBF and OEF measurements. For each imaging time point, the FMRIB Linear Image Registration Tool34 coregistered CBF and OEF maps to the participant’s MPRAGE and FLAIR images. Visual inspection confirmed accurate alignment across all maps for each participant. A board-certified vascular (A.L.F.) or pediatric (K.P.G.) neurologist manually delineated, and a board-certified neuroradiologist (K.D.V.) confirmed, all infarcts on FLAIR maps using publically available Medical Image Processing, Analysis and Visualization software. All infarcted regions were removed from CBF and OEF measurements such that only viable tissue was included.
Regional OEF analysis
Visual inspection of a majority of OEF maps in the SCA cohort revealed a symmetric region of elevated OEF within the deep white matter bilaterally. To quantify regional OEF elevation before and after transfusion, OEF voxels with values >2 standard deviations of whole-brain OEF were defined as peak OEF voxels (95th percentile OEF, ∼40%). A 40% threshold was applied to each hemispheric OEF map from all patients receiving CTT, from which volumes of OEF >40% were calculated as a percentage of total hemispheric volume to account for variation in brain size and encephalomalacia. OEF thresholds of 38% and 36% were applied in a similar manner to ensure results were not unique to a single threshold. Because of potential hemispheric variability in OEF related to ipsilateral infarct burden and vasculopathy, regional peak OEF was analyzed by hemisphere rather than by participant.
Statistical analysis
Nonparametric methods were used for statistical comparisons. Wilcoxon signed-rank tests compared pre- and posttransfusion values. Univariate and multivariate modeling evaluated predictors of OEF and CBF. Spearman’s rank correlation coefficient examined the association of whole-brain CBF/OEF with 4 covariates: Hb, Hb S percentage, age, and sex. The 4 covariates were then entered into a generalized linear mixed model, accounting for repeated measures within participants, for prediction of whole-brain OEF and CBF. Pairwise Mann-Whitney U tests compared continuous variables among CTT, non-CTT SCA, and healthy control participants, and Kruskal-Wallis tests compared categorical predictors across all cohorts. For regional OEF analyses, percentages of hemispheric brain volume were compared pre- and posttransfusion using a generalized linear mixed model accounting for 2 hemispheres nested within a participant. A generalized linear mixed model accounting for repeated measures investigated if total Hb and/or Hb S predicted normalized volumes above the 40% threshold. Where there were multiple comparisons, P values were corrected by Benjamini-Hochberg procedure to control for family-wise errors. Statistical analysis was performed using SAS software (version 9.4; SAS Institute, Inc., Cary, NC).
Results
Patient characteristics
Twenty-one children with SCA receiving CTT for stroke prevention, 21 age-matched children with SCA not receiving CTT, and 13 healthy siblings were included in the analyses (Table 1). There were no significant differences in age or sex among the 3 cohorts. One patient was excluded from OEF analyses because of failure to perform the OEF sequence before transfusion. Two participants were excluded from CBF analyses, 1 because of a misplaced labeling plane for pseudocontinuous ASL and the other because of incorrect sequence parameters.
Patient characteristics
| . | SCA CTT cohort (n = 21) . | SCA non-CTT cohort (n = 21) . | Control cohort (n = 13) . | P . |
|---|---|---|---|---|
| Mean age (range) at scan, y | 13.1 (6-21) | 11.5 (6-21) | 11.2 (6-17) | .55 |
| Male sex, no. (%) | 9 (43) | 9 (43) | 9 (64) | .25 |
| Hb, no. (%) | ||||
| SS | 20 (95) | 19 (90) | NA | |
| S-β0 thalassemia | 1 (5) | 2 (10) | NA | |
| Vasculopathy, no. (%) | 7 (33) | 0 | 0 | |
| Indication for CTT, no. (%) | NA | NA | ||
| Overt stroke/TIA | 10 (48) | |||
| Silent stroke | 5 (24) | |||
| Abnormal TCD | 6 (29) | |||
| Type of CTT, no. (%) | NA | NA | ||
| Manual exchange transfusion | 17 (81) | |||
| Erythrocytapheresis | 4 (19) | |||
| Median Hb (25th-75th IQR), g/dL* | 8.5 (7.7-9.5) | 13.3 (12.7-14.2) | <.001† | |
| Pretransfusion | 9.1 (8.8-9.6) | |||
| Posttransfusion | 10.3 (10.1-11.0) | |||
| P | <.001 | |||
| Median Hb S (25th-75th IQR), % | 74.4 (67.8-81.3) | NA | <.001† | |
| Pretransfusion | 39.7 (31.7-48.1) | |||
| Posttransfusion | 24.3 (17.1-33.1) | |||
| P | <.001 | |||
| Median SpO2(25th-75th IQR), % | 97 (94-99) | 99 (98-100) | .03† | |
| Pretransfusion | 98 (97-98) | |||
| Posttransfusion | 98 (96-99) | |||
| P | .307 | |||
| Median dyshemoglobins (25th-75th IQR), % | 4.2 (4.1-4.4) | 1.8 (1.5-1.8) | <.001† | |
| Pretransfusion | 4.0 (3.7-4.4) | |||
| Posttransfusion | 3.6 (3.3-4.0) | |||
| P | .02 | |||
| Median calculated CaO2(25th-75th IQR), mL/dL | 10.6 (9.5-12.2) | 17.7 (17.0-18.6) | <.001† | |
| Pretransfusion | 11.9 (11.1-12.4) | |||
| Posttransfusion | 13.3 (12.4-14.0) | |||
| P | <.001 |
| . | SCA CTT cohort (n = 21) . | SCA non-CTT cohort (n = 21) . | Control cohort (n = 13) . | P . |
|---|---|---|---|---|
| Mean age (range) at scan, y | 13.1 (6-21) | 11.5 (6-21) | 11.2 (6-17) | .55 |
| Male sex, no. (%) | 9 (43) | 9 (43) | 9 (64) | .25 |
| Hb, no. (%) | ||||
| SS | 20 (95) | 19 (90) | NA | |
| S-β0 thalassemia | 1 (5) | 2 (10) | NA | |
| Vasculopathy, no. (%) | 7 (33) | 0 | 0 | |
| Indication for CTT, no. (%) | NA | NA | ||
| Overt stroke/TIA | 10 (48) | |||
| Silent stroke | 5 (24) | |||
| Abnormal TCD | 6 (29) | |||
| Type of CTT, no. (%) | NA | NA | ||
| Manual exchange transfusion | 17 (81) | |||
| Erythrocytapheresis | 4 (19) | |||
| Median Hb (25th-75th IQR), g/dL* | 8.5 (7.7-9.5) | 13.3 (12.7-14.2) | <.001† | |
| Pretransfusion | 9.1 (8.8-9.6) | |||
| Posttransfusion | 10.3 (10.1-11.0) | |||
| P | <.001 | |||
| Median Hb S (25th-75th IQR), % | 74.4 (67.8-81.3) | NA | <.001† | |
| Pretransfusion | 39.7 (31.7-48.1) | |||
| Posttransfusion | 24.3 (17.1-33.1) | |||
| P | <.001 | |||
| Median SpO2(25th-75th IQR), % | 97 (94-99) | 99 (98-100) | .03† | |
| Pretransfusion | 98 (97-98) | |||
| Posttransfusion | 98 (96-99) | |||
| P | .307 | |||
| Median dyshemoglobins (25th-75th IQR), % | 4.2 (4.1-4.4) | 1.8 (1.5-1.8) | <.001† | |
| Pretransfusion | 4.0 (3.7-4.4) | |||
| Posttransfusion | 3.6 (3.3-4.0) | |||
| P | .02 | |||
| Median calculated CaO2(25th-75th IQR), mL/dL | 10.6 (9.5-12.2) | 17.7 (17.0-18.6) | <.001† | |
| Pretransfusion | 11.9 (11.1-12.4) | |||
| Posttransfusion | 13.3 (12.4-14.0) | |||
| P | <.001 |
Bold P values indicate statistical significance.
IQR, interquartile range; NA, not applicable; TCD, transcranial Doppler; TIA, transient ischemic attack.
Adjusted for dyshemoglobins.
Comparison is Kruskal-Wallis of posttransfusion, non-CTT, and controls.
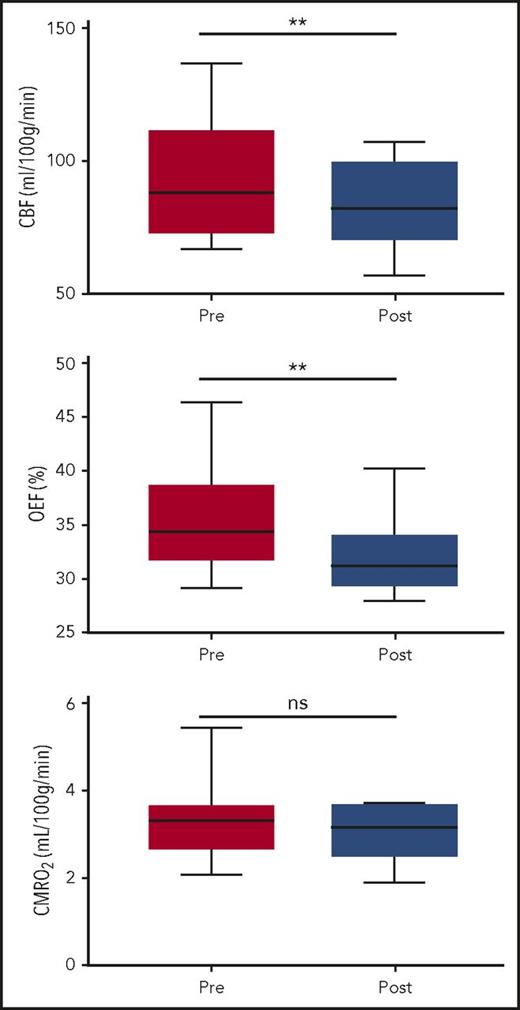
Transfusions reduce CBF and OEF
To understand the impact of transfusion on cerebral hemodynamics and oxygen metabolism in children with SCA, we measured CBF, OEF, and CMRO2 before and after transfusion. Transfusions increased median total Hb from 9.1 (IQR, 8.8-9.6) to 10.3 g/dL (IQR, 10.2-11.0; P < .001), whereas Hb S percentage decreased from 39.7% (IQR, 31.7%-48.1%) to 24.3% (IQR, 17.1%-33.1%; P < .001). SpO2 was unchanged at 98% (P = .307). Median CaO2 (1.36 × [Hb] × [SpO2]) increased from 12.2 (IQR, 11.7-12.7) to 13.7 mL/dL (IQR, 13.2-14.3; P < .001; Table 1).
Transfusions decreased whole-brain CBF and OEF, without significant change in CMRO2 (Figure 1). Median CBF decreased from 88.0 (IQR, 73.4-110.8) to 82.4 mL/100 g per minute (IQR, 70.9-99.6; P = .005). Median OEF decreased from 34.4% (IQR, 32.1%-38.5%) to 31.2% (IQR, 29.6%-33.8%; P < .001; Table 2). Examples of CBF and OEF maps are shown in Figure 2. Although collectively both CBF and OEF decreased by an average of 9% posttransfusion, the change in CBF only moderately correlated with change in OEF within individuals (ρ = 0.48; P = .05), suggesting that CBF and OEF changes in response to transfusion effects may vary independently. Tissue-segmented (gray vs white matter) CBF, OEF, and CMRO2 demonstrated a similar response to transfusions as whole-brain values (Table 2).
Exchange transfusions reduce CBF and OEF without changing CMRO2. Wilcoxon signed-rank test compared CBF, OEF, and CMRO2 before and after transfusion. CBF and OEF were significantly reduced after transfusion (P < .01); CMRO2 was not altered (P = .10). **P < .01. ns, not significant.
Exchange transfusions reduce CBF and OEF without changing CMRO2. Wilcoxon signed-rank test compared CBF, OEF, and CMRO2 before and after transfusion. CBF and OEF were significantly reduced after transfusion (P < .01); CMRO2 was not altered (P = .10). **P < .01. ns, not significant.
Transfusions decrease CBF and OEF without altering CMRO2
| . | Median (25th-75th IQR) . | P . | ||
|---|---|---|---|---|
| Pretransfusion . | Posttransfusion . | Δ . | ||
| CBF, mL/100 g per min | ||||
| Whole brain | 88.0 (73.4-110.8) | 82.4 (70.9-99.6) | −6.5 (1.2 to −18.7) | .005* |
| Gray matter | 108.7 (90.5-129.4) | 99.5 (84.4-122.4) | −11.9 (−4.3 to −23.6) | .01* |
| White matter | 59.5 (48.0-72.5) | 55.7 (48.8-60.7) | −2.5 (0.3 to −11.5) | .03* |
| OEF, % | ||||
| Whole brain | 34.4 (32.1-38.5) | 31.2 (29.6-33.8) | −2.0 (−1.0 to −5.1) | <.0001* |
| Gray matter | 35.1 (32.9-37.4) | 32.5 (30.6-33.9) | −2.3 (−0.4 to −4.5) | <.0001* |
| White matter | 33.5 (31.1-38.9) | 30.6 (28.8-33.8) | −2.5 (−0.9 to −5.8) | <.0001* |
| CMRO2, mL/100 g per min | ||||
| Whole brain | 3.2 (2.7-3.6) | 3.2 (2.5-3.7) | −0.1 (0.1 to −0.8) | .07 |
| Gray matter | 4.0 (3.3-4.3) | 4.0 (3.1-4.4) | −0.1 (0.0 to −0.6) | .08 |
| White matter | 2.4 (1.9-3.0) | 2.3 (1.9-2.5) | −0.1 (0.1 to −0.5) | .10 |
| . | Median (25th-75th IQR) . | P . | ||
|---|---|---|---|---|
| Pretransfusion . | Posttransfusion . | Δ . | ||
| CBF, mL/100 g per min | ||||
| Whole brain | 88.0 (73.4-110.8) | 82.4 (70.9-99.6) | −6.5 (1.2 to −18.7) | .005* |
| Gray matter | 108.7 (90.5-129.4) | 99.5 (84.4-122.4) | −11.9 (−4.3 to −23.6) | .01* |
| White matter | 59.5 (48.0-72.5) | 55.7 (48.8-60.7) | −2.5 (0.3 to −11.5) | .03* |
| OEF, % | ||||
| Whole brain | 34.4 (32.1-38.5) | 31.2 (29.6-33.8) | −2.0 (−1.0 to −5.1) | <.0001* |
| Gray matter | 35.1 (32.9-37.4) | 32.5 (30.6-33.9) | −2.3 (−0.4 to −4.5) | <.0001* |
| White matter | 33.5 (31.1-38.9) | 30.6 (28.8-33.8) | −2.5 (−0.9 to −5.8) | <.0001* |
| CMRO2, mL/100 g per min | ||||
| Whole brain | 3.2 (2.7-3.6) | 3.2 (2.5-3.7) | −0.1 (0.1 to −0.8) | .07 |
| Gray matter | 4.0 (3.3-4.3) | 4.0 (3.1-4.4) | −0.1 (0.0 to −0.6) | .08 |
| White matter | 2.4 (1.9-3.0) | 2.3 (1.9-2.5) | −0.1 (0.1 to −0.5) | .10 |
Wilcoxon signed-rank test was used for all comparisons. Bold P values indicate statistical significance.
P values remaining significant after Benjamani-Hochberg procedure for multiple-comparison adjustment.
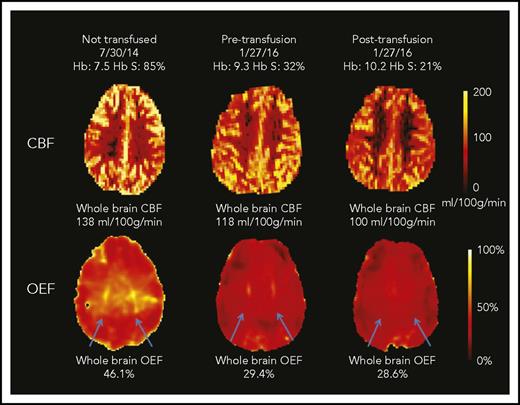
CBF and OEF maps from a child with SCA. This 7-year-old boy underwent an MRI scan before CTT initiation and again before and after an exchange transfusion (only CTT values included in cohort-level analyses). The whole-brain CBF was highest at his first scan (138 mL/100 g per minute). After 17 months of CTT, his pretransfusion CBF was lower than his initial scan (118 mL/100 g per minute; 14% drop) and further decreased after transfusion to 100 mL/100 g per minute (15% drop). The whole-brain OEF was highest at the first scan (46.1%), with dramatic reduction in OEF measured pretransfusion (29.4%; 38% drop) and only modest reduction posttransfusion (28.6%; 3% drop). His OEF maps were also notable for regionally elevated OEF in the deep white matter (blue arrows), which was most prominent before CTT initiation. This peak OEF was still detectable on the pretransfusion scan, although less prominent. After transfusion, the peak OEF region was absent, with restoration of homogeneous OEF across the brain.
CBF and OEF maps from a child with SCA. This 7-year-old boy underwent an MRI scan before CTT initiation and again before and after an exchange transfusion (only CTT values included in cohort-level analyses). The whole-brain CBF was highest at his first scan (138 mL/100 g per minute). After 17 months of CTT, his pretransfusion CBF was lower than his initial scan (118 mL/100 g per minute; 14% drop) and further decreased after transfusion to 100 mL/100 g per minute (15% drop). The whole-brain OEF was highest at the first scan (46.1%), with dramatic reduction in OEF measured pretransfusion (29.4%; 38% drop) and only modest reduction posttransfusion (28.6%; 3% drop). His OEF maps were also notable for regionally elevated OEF in the deep white matter (blue arrows), which was most prominent before CTT initiation. This peak OEF was still detectable on the pretransfusion scan, although less prominent. After transfusion, the peak OEF region was absent, with restoration of homogeneous OEF across the brain.
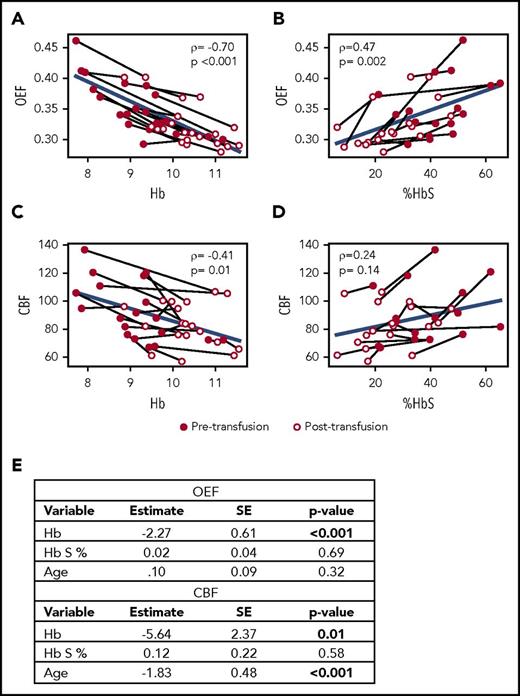
To evaluate factors influencing CBF and OEF in the CTT cohort, we modeled whole-brain CBF and OEF as a function of Hb, Hb S percentage, age, and sex in univariate and multivariate analyses (Figure 3). For OEF, Hb, and Hb S percentage were significantly correlated in univariate analyses, but only Hb remained an independent predictor in multivariate analysis. For CBF, age and Hb were significantly correlated in univariate analyses, and both remained independent predictors in multivariate analysis.
Hb is an independent predictor of OEF and CBF. Univariate correlations with Spearman’s rank test (ρ) of Hb (A) and Hb S percentage (B) with OEF and of Hb (C) and Hb S percentage (D) with CBF. Light lines connect individual patient values before and after transfusion. Blue lines indicate group-level regression. (E) Multivariate modeling of OEF and CBF as functions of Hb, Hb S percentage, age, and sex retain Hb (OEF and CBF) and age (CBF) as independent predictors. SE, standard error.
Hb is an independent predictor of OEF and CBF. Univariate correlations with Spearman’s rank test (ρ) of Hb (A) and Hb S percentage (B) with OEF and of Hb (C) and Hb S percentage (D) with CBF. Light lines connect individual patient values before and after transfusion. Blue lines indicate group-level regression. (E) Multivariate modeling of OEF and CBF as functions of Hb, Hb S percentage, age, and sex retain Hb (OEF and CBF) and age (CBF) as independent predictors. SE, standard error.
Transfusions reduce regions of peak OEF in deep white matter
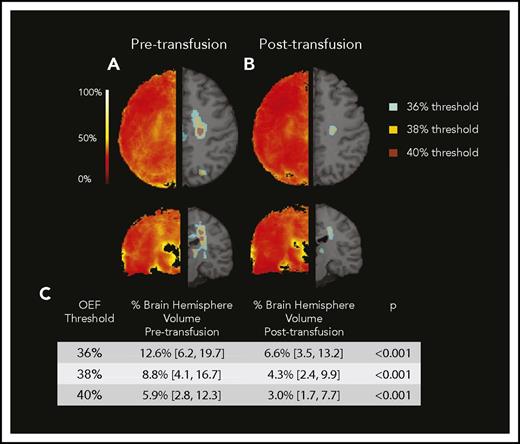
We noted a region of peak OEF within the supraventricular deep white matter in a majority of children with SCA receiving CTT (Figure 2 blue arrows; peak OEF is present pretransfusion and absent posttransfusion). To better define this region and understand the tissue-specific effects of CTT on regional OEF elevation, we averaged all pretransfusion and all posttransfusion OEF maps from the CTT cohort (Figure 4). On the averaged posttransfusion map, this region diminished but remained detectable. To account for hemispheric asymmetries in OEF resulting from vasculopathy or preexisting stroke burden, we evaluated the effect of transfusions on peak OEF within hemispheres, delineated by volume of peak OEF above 36%, 38%, and 40%. We examined 3 thresholds to mitigate a chance association between an arbitrary threshold and transfusion effect. We compared the normalized volume of peak OEF (percentage OEF volume of hemispheric volume) above each threshold before and after transfusion. Peak OEF volumes above each threshold were smaller after transfusion (Figure 4). To evaluate the influence of Hb and Hb S percentage on regionally elevated OEF, we modeled Hb and Hb S percentage as predictors of OEF volumes >40%, finding that Hb was an independent predictor (P < .001), whereas Hb S percentage was not (P = .45). In this model, OEF volume >40% decreased by 3% of total hemispheric volume for every 1-g/dL increase in Hb.
Regional peak OEF diminishes with transfusion. Averaged pretransfusion (A) and posttransfusion (B) OEF maps. Left side demonstrates averaged map. Right side demonstrates regions above successive thresholds as applied to averaged maps. (C) Median percentage of hemispheric brain volume above OEF thresholds decreased after transfusion.
Regional peak OEF diminishes with transfusion. Averaged pretransfusion (A) and posttransfusion (B) OEF maps. Left side demonstrates averaged map. Right side demonstrates regions above successive thresholds as applied to averaged maps. (C) Median percentage of hemispheric brain volume above OEF thresholds decreased after transfusion.
Transfusions reduce but do not normalize CBF and OEF
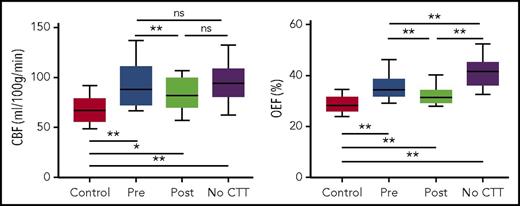
To contextualize the impact of CTT on cerebral pathophysiology, we compared pre and posttransfusion values of CBF and OEF with those of age-matched children with SCA not receiving CTT and with those of healthy controls. Children with SCA receiving CTT had CBF (both pre- and posttransfusion) similar to that of children with SCA not receiving CTT (Figure 5; supplemental Table, available on the Blood Web site) but higher than that of controls. OEF in children receiving CTT, however, fell between the extreme values of controls and children with SCA not receiving CTT. This suggests that CTT maintains OEF values lower than those in nontransfused children with SCA throughout the transfusion cycle (both pre- and posttransfusion), thereby reducing chronic cerebral metabolic stress. All children with SCA, regardless of CTT, demonstrated higher CBF and OEF compared with healthy controls, indicating that CTT does not normalize values to control levels (Figure 5).
CBF and OEF in a CTT SCA cohort lie between non-CTT SCA and healthy control cohorts. Mann-Whitney U tests directly compared the pre- and posttransfusion values with the control and also with the SCA nontransfused (no CTT) values. Wilcoxon signed-rank tests compared pre- and posttransfusion values. P values were corrected with Benjamani-Hochberg procedure for multiple comparisons. *P < .05 after correction, **P < .01 after correction.
CBF and OEF in a CTT SCA cohort lie between non-CTT SCA and healthy control cohorts. Mann-Whitney U tests directly compared the pre- and posttransfusion values with the control and also with the SCA nontransfused (no CTT) values. Wilcoxon signed-rank tests compared pre- and posttransfusion values. P values were corrected with Benjamani-Hochberg procedure for multiple comparisons. *P < .05 after correction, **P < .01 after correction.
Discussion
This prospective imaging study used novel, noninvasive MR methods allowing tissue-level quantification of OEF and CMRO2 for the first time in pediatric SCA. We rigorously evaluated transfusion-induced physiologic changes by obtaining 2 MRI scans in 21 children within 24 hours of transfusion. As expected, red-cell exchange transfusions raised Hb and lowered Hb S percentage, thereby increasing CaO2. We found transfusions lowered CBF and OEF while maintaining CMRO2, suggesting reduction of cerebral metabolic stress and demonstrating a potential mechanism by which CTT decreases infarction in this high-risk population. Furthermore, we found transfusions reduced the volume of peak OEF, indicating that CTT may protect tissue with heightened ischemic vulnerability.
In healthy children, CBF is elevated above adult values to meet the increased metabolic demands of the developing brain, presumably through autoregulatory arteriolar dilation.35 Prior studies found that CBF elevation in children with SCA is higher than the expected developmental peak, likely as a compensatory mechanism to maintain oxygen delivery in the setting of severe, chronic anemia.36-38 Similarly, we found higher CBF in both transfused and nontransfused children with SCA compared with healthy children. Exchange transfusion lowered CBF, suggesting a reduction in hemodynamic stress resulting from increased CaO2. One mechanism by which transfusions may lower CBF is restoration of autoregulatory cerebrovascular reactivity, as recently demonstrated by Kosinski et al,38 who performed carbon dioxide challenge in children with SCA before and after transfusion.
Increasing OEF serves to maintain tissue oxygenation in response to oxygen-limiting pathologies, possibly a combination of blood flow impairment (ie, micro- or macrovasculopathy) as well as decreased arterial oxygen content (ie, anemia).31 Adults with non-SCA anemia resulting from chronic renal failure had elevated whole-brain OEF, which decreased after administration of erythropoietin and increase in Hb.39 The immediate effects of transfusions on OEF have not previously been reported in SCA. We found that elevated OEF acutely dropped after exchange transfusions. Together, the CBF and OEF responses to transfusion suggest that transfusions lower ischemia risk by reducing cerebral metabolic stress and restoring compensatory reserve mechanisms.
Recent developments in advanced MRI methods have enabled OEF measurements without the concern of radiation imposed by PET.40-42 A strength of our study is acquisition of voxel-wise OEF, enabling examination of regional oxygen metabolism,43,44 unlike other OEF MRI methods limited to a single measurement in the superior sagittal sinus.45 We identified a region of increased OEF in the deep supraventricular white matter, corresponding to the internal borderzone, which we and others have demonstrated to be at highest risk of infarction in SCA.46,47 We propose that in addition to flow-limiting pathologies, oxygen-limiting pathologies would further increase ischemic vulnerability of the internal borderzone (or watershed region), as signaled by high OEF. In children with SCA, CaO2-lowering stressors could be common SCA complications, such as worsening of anemia or hypoxemia during acute chest syndrome. Lending support to this, Dowling48 observed acute silent cerebral ischemic events within this deep white matter in severely anemic children with and without SCA. Furthermore, we previously found that peak OEF in the internal borderzone from a nontransfused SCA cohort correlated and colocalized with high stroke density from an independent SCA cohort.15,21 Here, we evaluated the impact of transfusion on peak OEF by comparing tissue volumes with OEF >36%, 38%, and 40% before and after transfusion. Regardless of the OEF threshold applied, the volume of peak OEF decreased after transfusion. Supporting the contention that anemia drives regional vulnerability, we found Hb to be an independent predictor of volume of high OEF, whereas Hb S percentage was not, suggesting that raising Hb and CaO2 with transfusions diminishes regions of highest ischemic vulnerability.
In addition to metabolic and hemodynamic alterations of a single exchange transfusion, we contextualized the effects of CTT by comparing OEF and CBF with those of age-matched children with SCA not receiving CTT and those of a healthy sibling control population. Both pre- and posttransfusion values for OEF were lower than those of nontransfused patients with SCA but higher than those of healthy controls. Thus, assuming Hb concentration is lowest and OEF is highest immediately before transfusion, CTT may increase metabolic reserve throughout the transfusion cycle. If longitudinal studies demonstrate that OEF predicts future infarcts in SCA, frequency of CTT could be individualized by titrating transfusions to OEF levels rather than empiric Hb S goals.
Surprisingly, neither pre- nor posttransfusion CBF was statistically different between children receiving CTT and nontransfused children with SCA. Although the variance of CBF is, in part, due to age-related CBF variation, this is unlikely to explain the lack of difference, because the cohorts were age matched. It is possible that before CTT initiation, the CTT cohort had an even higher baseline CBF with subsequent reduction in CBF. Blood flow velocities, measured by transcranial Doppler, often decrease after 1 year of CTT.49,50 Hydroxyurea, received by 66% of our non-CTT cohort, increases Hb and may mimic hemodynamic-relieving effects of CTT, thus diminishing the CBF differences between the 2 cohorts.50,51 Alternatively, compensatory responses to low CaO2 in SCA may occur sequentially. Studies in adults with non-SCA carotid occlusion identified classic responses to reduced oxygen delivery, first noting a change in CBF (stage 1 hemodynamic impairment), followed by OEF elevation (stage 2), which occurred in a subset of individuals and was strongly predictive of future ischemic stroke.17,52 However, unlike in carotid occlusion, where flow is the major perturbing pathophysiology, in SCA the reduction of oxygen delivery is driven by low Hb as well as endothelial damage and resultant microvasculopathy, which may result in altered or additive compensatory responses. Although these compensatory stages likely overlap, OEF may be the first to normalize when metabolic stress is relieved, whereas CBF may remain elevated, as demonstrated in Figure 2. Further work is needed to understand the timing of compensatory responses in the setting of multifactorial pathologies, such as SCA. We postulate that OEF, as a downstream compensatory mechanism demonstrating less individual variability, may be more closely tied to oxygen metabolic stress and thus a more accurate biomarker of ischemia risk than CBF alone.
Although degree of anemia is an independent predictor of stroke risk across nontransfused SCA populations,2,8,53 specific transfusion Hb and Hb S percentage targets for optimal stroke prevention have been understudied. In 1976, Russell et al54 reported targets of total Hb >10 gm/dL and Hb S <30% decreased stroke recurrence, improved arteriopathy, and normalized CBF in 3 children undergoing CTT for 1 year. Larger studies implemented more aggressive transfusion goals, raising Hb to 12 to 14 g/dL and reducing Hb S to <20% to 30%.3,13 Current guidelines recommend that exchange transfusions for stroke prevention maintain Hb S percentage <30%,55 without a clear target for Hb.7,9,56-58 Bush et al59 found CaO2 (ie, Hb), but not Hb S percentage, to influence CBF. We also found through multivariate modeling that Hb was an independent predictor of CBF and OEF, but Hb S percentage was not, suggesting that CaO2, driven largely by total Hb, is closely tied to cerebral metabolic reserve and thereby ischemic vulnerability. However, stroke occurrences in SCA are multifactorial, including not only regional vulnerability but also thromboembolic mechanisms related to vasculopathy and hypercoagulability.60-62 Thus, it is likely that Hb S percentage may contribute to other stroke mechanisms (eg, rheology, aggregation, adhesion, endotheliopathy). Because Hb S levels also play a role in the severity of anemia, isolating the independent effects of total Hb and Hb S percentage should be done cautiously. Larger studies, varying both Hb and Hb S percentage targets, are needed to parse individual effects of Hb and Hb S percentage and their interaction to optimize transfusion goals.58
Although this is the largest study in pediatric SCA examining cerebral physiology before and after transfusion, its sample size precludes comprehensive evaluation of factors driving CBF and OEF responses to transfusion. This cross-sectional study cannot assess whether CTT-based change in cerebral metabolic reserve is linked to effective stroke prevention; however, participants are undergoing follow-up longitudinally to evaluate stroke risk. A strength of our study is that we measured blood T1 in each participant instead of assuming a T1 value for CBF quantification, because T1 is dependent on age, hematocrit, and possibly conformation changes of sickle cells, all of which vary in our population. Because of the measurement errors induced by large in-flow effects of arterial blood, we performed in vivo blood T1 measurements in the sagittal sinus of each individual, as measured in other published work.63,64 Although superior to assuming literature-based values, 1 limitation to this method is that it does not directly measure T1 of arterial blood. Blood oxygenation influencing T1 will likely differ between veins and capillaries, and whether these differences are similar in children with and without SCA has not been studied, to the best of our knowledge. Our transfusion cohort included patients with vasculopathy, which may reduce the accuracy of regional CBF quantification. Although vasculopathy will affect CBF measurements, MRI measurements with ASL remain reliable in the presence of vasculopathy.65 Finally, recent data suggest that individuals with SCA may have reduced labeling efficiency by ∼20% when measuring CBF using ASL.66 Labeling efficiency is scanner, site, and patient specific, thus warranting further investigation. In our study, assuming labeling efficiency is reduced in SCA, this would have caused underestimation of CBF in SCA and thus would have been unlikely to change either the finding of elevated CBF in children with SCA compared with controls or differences between pre- and posttransfusion values.
In conclusion, in children with SCA undergoing CTT, red-cell exchange transfusions reduce CBF and OEF, providing a mechanism by which CTT reduces infarct risk. Transfusions diminished peak OEF in the deep white matter of the internal borderzone, suggesting a reduction in regional ischemic vulnerability. Understanding the physiologic effects of CTT in children with SCA may enable individualized transfusion approaches, as well as lay the foundation for alternative therapies that mimic the beneficial effects of CTT.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff at the Center for Clinical Imaging Research at Washington University for their assistance with image acquisition.
This work was supported by the Child Neurology Foundation (K.P.G.); the Pediatric Critical Care and Trauma Scientist Development Program from the National Institutes of Health (NIH), Eunice Kennedy Shriver National Institute of Child Health and Human Development (K12 HD04734) (K.P.G.); Washington University St. Louis CTSA from the NIH, National Center for Advancing Translational Sciences (UL1 TR000448) (K.P.G. and M.E.F.); Hematology K12 (5K12H2087107) (M.E.F.); NIH, National Institute of Neurological Disorders and Stroke grants K23NS099472 (K.P.G.), R01NS085419 (J.-M.L.), and R01NS082561 (H.A.); NIH, National Heart, Lung, and Blood Institute grant R01HL129241 (A.L.F.); and NIH, Eunice Kennedy Shriver National Institute of Child Health and Human Development Intellectual and Developmental Disabilities Research Center at Washington University (U54 HD087011) (J.S.S.).
Authorship
Contribution: K.P.G. and A.L.F. designed the experiment, collected, analyzed, and interpreted data, and prepared the manuscript; M.E.F. and M.L.H. collected and interpreted the data; J.-M.L. designed the experiment, analyzed and interpreted data, and prepared the manuscript; M.M.B. analyzed data and performed statistical analyses; D.K.R., H.A., and C.E. collected, analyzed, and interpreted data; K.D.V., R.C.M., J.S.S., and Y.C. analyzed and interpreted data; L.S.C. collected data; A.D. contributed to the design of the experiment; and all authors critically reviewed and approved the final version of the manuscript.
Conflict-of-interest disclosure: M.L.H. is a member of the Scientific Advisory Board of the Sickle Cell Transplant Alliance for Research. M.L.H.'s spouse is employed at Pfizer, Inc. The remaining authors declare no competing financial interests.
Correspondence: Andria L. Ford, Department of Neurology, Washington University School of Medicine, 600 South Euclid Ave, Campus Box 8111, St. Louis, MO 63110; e-mail: forda@wustl.edu; and Jin-Moo Lee, Department of Neurology, Washington University School of Medicine, 600 South Euclid Ave, Campus Box 8111, St. Louis, MO 63110; email: leejm@wustl.edu.
References
Author notes
K.P.G. and M.E.F. contributed equally to this work.
J.-M.L. and A.L.F. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal