Key Points
FF-10101 has selective and potent inhibitory activities against FLT3 by forming a covalent bond to the C695 residue.
FF-10101 shows high efficacy against AML cells with FLT3 mutations including quizartinib-resistant activation loop mutations.
Abstract
An activating mutation of Fms-like tyrosine kinase 3 (FLT3) is the most frequent genetic alteration associated with poor prognosis in acute myeloid leukemia (AML). Although many FLT3 inhibitors have been clinically developed, no first-generation inhibitors have demonstrated clinical efficacy by monotherapy, due to poor pharmacokinetics or unfavorable safety profiles possibly associated with low selectivity against FLT3 kinase. Recently, a selective FLT3 inhibitor, quizartinib, demonstrated favorable outcomes in clinical studies. However, several resistant mutations emerged during the disease progression. To overcome these problems, we developed a novel FLT3 inhibitor, FF-10101, designed to possess selective and irreversible FLT3 inhibition. The co-crystal structure of FLT3 protein bound to FF-10101 revealed the formation of a covalent bond between FF-10101 and the cysteine residue at 695 of FLT3. The unique binding brought high selectivity and inhibitory activity against FLT3 kinase. FF-10101 showed potent growth inhibitory effects on human AML cell lines harboring FLT3 internal tandem duplication (FLT3-ITD), MOLM-13, MOLM-14, and MV4-11, and all tested types of mutant FLT3-expressing 32D cells including quizartinib-resistant mutations at D835, Y842, and F691 residues in the FLT3 kinase domain. In mouse subcutaneous implantation models, orally administered FF-10101 showed significant growth inhibitory effect on FLT3-ITD-D835Y- and FLT3-ITD-F691L–expressing 32D cells. Furthermore, FF-10101 potently inhibited growth of primary AML cells harboring either FLT3-ITD or FLT3-D835 mutation in vitro and in vivo. These results indicate that FF-10101 is a promising agent for the treatment of patients with AML with FLT3 mutations, including the activation loop mutations clinically identified as quizartinib-resistant mutations.
Introduction
Mutation of the Fms-like tyrosine kinase 3 (FLT3) gene is the most frequent genetic alteration in acute myeloid leukemia (AML)1,2 and mainly consists of internal tandem duplication within the juxtamembrane domain (FLT3-ITD) and point mutations, deletions, and insertions in the codons surrounding D835 in the tyrosine kinase domain (FLT3-TKD).3-5 Both mutations cause constitutive activation of FLT3 and induce activations of multiple intracellular signaling molecules, leading to autonomous cell proliferation and activation.4 FLT3-ITD is closely associated with a poor prognosis in patients with AML.5-7 It has been reported that allogeneic hematopoietic stem cell transplantation (allo-HSCT) at the first complete remission (CR) is beneficial for patients with AML with FLT3 mutations8,9 ; however, all patients cannot undergo allo-HSCT, and FLT3 mutation is still a risk factor for relapse even in patients who underwent allo-HSCT. Therefore, development of an approved FLT3 inhibitor is highly desired. Many FLT3 inhibitors have been clinically developed to date, but no FLT3 inhibitor has yet been approved for use as monotherapy. In particular, clinical responses of the first-generation FLT3 inhibitors as monotherapy were limited because of unfavorable safety profiles, which might mainly be caused by their multikinase inhibition profiles and poor pharmacokinetic (PK) properties.10-12 Recently, an international, prospective, randomized, placebo-controlled double-blind trial (CALGB 10603/RATIFY) showed that the addition of midostaurin to standard chemotherapy significantly improved overall survival in young adults with AML with FLT3 mutations.13,14 However, it is difficult to apply this combination regimen in elderly patients and/or in those with comorbidities.
Because the first-generation FLT3 inhibitors were not originally screened for sensitivity and selectivity to the activated FLT3 kinase, the discovery and clinical development of novel FLT3 inhibitors in the second generation are required.
For improvement in clinical safety and efficacy, second-generation FLT3 inhibitors, which are more selective and potent against FLT3 kinase, have been developed. Quizartinib has been screened for the affinity against FLT3 using the KinomeScan technique and has a high selectivity and sensitivity to FLT3. Although its 50% inhibitory concentration (IC50) value against dephosphorylation of FLT3-ITD is 1.1 nM, it does not have a potency against FLT3-TKD mutations.12,15 In clinical studies, quizartinib showed strong efficacy in patients with AML with FLT3-ITD, including CR; however, it is noteworthy that many responded patients remained at incomplete CR (CRi) and did not achieve CR with the recovery of normal hematopoiesis.16 The high CRi rate is thought to have been caused by the strong suppression of normal hematopoietic stem/progenitor cells through the KIT inhibitory effect of quizartinib.17 Furthermore, it has been reported that another mutation in the kinase domain (D835Y, D835V, D835F, or F691L) was additionally acquired at relapse in 8 patients with FLT3-ITD who achieved CR with quizartinib monotherapy.18 These clinical results indicate that selective and continuous FLT3 inhibition could provide patients with CR, but potency against both FLT3-ITD and FLT3-TKD mutations is required for FLT3 inhibitors.
We developed a novel FLT3 inhibitor, FF-10101, which shows covalent and selective binding to FLT3. The unique binding mode of FF-10101, characterized by forming a covalent bond to the cysteine residue at the 695 position (C695) while maintaining the binding ability to the active or inactive conformation of FLT3, brings about its potency against FLT3-ITD as well as FLT3-TKD mutations, including the quizartinib-resistant mutations observed in clinical studies. In addition, FF-10101 shows potent growth inhibitory effects on primary AML cells with FLT3 mutations in both in vitro and mouse models xenografted with primary AML cells (patient-derived xenograft [PDX]).
Methods
Compounds
FF-10101((S,E)-N-{1-[(5-{2-[(4-cyanophenyl)amino]-4-(propylamino)pyrimidin-5-yl}pent-4-yn-1-yl)amino]-1-oxopropan-2-yl}-4-(dimethylamino)-N-methylbut-2-enamide) was originally designed and synthesized by the FUJIFILM Corporation. An FF-10101 analogue with a single bond instead of a double bond, incapable of forming the covalent bond with FLT3 (FLA-9430), was also synthesized. Quizartinib, midostaurin, and crenolanib were purchased from Selleck Chemicals (Houston, TX), Enzo Life Sciences (Plymouth Meeting, PA), and MedChem Express (Monmouth Junction, NJ), respectively.
Co-crystal structure of FLT3 bound to FF-10101
Protein expression, purification, and crystallization have been described previously.19 Crystal was soaked overnight with a crystallization buffer containing FF-10101 and cryoprotected with 25% glycerol before freezing. Diffraction data were collected at the Photon Factory BL-17A. The FLT3/FF-10101 co-crystal structure was solved by molecular replacement with the program MOLREP using the apo-FLT3 structure (PDB ID: 1RJB) as a starting model. The structure was refined in REFMAC. The coordinates are deposited in the Protein data bank (PDB ID: 5X02).
Washout FLT3 enzyme assays
GST-tagged FLT3 (Carna Biosciences, Inc., Kobe, Japan) was immobilized in a 96-well plate pretreated with anti-GST antibody (Rockland Immunochemicals Inc., Limerick, PA). After 1-hour preincubation with excess concentration of a FLT3 inhibitor (5 μM) followed by 5-time wash with Tris-buffered saline with Tween 20 (TBST), enzyme assay was started by the addition of 1.2 mM adenosine triphosphate (ATP) and 3 μM substrate peptide labeled with FAM (fluorescein). Phosphorylated substrate was detected by mobility shift assay with the LabChip EZ Reader (PerkinElmer, Boston, MA).
Biochemical kinase inhibition assays
Inhibitory activity of FF-10101 against 216 kinases was evaluated with ProfilerPro Kinase Selectivity Assay Kits (PerkinElmer) in accordance with the manufacturer’s protocol. ATP concentrations around Km were used for the assay. The IC50 values were calculated with XLfit software (ID Business Solutions, Guildford, United Kingdom).
In vitro cellular assays
To confirm the covalent binding of FF-10101 to C695 in the FLT3 molecule, HEK293T cells transiently expressing FLT3-ITD or FLT3-ITD-C695S were incubated with various concentrations of FF-10101 and FLA-9430 at 37°C for 1 hour. Phosphorylation status of FLT3 was determined by Phospho-FLT3 (Tyr591) Sandwich ELISA Kit (Cell Signaling Technology, Danvers, MA) in accordance with the manufacturer’s protocol. Details are described in supplemental Methods, available on the Blood Web site.
Western blot
Western blot analyses were performed as previously described.20 For a phosphorylation assay in the presence of FLT3 ligand (FL), wild-type-FLT3/FLT3-ITD–coexpressing 32D cells were preincubated with FLT3 inhibitors for 2 hours. After the pre-incubation, 10 ng/mL FL (R&D Systems, Inc., Minneapolis, MN) was added for 20 minutes. Phosphorylated and total FLT3 proteins were detected with anti-phospho FLT3 and total FLT3 antibodies, respectively (supplemental Methods).
Colony formation assays
Mononuclear cells were isolated from cord blood (CB) by gradient density centrifugation with the Ficoll-Paque Plus (GE Healthcare, Chicago, IL). Inhibitory effects of FLT3 inhibitors were evaluated as previously described.21
In vivo efficacy studies
For the mouse xenograft model using a leukemia cell line, MOLM-13 cells (3 × 106 cells/mouse) were inoculated into the tail vein of NOD/SCID (NOD.CB17-PrkdcSCID/ShiJic) mice (CLEA, Tokyo, Japan), which were pretreated with cyclophosphamide for 2 days and anti-Asialo GM1 antibody for 1 day before injection for immunosuppression. After oral administration of FF-10101 or quizartinib once daily for 8 days, BM cells from bilateral femurs were collected. The percentage of human CD45-positive viable cells among the BM cells was measured by flow cytometry after staining with anti-human CD45 antibody (BD Biosciences) and PI. Human CD45-positive cells were defined as residual leukemia in the femurs. PDX models were generated as previously reported.22 FF-10101 and quizartinib were orally administered. Peripheral blood (PB) was collected from the tail vein at several time points, and the percentage of AML cells was determined by flow cytometry. The percentage of AML cells in BM was determined following the same method using the MOLM-13-xenograft-mouse model. For mouse subcutaneous implantation models, refer to supplemental Methods. Animal experiments were performed in accordance with the code of Pharmaceutical and Healthcare Research Laboratories for the Care and Use of Laboratory Animals, FUJIFILM Corporation, and the guidelines of the Institute for Laboratory Animal Research, Nagoya University.
PK and PD studies
PK and pharmacodynamic (PD) studies were performed separately from the efficacy studies. MOLM-13–bearing NOD/SCID mice were prepared by the same method as the efficacy study. Single administration of FF-10101 was given to the mice at 15 days after inoculation of MOLM-13 cells. The mice were suggested to have ∼20% of MOLM-13 cells in the bone marrow (BM) because the mice were examined at a time point close to the end point of the efficacy study. Heparinized whole-blood and BM samples were collected at 2, 4, 8, and 16 hours after administration. In the AML-PDX model, mice were examined at 35 days after implantation of primary AML cells and had a mean rate of 98.7% of human CD45-positive cells in the BM. Heparinized whole-blood and BM samplings were performed at 4, 8, 10, 14, 18, and 24 hours after single oral administration. In mice that received repeated administration at 10 hours after the first administration at 10 mg/kg of FF-10101, blood samplings were performed at 10, 18, and 24 hours and BM sampling at 24 hours after the administration. Concentrations of FF-10101 in plasma and BM were determined by liquid chromatography/mass spectrometry/mass spectrometry (LC/MS/MS). Phosphorylated and total protein levels in BM were analyzed by western blot analysis.
Information on additional methods is described in supplemental Methods.
Results
Covalent binding of FF-10101 to the C695 residue of FLT3 contributes to increased and irreversible inhibition of kinase activity of FLT3
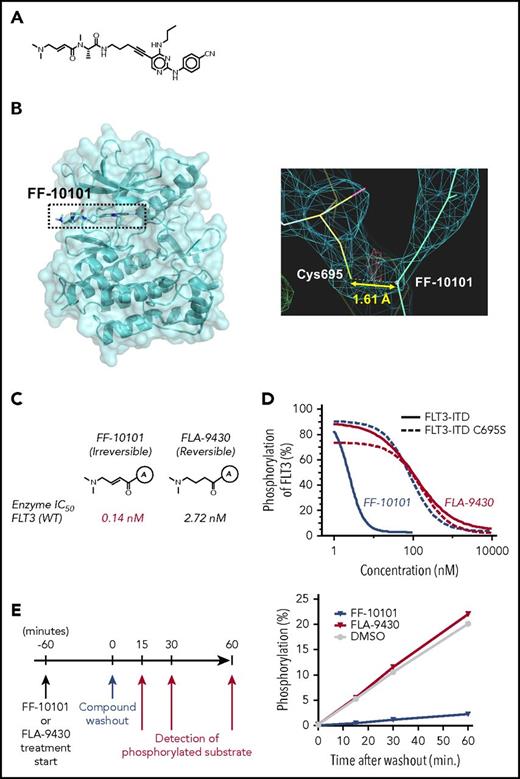
We designed FF-10101 to specifically form a covalent bond to the FLT3 C695 residue to enable it to possess high-inhibitory activity against FLT3 (Figure 1A). The covalent bond formation was revealed by a refined electron density map in X-ray analysis of co-crystal structure of FLT3 protein bound to FF-10101 (Figure 1B).
Chemical structure of FF-10101, and potent and irreversible inhibition of FLT3 by FF-10101 through binding interaction with cysteine residue at 695 (C695). (A) Chemical structure of FF-10101. (B) X-ray analysis of the co-crystal structure of the FLT3 protein bound to FF-10101 revealed covalent binding of FF-10101 to C695 of FLT3 molecule. (C) FF-10101 has a double bond to form a covalent bond to the C695 of the FLT3 molecule. FLA-9430 has the same chemical structure as FF-10101, with the exception of having a single bond instead of a double bond. Marked differences in inhibitory activities against the wild-type FLT3 enzyme between FF-10101 and FLA-9430 were shown, with IC50 values of 0.14 nM and 2.72 nM, respectively. (D) FLT3-ITD–expressing or FLT3-ITD-C695S–expressing HEK293T cells were incubated with various concentrations of FF-10101 or FLA-9430 at 37°C for 1 hour. Phosphorylation status of FLT3 was assessed as described in “Methods.” Percent inhibition of FLT3 phosphorylation at each concentration was plotted to calculate IC50 values using XLfit software. (E) FLT3 immobilized in a 96-well plate was preincubated with an excess concentration of FLT3 inhibitor (5 μM) for 1 hour followed by 5-time wash with TBST. After washing out theFLT3 inhibitors, ATP and substrate peptide labeled with FAM were added. Phosphorylated substrate was detected at each time point by a mobility shift assay.
Chemical structure of FF-10101, and potent and irreversible inhibition of FLT3 by FF-10101 through binding interaction with cysteine residue at 695 (C695). (A) Chemical structure of FF-10101. (B) X-ray analysis of the co-crystal structure of the FLT3 protein bound to FF-10101 revealed covalent binding of FF-10101 to C695 of FLT3 molecule. (C) FF-10101 has a double bond to form a covalent bond to the C695 of the FLT3 molecule. FLA-9430 has the same chemical structure as FF-10101, with the exception of having a single bond instead of a double bond. Marked differences in inhibitory activities against the wild-type FLT3 enzyme between FF-10101 and FLA-9430 were shown, with IC50 values of 0.14 nM and 2.72 nM, respectively. (D) FLT3-ITD–expressing or FLT3-ITD-C695S–expressing HEK293T cells were incubated with various concentrations of FF-10101 or FLA-9430 at 37°C for 1 hour. Phosphorylation status of FLT3 was assessed as described in “Methods.” Percent inhibition of FLT3 phosphorylation at each concentration was plotted to calculate IC50 values using XLfit software. (E) FLT3 immobilized in a 96-well plate was preincubated with an excess concentration of FLT3 inhibitor (5 μM) for 1 hour followed by 5-time wash with TBST. After washing out theFLT3 inhibitors, ATP and substrate peptide labeled with FAM were added. Phosphorylated substrate was detected at each time point by a mobility shift assay.
We compared inhibitory effects on FLT3 phosphorylation between FF-10101 and FLA-9430, the latter of which cannot form the covalent bond with FLT3, using HEK293T cells transiently expressing FLT3-ITD or FLT3-ITD-C695S. FF-10101 more potently inhibited the phosphorylation of FLT3-ITD than that of FLT3-ITD-C695S (IC50 values 2.4 nM and 79 nM, respectively). In contrast, FLA-9430 showed the same inhibitory effects on phosphorylation of FLT3-ITD and FLT3-ITD-C695S, and the potency was far less than FF-10101 (IC50 values of 99 nM and 88 nM, respectively) (Figure 1C-D). These results demonstrated that the covalent bond of FF-10101 to the C695 residue contributes to increased inhibition of FLT3 kinase activity. In addition, 60-minute treatment of FLT3 enzyme with FF-10101 followed by washout showed sustained inhibition of kinase activity, whereas FLA-9430 treatment failed to inhibit kinase activity after washout (Figure 1E). These results indicated that the covalent bond formation of FF-10101 induced irreversible inhibition of FLT3 for at least 60 minutes after washout.
FF-10101 potently and selectively inhibits the growth of mutant FLT3-expressing leukemia cells in vitro
In vitro kinase assay results revealed that FF-10101 potently inhibited kinase activities of wild-type FLT3 and FLT3-D835Y with IC50 values of 0.20 nM and 0.16 nM, respectively (Table 1). In addition, FF-10101 demonstrated high kinase selectivity to wild-type FLT3 with at least 30-fold margins against the other kinases except for FMS and KIT with IC50 values of 0.94 nM and 2.0 nM, respectively (Table 1; supplemental Table 1).
Kinase selectivity profile of FF-10101
| Kinase . | IC50, nM . | Margin of selectivity . |
|---|---|---|
| FLT3 | 0.20 | 1.0 |
| FLT3 (D835Y) | 0.16 | 0.80 |
| FMS | 0.94 | 4.7 |
| KIT | 2.0 | 10 |
| MINK | 6.8 | 34 |
| KHS1 | 9.1 | 46 |
| KIT (T670I) | 11 | 55 |
| FGR | 12 | 60 |
| IRAK1 | 13 | 65 |
| MELK | 16 | 80 |
| HGK | 17 | 85 |
| Kinase . | IC50, nM . | Margin of selectivity . |
|---|---|---|
| FLT3 | 0.20 | 1.0 |
| FLT3 (D835Y) | 0.16 | 0.80 |
| FMS | 0.94 | 4.7 |
| KIT | 2.0 | 10 |
| MINK | 6.8 | 34 |
| KHS1 | 9.1 | 46 |
| KIT (T670I) | 11 | 55 |
| FGR | 12 | 60 |
| IRAK1 | 13 | 65 |
| MELK | 16 | 80 |
| HGK | 17 | 85 |
Kinase inhibitory activity of FF-10101 was evaluated against 216 kinases. Each enzyme was incubated with FF-10101 dilutions in the presence of ATP at 28°C for 90 minutes. Percent inhibition of enzyme activity was determined, and IC50 values were calculated in accordance with the manufacturer’s protocol. Kinases with IC50 values of less than 20 nM are listed.
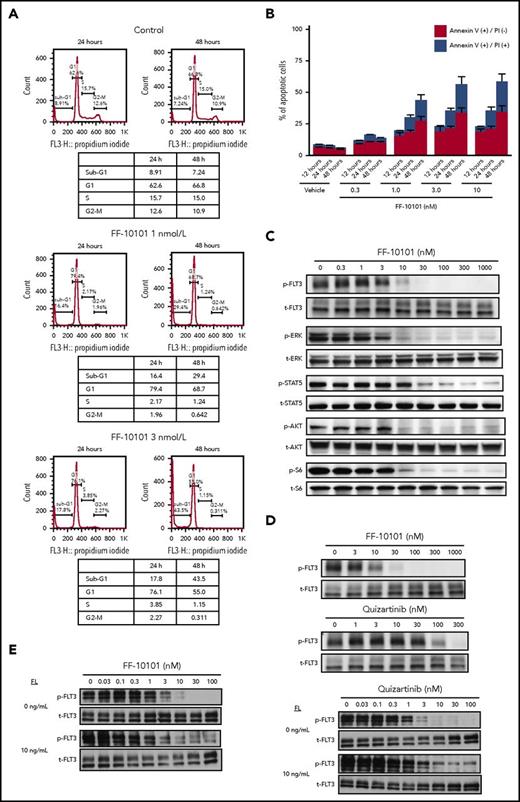
We evaluated the growth inhibitory effects of FF-10101 on human leukemia cell lines and FLT3-ITD–expressing 32D cells. FF-10101 potently inhibited growth of FLT3-ITD mutated cell lines MV4-11, MOLM-13, and MOLM-14, with GI50 values of 0.83, 1.1, and 1.5 nM, respectively. Although a weak growth inhibitory effect was observed in FLT3 nonmutated cell lines Kasumi-1 and EOL-1, their GI50 values were 26 nM and 72 nM, respectively. The growth inhibitory effect of FF-10101 on AML cells with FLT3-ITD was comparable to that of quizartinib (Table 2). Cell cycle analysis revealed that FF-10101 increased sub-G1 population in MV4-11 cells in a dose- and time-dependent manner (Figure 2A). Consistent with the increase of sub-G1 population, FF-10101 induced apoptosis to MV4-11 cells in a time- and dose-dependent manner (Figure 2B). FF-10101 inhibited phosphorylation of FLT3 and its downstream molecules ERK, STAT5, AKT, and S6 in a dose-dependent manner in MV4-11 cells (Figure 2C). These results confirmed the proof of concept that FF-10101 inhibits the growth of leukemia cells with FLT3-ITD through dephosphorylation of FLT3 followed by apoptosis. Of note is that FF-10101 possessed higher inhibitory effects on FLT3 phosphorylation even in 100% human plasma compared with quizartinib (Figure 2D). Furthermore, FF-10101 exerted high inhibitory activity against FLT3 phosphorylation in the presence of FL, although this activity was slightly reduced as compared with inhibitory activity in the absence of FL. Quizartinib showed similar or further reduced activity in the presence of FL (Figure 2E).
Cell growth-inhibitory profile of FF-10101 and quizartinib
| Cell lines . | GI50, nM . | |
|---|---|---|
| FF-10101 . | Quizartinib . | |
| MV4-11 | 0.83 | 0.95 |
| MOLM-13 | 1.1 | 1.6 |
| MOLM-14 | 1.5 | 2.4 |
| Kasumi-1 | 26 | 16 |
| EOL-1 | 72 | 0.46 |
| THP-1 | >1000 | >1000 |
| K562 | >1000 | >1000 |
| Cell lines . | GI50, nM . | |
|---|---|---|
| FF-10101 . | Quizartinib . | |
| MV4-11 | 0.83 | 0.95 |
| MOLM-13 | 1.1 | 1.6 |
| MOLM-14 | 1.5 | 2.4 |
| Kasumi-1 | 26 | 16 |
| EOL-1 | 72 | 0.46 |
| THP-1 | >1000 | >1000 |
| K562 | >1000 | >1000 |
Mean GI50 values from 3 independent experiments are indicated in the table. Leukemia cell lines were incubated with various concentrations of FLT3 inhibitors at 37°C for 2 d followed by determination of GI50 values.
Mode of action of FF-10101. (A-B) Cells were treated with FF-10101 for the indicated hours, and cell cycle and apoptosis analyses were performed using BD Cycletest Plus DNA Reagent Kit and TACS annexin V Kit, respectively. (C-D) Cells were treated with FF-10101 in culture medium (C) or 100% human plasma (D) for 1 hour. Phospho- and total-FLT3 were detected by western blot analysis after immunoprecipitation with total-FLT3 antibody. Whole-cell lysates were also subjected to western blot analysis, for detection of phosphorylation status of downstream molecules of FLT3. (E) wild-type FLT3/FLT3-ITD–coexpressing 32D cells were preincubated with FLT3 inhibitors for 2 hours. After preincubation, 10 ng/mL of FL was added and further incubated for 20 minutes. Whole-cell lysates were subjected to western blot analysis for detection of phosphorylated and total FLT3 proteins.
Mode of action of FF-10101. (A-B) Cells were treated with FF-10101 for the indicated hours, and cell cycle and apoptosis analyses were performed using BD Cycletest Plus DNA Reagent Kit and TACS annexin V Kit, respectively. (C-D) Cells were treated with FF-10101 in culture medium (C) or 100% human plasma (D) for 1 hour. Phospho- and total-FLT3 were detected by western blot analysis after immunoprecipitation with total-FLT3 antibody. Whole-cell lysates were also subjected to western blot analysis, for detection of phosphorylation status of downstream molecules of FLT3. (E) wild-type FLT3/FLT3-ITD–coexpressing 32D cells were preincubated with FLT3 inhibitors for 2 hours. After preincubation, 10 ng/mL of FL was added and further incubated for 20 minutes. Whole-cell lysates were subjected to western blot analysis for detection of phosphorylated and total FLT3 proteins.
FF-10101 inhibited phosphorylation of KIT in Kasumi-1 cells at comparable levels to quizartinib, indicating that the weak growth inhibition was brought on by KIT inhibition (supplemental Figure 1). In addition, FF-10101 inhibited proliferation of 32D cells expressing wild-type KIT with a GI50 value of 12 nM more weakly than quizartinib, with a GI50 value of 3.3 nM corroborated by inhibition of phosphorylation of KIT in the cells, whereas FF-10101 showed higher inhibitory activity on proliferation of 32D cells expressing KIT-D816V than quizartinib (supplemental Table 2; supplemental Figure 2).
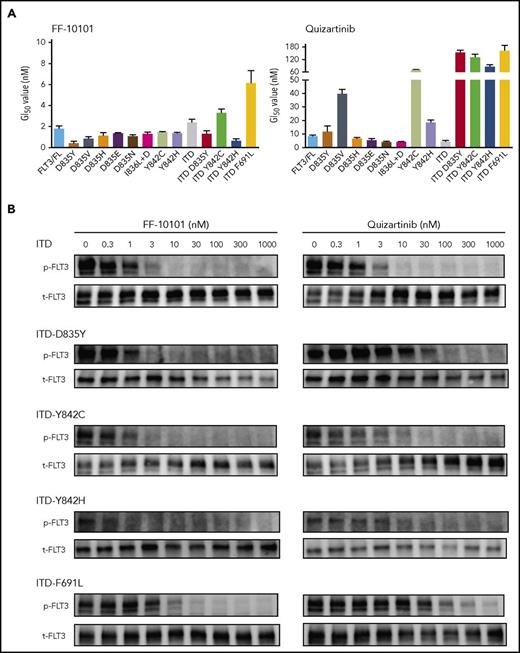
We next evaluated the inhibitory effects of FF-10101 on various types of FLT3-TKD mutations and FLT3-ITD with D835Y, Y842C, Y842H, or F691L mutation, some of which were clinically identified in quizartinib-resistant AML cells. FF-10101 showed growth inhibitory effects on all tested types of FLT3-TKD mutation- and FLT3-ITD with D835Y, Y842C, Y842H, or F691L mutation-expressing 32D cells, with GI50 values from 0.43 nM to 6.1 nM (Figure 3A). Consistently, FF-10101 potently inhibited FLT3 phosphorylation in quizartinib-resistant FLT3 mutants FLT3-ITD-D835Y, FLT3-ITD-Y842C, FLT3-ITD-Y842H, or FLT3-ITD-F691L–expressing 32D cells (Figure 3B). Furthermore, growth inhibitory effects of FF-10101 on FLT3-TKD mutations were substantially higher than those of midostaurin and crenolanib (Table 3). These results indicated that FF-10101 is a potent and selective FLT3 inhibitor that is invulnerable to various FLT3 mutations including quizartinib-resistant mutations.12,18
Inhibitory effect of FF-10101 on drug-resistant FLT3 mutations. (A) 32D transfectants stably expressing wild-type FLT3 or mutated FLT3s were treated with FF-10101 or quizartinib for 2 days. After treatment, cell viability was assessed by MTS assay. Three independent experiments were performed, and mean ± standard error of IC50 values of cell viability for each cell lines are shown in this graph. (B) 32D transfectants were treated with FF-10101 or quizartinib for 2 hours. Cell lysates were prepared and subjected to western blot analysis to assess the inhibitory effect of FF-10101 on phosphorylation of mutant FLT3.
Inhibitory effect of FF-10101 on drug-resistant FLT3 mutations. (A) 32D transfectants stably expressing wild-type FLT3 or mutated FLT3s were treated with FF-10101 or quizartinib for 2 days. After treatment, cell viability was assessed by MTS assay. Three independent experiments were performed, and mean ± standard error of IC50 values of cell viability for each cell lines are shown in this graph. (B) 32D transfectants were treated with FF-10101 or quizartinib for 2 hours. Cell lysates were prepared and subjected to western blot analysis to assess the inhibitory effect of FF-10101 on phosphorylation of mutant FLT3.
Cell growth-inhibitory profile of FF-10101 and other FLT3 inhibitors
| 32D cell lines . | GI50, nM . | |||
|---|---|---|---|---|
| FF-10101 . | Quizartinib . | Crenolanib . | Midostaurin . | |
| FLT3-ITD | 1.9 | 2.0 | 32 | 32 |
| FLT3-ITD-D835Y | 0.81 | 89 | 44 | 16 |
| FLT3-ITD-Y842C | 3.5 | 66 | 12 | 35 |
| FLT3-ITD-Y842H | 5.3 | 92 | 11 | 35 |
| FLT3-ITD-F691L | 10 | 220 | 290 | 35 |
| FLT3-D835Y | 0.32 | 5.7 | 2.8 | 2.9 |
| FLT3-D835H | 1.1 | 4.1 | 16 | 8.7 |
| Parent/IL-3 | 2300 | 2000 | 1200 | 270 |
| 32D cell lines . | GI50, nM . | |||
|---|---|---|---|---|
| FF-10101 . | Quizartinib . | Crenolanib . | Midostaurin . | |
| FLT3-ITD | 1.9 | 2.0 | 32 | 32 |
| FLT3-ITD-D835Y | 0.81 | 89 | 44 | 16 |
| FLT3-ITD-Y842C | 3.5 | 66 | 12 | 35 |
| FLT3-ITD-Y842H | 5.3 | 92 | 11 | 35 |
| FLT3-ITD-F691L | 10 | 220 | 290 | 35 |
| FLT3-D835Y | 0.32 | 5.7 | 2.8 | 2.9 |
| FLT3-D835H | 1.1 | 4.1 | 16 | 8.7 |
| Parent/IL-3 | 2300 | 2000 | 1200 | 270 |
Mean GI50 values from 2 or more independent experiments are indicated in the table. 32D-transfectant cell lines were incubated with various concentrations of FLT3 inhibitors at 37°C for 2 d followed by determination of GI50 values.
In vivo efficacy of FF-10101
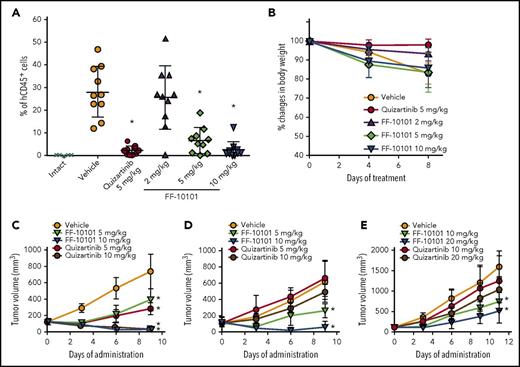
We intravenously inoculated MOLM-13 cells into NOD/SCID mice. Oral administration of FF-10101 significantly decreased the percentage of human CD45-positive cells in BM, compared with the vehicle administration group (mean number of human CD45-positive cells was 6.7% and 2.7% at 5 and 10 mg/kg, respectively, in the FF-10101 administration group vs 28.4% in the vehicle administration group) (Figure 4A). The anti-leukemic effect was the same as that of 5 mg/kg of quizartinib (2.4% in the quizartinib administration group). No significant difference was observed in body weight changes among the groups during 8 days of administration (Figure 4B). To evaluate the in vivo effect of FF-10101 on quizartinib-resistant FLT3 mutations, we subcutaneously inoculated FLT3-ITD–, FLT3-ITD-D835Y–, and FLT3-ITD-F691L–expressing 32D cells into NOD/SCID mice. Oral administration of FF-10101 showed the same inhibitory effects on the tumor with FLT3-ITD as quizartinib (Figure 4C). However, FF-10101 more potently inhibited the growth of tumors with FLT3-ITD-D835Y and FLT3-ITD-F691L than quizartinib, although the inhibitory effects against FLT3-ITD-F691L were moderate (Figure 4D-E).
Anti-leukemic effects of FF-10101 in in vivo models. (A-B) MOLM-13 cells were injected into the tail vein of NOD/SCID mice. Mice were divided into 5 groups (n = 10/group) at 8 days after the injection. FF-10101 (2, 5, and 10 mg/kg) or quizartinib (5 mg/kg) was orally administered to mice once daily for 8 days. Mice were weighed at days 0, 4, and 8, and relative body weight to initial body weight (day 0) was represented as percent changes. At day 8, mice were euthanized and the percentage of human CD45-positive cells in the BM was determined by flow cytometry. The percentage of human CD45-positive cells in each mouse was plotted (panel A). No significant differences in body weight among groups were observed (panel B). Bars show mean ± standard deviation (SD). *P < .05 compared with control by Dunnett's Multiple Comparison Test. (C-E) 32D cells stably expressing FLT3-ITD, FLT3-ITD-D835Y, or FLT3-ITD-F691L were subcutaneously implanted into SCID mice. Mice were randomly divided into 5 groups (n = 5/group) when mean subcutaneous tumor volume reached 100 to 300 mm3. FF-10101 and quizartinib were orally administered at 5 and 10 mg/kg daily for 9 days for FLT3-ITD (panel C) and FLT3-ITD-D835Y (panel D), and at 10 and 20 mg/kg daily for 11 days for FLT3-ITD-F691L (panel E). Tumor volume was measured twice per week. No differences in body weight among groups were observed during the studies (data not shown). Data of tumor volume are represented as mean ± SD. *P < .05 compared with control by Dunnett's Multiple Comparison Test.
Anti-leukemic effects of FF-10101 in in vivo models. (A-B) MOLM-13 cells were injected into the tail vein of NOD/SCID mice. Mice were divided into 5 groups (n = 10/group) at 8 days after the injection. FF-10101 (2, 5, and 10 mg/kg) or quizartinib (5 mg/kg) was orally administered to mice once daily for 8 days. Mice were weighed at days 0, 4, and 8, and relative body weight to initial body weight (day 0) was represented as percent changes. At day 8, mice were euthanized and the percentage of human CD45-positive cells in the BM was determined by flow cytometry. The percentage of human CD45-positive cells in each mouse was plotted (panel A). No significant differences in body weight among groups were observed (panel B). Bars show mean ± standard deviation (SD). *P < .05 compared with control by Dunnett's Multiple Comparison Test. (C-E) 32D cells stably expressing FLT3-ITD, FLT3-ITD-D835Y, or FLT3-ITD-F691L were subcutaneously implanted into SCID mice. Mice were randomly divided into 5 groups (n = 5/group) when mean subcutaneous tumor volume reached 100 to 300 mm3. FF-10101 and quizartinib were orally administered at 5 and 10 mg/kg daily for 9 days for FLT3-ITD (panel C) and FLT3-ITD-D835Y (panel D), and at 10 and 20 mg/kg daily for 11 days for FLT3-ITD-F691L (panel E). Tumor volume was measured twice per week. No differences in body weight among groups were observed during the studies (data not shown). Data of tumor volume are represented as mean ± SD. *P < .05 compared with control by Dunnett's Multiple Comparison Test.
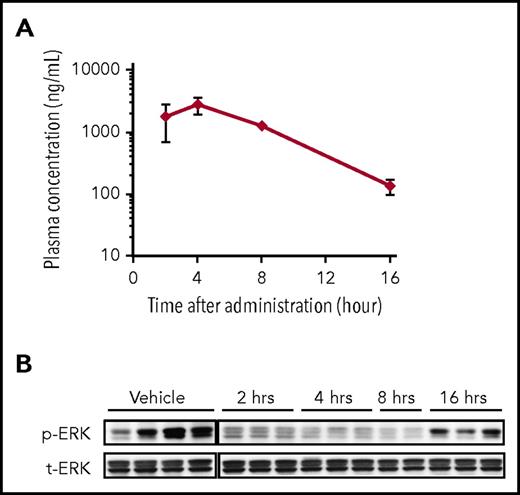
We performed a PK/PD study in the MOLM-13 xenograft model separately from the efficacy study (Figure 5A-B). Single oral administration of FF-10101 at 5 mg/kg was given to mice at 15 days after intravenous injection of MOLM-13 cells. Plasma concentration of FF-10101 time-dependently increased and reached a maximum concentration at 4 hours after administration (Figure 5A). In association with a higher plasma concentration of FF-10101 (≥1000 ng/mL) at 2, 4, and 8 hours, phosphorylation of ERK in BM was clearly inhibited (Figure 5B).
PK/PD study in the MOLM-13 xenograft model. (A-B) PK/PD study was conducted in the MOLM-13 xenograft model separately from the efficacy study. Single oral administration of FF-10101 at 5 mg/kg was given to mice at 15 days after intravenous injection of MOLM-13 cells. Blood and BM were sampled at 2, 4, 8, and 16 hours after administration (n = 3/each time point). Four mice were administered as the vehicle administration group and were euthanized after 16 hours. Plasma concentrations of FF-10101 at each time point were analyzed by LC/MS/MS (panel A). ERK phosphorylation levels in BM were analyzed by western blotting (panel B). Bars show mean ± SD.
PK/PD study in the MOLM-13 xenograft model. (A-B) PK/PD study was conducted in the MOLM-13 xenograft model separately from the efficacy study. Single oral administration of FF-10101 at 5 mg/kg was given to mice at 15 days after intravenous injection of MOLM-13 cells. Blood and BM were sampled at 2, 4, 8, and 16 hours after administration (n = 3/each time point). Four mice were administered as the vehicle administration group and were euthanized after 16 hours. Plasma concentrations of FF-10101 at each time point were analyzed by LC/MS/MS (panel A). ERK phosphorylation levels in BM were analyzed by western blotting (panel B). Bars show mean ± SD.
Efficacy of FF-10101 against primary AML cells
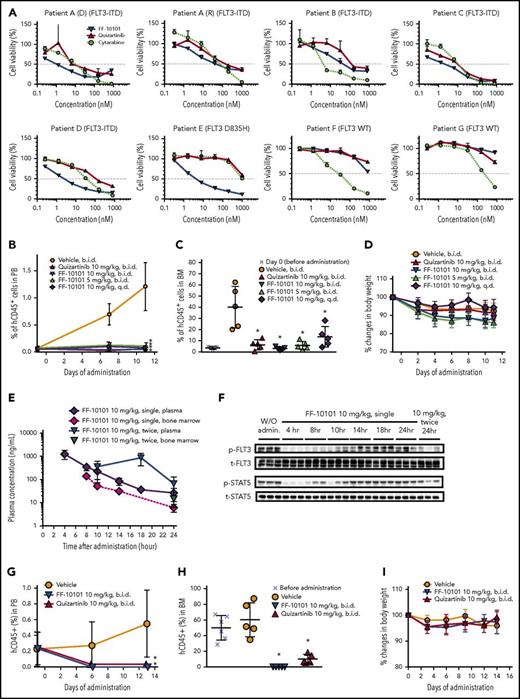
We evaluated the anti-leukemia effects of FF-10101 on 6 primary AML cells with FLT3 mutations and 2 with wild-type FLT3 in vitro. In all tested primary AML cells with FLT3 mutations, FF-10101 potently and dose-dependently reduced cell viability at comparable levels to quizartinib irrespective of the allelic ratio of FLT3-ITD to wild-type FLT3 (Figure 6A, patient A-D; supplemental Table 3). It is notable that FF-10101 demonstrated a potent inhibitory effect on primary AML cells with FLT3-D835H, on which quizartinib and cytarabine showed little inhibitory effects (Figure 6A, patient E). In addition, FF-10101 retained the inhibitory activity against paired newly diagnosed and relapsed AML cells, although the relapsed cells had a complex karyotype (Figure 6A, patient A (D) and (R); supplemental Table 3). Potent inhibitory activity of FF-10101 was also verified in relapsed AML cells (Figure 6A, patient C). In contrast, inhibitory effects of FF-10101 were low on AML cells with wild-type FLT3 (Figure 6A, patient F-G).
Anti-leukemic effects of FF-10101 against primary AML cells in vitro and in vivo. (A) Primary AML cells were incubated with FLT3 inhibitors for 1 week. Cell viability (%) assessed with CellTiter-Glo reagent was plotted at each concentration. Data are represented as mean ± SD. Paired samples at diagnosis (D) and relapse (R) were analyzed for patient A. Samples of patients B, D, F, and G were obtained at diagnosis, and those of patient C and patient E were in relapse. (B-D) Primary AML cells with FLT3-ITD were inoculated into NOG mice via the tail vein. Mice were divided into 6 groups (n = 5/group) based on the percentage of human CD45-positive cells in PB. Five mice were euthanized 27 days after the implantation (day 0) for assessment of engrafted cells in BM at the time of initiation of drug administration. FF-10101 was orally administered at 5 mg/kg twice daily, 10 mg/kg once daily, and 10 mg/kg twice daily, and quizartinib at 10 mg/kg twice daily, for 11 days. PB was sampled at the indicated time points for monitoring the percentage of human CD45-positive cells (panel B). After 11-day treatment, BM was collected from bilateral femurs for determination of the percentage of human CD45-positive cells (panel C). Mice were weighed at days −1, 2, 4, 6, 8, 10, and 11, and relative body weight to initial body weight (day −1) was represented as percent changes. No significant differences in body weight among groups were observed (panel D). Bars show mean ± SD. (E-F) PK/PD study was conducted in the xenograft model with the primary AML cells shown in Figure 6B-D. Single or repeated oral administration of FF-10101 at 10 mg/kg was given to mice with excess blasts at 35 days after intravenous injection of primary AML cells. Plasma and BM concentrations of FF-10101 at indicated time points were analyzed by LC/MS/MS (panel E). BM lysates were subjected to western blotting for detection of FLT3 (t-FLT3), STAT5 (t-STAT5), and their phosphorylated forms (p-FLT3 and p-STAT5) (panel F). (G-I) Primary AML cells with FLT3-D835H mutation were xenografted into NOG mice. Mice were divided into 4 groups (n = 5 or 6/group) based on the percentage of human CD45-positive cells in PB. Six mice were euthanized for identification of engrafted cells in BM before initiation of administration. FF-10101 and quizartinib were orally administered at 10 mg/kg twice daily for 14 days. The percentage of human CD45-positive cells in PB was monitored once per week (panel G). After 14-day administration, the percentage of human CD45-positive cells in BM was determined by flow cytometry (panel H). Mice were weighed at days 0, 3, 6, 9, 12, and 14, and relative body weight to initial body weight (day 0) was represented as percent changes. No significant differences in body weight among groups were observed (panel I). Bars show mean ± SD. *P < .05 compared with control by Dunnett's Multiple Comparison Test.
Anti-leukemic effects of FF-10101 against primary AML cells in vitro and in vivo. (A) Primary AML cells were incubated with FLT3 inhibitors for 1 week. Cell viability (%) assessed with CellTiter-Glo reagent was plotted at each concentration. Data are represented as mean ± SD. Paired samples at diagnosis (D) and relapse (R) were analyzed for patient A. Samples of patients B, D, F, and G were obtained at diagnosis, and those of patient C and patient E were in relapse. (B-D) Primary AML cells with FLT3-ITD were inoculated into NOG mice via the tail vein. Mice were divided into 6 groups (n = 5/group) based on the percentage of human CD45-positive cells in PB. Five mice were euthanized 27 days after the implantation (day 0) for assessment of engrafted cells in BM at the time of initiation of drug administration. FF-10101 was orally administered at 5 mg/kg twice daily, 10 mg/kg once daily, and 10 mg/kg twice daily, and quizartinib at 10 mg/kg twice daily, for 11 days. PB was sampled at the indicated time points for monitoring the percentage of human CD45-positive cells (panel B). After 11-day treatment, BM was collected from bilateral femurs for determination of the percentage of human CD45-positive cells (panel C). Mice were weighed at days −1, 2, 4, 6, 8, 10, and 11, and relative body weight to initial body weight (day −1) was represented as percent changes. No significant differences in body weight among groups were observed (panel D). Bars show mean ± SD. (E-F) PK/PD study was conducted in the xenograft model with the primary AML cells shown in Figure 6B-D. Single or repeated oral administration of FF-10101 at 10 mg/kg was given to mice with excess blasts at 35 days after intravenous injection of primary AML cells. Plasma and BM concentrations of FF-10101 at indicated time points were analyzed by LC/MS/MS (panel E). BM lysates were subjected to western blotting for detection of FLT3 (t-FLT3), STAT5 (t-STAT5), and their phosphorylated forms (p-FLT3 and p-STAT5) (panel F). (G-I) Primary AML cells with FLT3-D835H mutation were xenografted into NOG mice. Mice were divided into 4 groups (n = 5 or 6/group) based on the percentage of human CD45-positive cells in PB. Six mice were euthanized for identification of engrafted cells in BM before initiation of administration. FF-10101 and quizartinib were orally administered at 10 mg/kg twice daily for 14 days. The percentage of human CD45-positive cells in PB was monitored once per week (panel G). After 14-day administration, the percentage of human CD45-positive cells in BM was determined by flow cytometry (panel H). Mice were weighed at days 0, 3, 6, 9, 12, and 14, and relative body weight to initial body weight (day 0) was represented as percent changes. No significant differences in body weight among groups were observed (panel I). Bars show mean ± SD. *P < .05 compared with control by Dunnett's Multiple Comparison Test.
We evaluated the in vivo efficacy of FF-10101 in the PDX models of AML cells with FLT3 mutations. Primary AML cells were intravenously inoculated into NOG mice, and drug administration was started when AML cells were detectable as human CD45-positive cells in mouse PB. Oral administration of FF-10101 showed significant anti-leukemic effects at 5 mg/kg and 10 mg/kg twice daily for 11 days in the PDX model of AML cells with FLT3-ITD (Figure 6B-D). On the last day of treatment, the mean percentage of AML cells in PB was 0.100% and 0.050% in the 5- and 10-mg/kg FF-10101 administration groups, respectively, and 1.21% in the vehicle treatment group (Figure 6B). In BM, 5.74% and 3.12% of AML cells were detected in the 5- and 10-mg/kg FF-10101 administration groups, respectively, and 40.0% in the vehicle control group (Figure 6C; supplemental Figure 3). The inhibitory effect of FF-10101 on primary AML cells with FLT3-ITD was the same as that of quizartinib in this model. No significant difference was observed in body weight changes among the groups during 11 days of administration (Figure 6D). We conducted a PK/PD study in the same model separately from the efficacy study (Figure 6E-F). Single oral administration of 10 mg/kg of FF-10101 inhibited phosphorylation of FLT3 and STAT5 in AML cells engrafted in BM for 8 to 10 hours, consistent with the results in Figure 5B. Although the inhibitory effect of FF-10101 on FLT3 phosphorylation was observed for 14 hours after single administration, 10 mg/kg of FF-10101 administered twice a day successfully inhibited phosphorylation of FLT3 in AML cells for 24 hours after first administration. This result indicated that oral administration of FF-10101 twice daily was required for continuous inhibition of FLT3 in the mouse model. The anti-leukemic effect of FF-10101 was further evaluated in the PDX model of AML cells with FLT3-D835H (Figure 6G-I). Before drug administration, the mean percentage of AML cells in PB and BM was 0.449% and 50.0%, respectively. Oral administration of FF-10101 at 10 mg/kg twice daily for 2 weeks significantly reduced AML cells in PB (0%) (Figure 6G) and BM (0.116%) (Figure 6H; supplemental Figure 4). In contrast, weaker but significant reduction of AML cells was observed in the quizartinib administration group (mean number of human CD45-positive cells: 0% in PB and 10.0% in BM) (Figure 6G-H), although the apparent lack of inhibitory activity against the same AML cells was observed (Figure 6A). The inhibitory effect of quizartinib might be brought on by AC886, an active metabolite of quizartinib, which is reported to have a similar kinase selectivity profile and be as potent as quizartinib.23 No significant difference was observed in body weight changes among the groups during 14 days of administration (Figure 6I).
Influence of FF-10101 on normal hematopoiesis
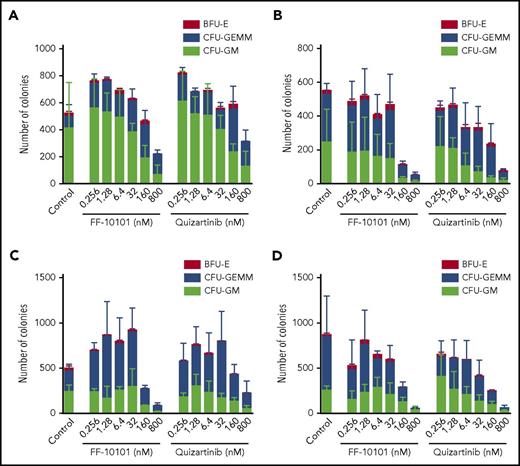
We finally evaluated the effect of FF-10101 on normal hematopoiesis using human CB mononuclear cells. Mononuclear cells were isolated from 4 independent CB samples and incubated with increasing concentrations of FLT3 inhibitors for 10 or 14 days. Treatment with FF-10101 at 160 nM markedly reduced the number of colonies by ∼50% or more in 3 of 4 CB samples. The inhibitory effects were comparable to or a little more potent than those of quizartinib (Figure 7A-D). The distributions of BFU-E, CFU-GEMM, and CFU-GM colonies were not largely affected by treatment with FF-10101.
Inhibitory effects of FF-10101 on colony formation of human mononuclear cells. (A-D) Mononuclear cells isolated from 4 independent CB samples were placed in methylcellulose semisolid medium with increasing concentrations of FLT3 inhibitors (n = 3) and incubated at 37°C for 10 (panel A-B) or 14 days (panel C-D). After incubation, CFU-GM, CFU-GEMM, and BFU-E colonies were manually counted. Mean colony numbers ± SD in each colony are shown.
Inhibitory effects of FF-10101 on colony formation of human mononuclear cells. (A-D) Mononuclear cells isolated from 4 independent CB samples were placed in methylcellulose semisolid medium with increasing concentrations of FLT3 inhibitors (n = 3) and incubated at 37°C for 10 (panel A-B) or 14 days (panel C-D). After incubation, CFU-GM, CFU-GEMM, and BFU-E colonies were manually counted. Mean colony numbers ± SD in each colony are shown.
Discussion
Although many FLT3 inhibitors have been developed, several problems, such as poor PK properties, toxicities, and resistance, have been apparent in clinical trials.10-12 Recently, quizartinib achieved better clinical responses than the first-generation FLT3 inhibitors in monotherapy, which indicates that selective and sustained inhibition of FLT3 could eliminate AML cells with FLT3 mutations.16,23,24 However, acquired clinical resistance to quizartinib has been an emerging issue in treatment, requiring development of another FLT3 inhibitor that can conquer these resistant mutations.18
FLT3 inhibitors are generally classified into type I and type II inhibitors, according to the potency against active and inactive conformations of FLT3.25,26 Many type II inhibitors, including quizartinib and sorafenib, are designed to favorably bind to the back pocket of the inactive conformation of FLT3, defined as Asp-Phe-Gly (DFG)-out to increase the affinity to the ATP binding site of FLT3.18,27 However, such inhibitors show low activity against various mutations at the D835 and Y842 residues because those mutations induce the conformation of FLT3 to the active form, resulting in loss of affinity of the inhibitors to FLT3.28,29 In contrast, type I inhibitors, including midostaurin and crenolanib, can bind to active conformation of FLT3 caused by D835 or Y842 mutations.30,31 However, many type I inhibitors have more broad kinase inhibitory activities than type II inhibitors because of the similarity of binding sites among kinases, which results in the cause of off-target effects.12
To conquer these problems, we designed FF-10101 to form a covalent binding between the C695 residue of the FLT3 molecule and an acryloyl group in the chemical structure. In fact, the covalent binding was confirmed by the co-crystal structure of the FLT3 protein bound to FF-10101. We demonstrated that the C695 residue plays an important role in the potent and selective inhibitory activity of FF-10101 compared with FLA-9430, which is incapable of covalent binding. FF-10101 has a high selectivity to wild-type FLT3 and FLT3-D835Y, with at least a 30-fold higher activity than those of the other tested 212 kinases except for KIT and FMS, which belong to the same type III receptor kinase family as FLT3 and PDGFR32 and conserve amino acid sequence identical to that around the cysteine residue 695 of FLT3. It is important to note that FF-10101 possesses the ability to bind not only to inactive conformation verified by the co-crystal structure (data not shown), but also to active conformation as corroborated by inhibition of activation loop mutations including FLT3-D835Y. The unique binding of FF-10101 provides broad inhibitory effects on various FLT3 mutations, including the secondary mutations (at D835 and F691), conferring clinical resistance to quizartinib; the potency is highest among the developed FLT3 inhibitors, including midostaurin and crenolanib.33,34 The strong potency of FF-10101 against various FLT3 mutations prompts us to highly expect better clinical efficacy in patients with AML with FLT3 mutations than those of previously developed FLT3 inhibitors.
Of further interest, the effect of FF-10101 at 5 mg/kg twice daily was more potent than that at 10 mg/kg once daily with the same daily dose in the PDX model of AML cells with FLT3-ITD. In this model, the PD analysis of FF-10101 revealed that repeated administration at 10 hours after the first dose led to sustained inhibition of FLT3 phosphorylation for 24 hours, although single administration failed to achieve the sustained inhibition. These results suggested that higher effects at 5 and 10 mg/kg twice daily vs 10 mg/kg once daily were brought by sustained inhibition of FLT3 phosphorylation by repeated administration. Because the sustained and marked inhibition of FLT3 phosphorylation is associated with high overall response in clinical practice, in a phase 1 study we intend to determine the dosing schedule of FF-10101 that achieves the sustained and marked inhibition of FLT3 phosphorylation.23 On the other hand, such a dosing schedule of FF-10101 might induce myelosuppression leading to CRi, based on the results that FF-10101 inhibited colony formation of human mononuclear cells at comparable levels to or more potently than quizartinib, albeit with margins of 10-fold or more between the inhibitory effects against primary AML cells and human mononuclear cells.
It has been reported that the C481S mutation in BTK, which induces resistance to ibrutinib, has been identified in patients who experienced relapse after ibrutinib treatment.35 We also observed that FF-10101 has little potency against FLT3-ITD-C695S. Although mutation at the C695 residue in FLT3 has not been identified in any patients, even after treatment with FLT3 inhibitors, we should carefully monitor the emergence of this mutation during treatment with FF-10101.
In conclusion, we confirmed that FF-10101 has excellent anti-leukemia activity in AML cells with various FLT3 mutations both in vitro and in PDX models. We are presently conducting a phase 1 study to evaluate the safety and efficacy of FF-10101.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kanako Sugiyama and Sam-Yong Park of Yokohama City University for production of the FLT3 protein, and Manami Kira and Satomi Yamaji for their secretarial and technical assistance. The authors also express their sincere appreciation for experimental and administrative support from the Division of Experimental Animals, Nagoya University Graduate School of Medicine, and the Division of Medical Research Engineering, Nagoya University Graduate School of Medicine.
This work was supported in part by Grants-in-Aid from the Newly Extended Technology Transfer Program of the Japan Science and Technology Agency; the Scientific Research Program of the Ministry of Education, Culture, Sports, Science and Technology of Japan; and Nagoya University Hospital Funding for Clinical Research.
Authorship
Contribution: T.Y. designed experiments, performed animal studies, analyzed kinase selectivity, and wrote the manuscript; T. Nakatani designed experiments, performed cell proliferation assays and animal studies, and wrote the manuscript; K.U. performed western blot, cell cycle analysis, cell proliferation assay, and animal studies; H.O. performed western blot, cell proliferation assay, and animal studies; W.S. performed cell phosphorylation assay; N.K. performed cell proliferation and cell phosphorylation assays; K.S. performed apoptosis assay; N.F. performed crystallization and X-ray analysis of the co-crystal structure; T.D. performed cell proliferation assay; M.T., D.T., and A.H. designed and synthesized FF-10101 and FLA-9430 compounds; A.A. performed genetic analysis in clinical samples and established PDX mouse model; F.C. prepared mutant FLT3 complementary DNA and established mutant FLT3-expressing cells; Y.A. performed genetic analysis in clinical samples; Y.I. designed experiments, collected clinical samples and clinical data, and generated figures; F.H. provided input on experiments; S.H. designed experiments and provided input on experiments; T. Naoe designed experiments and provided input on experiments; and H.K. designed experiments, collected clinical samples, and edited the manuscript.
Conflict-of-interest disclosure: T.Y., T. Nakatani, K.U., H.O., W.S., N.K., K.S., N.F., T.D., M.T., D.T., A.H., and S.H. are employees of the FUJIFILM Corporation. T. Naoe received research funding from Sumitomo Dainippon Pharma Co., Ltd., FUJIFILM, Otsuka Pharmaceutical Co., Ltd., Pfizer Inc., and Toyama Chemical Co., Ltd.; patent royalties/licensing fees from Chugai Pharmaceutical Co., Ltd., FUJIFILM, and Kyowa Hakko Kirin Co., Ltd.; and honoraria from Amgen Astellas BioPharma K.K., Astellas Pharma Inc., Alexion Pharmaceuticals, Inc., Bristol-Myers Squibb, Daiichi Sankyo, Eisai Co., Ltd., Kyowa Hakko Kirin Co., Ltd., Meiji Holdings Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Otsuka Pharmaceutical Co., Ltd., and Sysmex Corporation. H.K. received research funding from Chugai Pharmaceutical Co., Ltd., Bristol-Myers Squibb, Kyowa Hakko Kirin Co., Ltd., Zenyaku Kogyo Co., Ltd., FUJIFILM, Nippon Boehringer Ingelheim Co., Ltd., Astellas Pharma Inc., and Celgene Corporation; consulting fees from Astellas Pharma Inc. and Daiichi Sankyo Co. Ltd.; and honoraria from Bristol-Myers Squibb and Pfizer Inc. The remaining authors declare no competing financial interests.
Correspondence: Takeshi Yamaura, Pharmaceutical and Healthcare Research Laboratories, FUJIFILM Corporation, 577 Ushijima, Kaisei-machi, Ashigarakami-gun, Kanagawa 258-8577, Japan; e-mail: takeshi.yamaura@fujifilm.com; and Hitoshi Kiyoi, Department of Hematology and Oncology, Nagoya University Graduate School of Medicine, Tsurumai-cho 65, Showa-ku, Nagoya 466-8550, Japan; e-mail: kiyoi@med.nagoya-u.ac.jp.