Key Points
Hh signaling has been selectively extinguished in the mouse osteoblastoid lineage.
Removal of Smo from osteoblasts results in a profound B-lymphopoietic defect.
Abstract
The stromal signals that promote B lymphopoiesis remain poorly understood. Hedgehog (Hh) signaling promotes B lymphopoiesis in a non–cell-autonomous fashion in vitro, and depletion of the Hh effector Smoothened (Smo) from stromal cells is associated with the loss of osteoblastoid markers. These observations suggested that Hh signaling in the osteoblastoid lineage promotes B lymphopoiesis in vivo. To test this, we employed a mouse model for conditional ablation of Smo in the osteoblastoid lineage. Depletion of Smo from osteoblastoid cells is associated with profound and selective reductions in the number and proportion of bone marrow B-lymphoid progenitors. Upon partial bone marrow ablation, mutant animals exhibit delayed repopulation of the B-lymphoid compartment after the early lymphoid progenitor stage. Primary osteoblasts from mutant mice are defective in supporting B lymphopoiesis in vitro, whereas hematopoietic progenitors from mutant mice exhibit normal differentiation. We conclude that efficient B lymphopoiesis in vivo is dependent on the maintenance of Hh signaling in the osteoblastoid lineage.
Introduction
The morphogen Hedgehog (Hh) plays critical roles in development and stem cell maintenance.1 Hh signaling proceeds through the essential activator Smoothened (Smo), which in the absence of ligand is inhibited by the receptor Patched (Ptch); the binding of ligand relieves inhibition of Smo, which in turn stimulates downstream transcription.2 Hh signaling has been implicated in the expansion3 and differentiation4,5 of hematopoietic stem cells, but cell-autonomous Hh signaling is not required for B lymphopoiesis in the mouse.6-8 We have shown that extinction of Hh signaling in stromal cells impairs their ability to support B lymphopoiesis from hematopoietic stem progenitor cells in vitro. Moreover, depletion of Smo from stromal cells is associated with downregulation of markers associated with osteoblastoid identity.6 Taken together, these observations prompted the hypothesis that B lymphopoiesis in the bone marrow (BM) is promoted by Hh signaling in stromal osteoblasts (OBs). We have now tested this by employing a mouse model in which Smo is selectively removed from the osteoblastoid lineage.
Methods
Animals
The transgenic mouse strain B6.Cg-Tg(Sp7-tTa, tetO-EGFP/cre)1Amc/J, hereafter termed Osx1-GFP::Cre, was obtained from The Jackson Laboratory (Bar Harbor, ME). Osx1-GFP::Cre+/0, Smofl/fl and Osx1-GFP::Cre+/0, Smo+/+ mice were obtained by interbreeding Osx1-GFP::Cre+/0 and Smofl/+ mice (gift of N. Watkins, Garvan Institute). C57BL/6 mice bearing a GFP cassette within the Rag1 locus were gifts of K. Medina (Mayo Medical School, Rochester, MN). All mouse lines were constructed on a C57BL/6 background. For partial BM ablation, mice were treated with 5-fluorouracil (5-FU; APP Pharmaceuticals) at 150 mg/kg by intraperitoneal injection. Mice were housed in accordance with policies of the Johns Hopkins Animal Care and Use Committee.
Flow cytometry
All antibodies were from BD Biosciences. Data were collected using an LSRII flow cytometer (BD Biosciences) and analyzed with FlowJo v10.1 software (Tree Star).
Hematopoietic stem progenitor cell differentiation
Isolation of primary OBs
Bone cells were isolated from 6- to 10-week-old mice as described previously,10 except that the final incubation was in medium containing 20% fetal bovine serum, 50 µg/mL ascorbic acid, and 10 mM β-glycerophosphate. OB differentiation was induced as described elsewhere.11 For immunofluorescence analysis, cells were fixed in methanol, incubated with 1.5% blocking serum in phosphate-buffered saline for 1 hour, and then with anti-mouse osteocalcin antibody (Takara) overnight at 4°C. Primary antibody was detected with a phycoerythrin-conjugated secondary antibody.
Results and discussion
Disruption of Hh signaling in osteoblastoid cells specifically impairs B-cell development
To test the prediction that Hh signaling in OBs promotes B lymphopoiesis, we deleted Smo by Cre-mediated excision in the mouse osteoblastoid lineage (Figure 1A; supplemental Figure 1A-C, available on the Blood Web site). In these animals, Cre was expressed from the osteoblastoid-specific Osx1 promoter.12 OB cells prepared from Osx1-Cre, Smofl/fl (SmoOb-ko) mice showed homogeneous staining for osteocalcin, a specific OB marker, and the identity of these cells was confirmed by flow cytometry (Figure 1B; supplemental Figure 2). Excision of floxed Smo alleles was observed in OB cells from SmoOb-ko mice, but not in OB cells from Osx1-Cre, Smo+/+ (Cre control) mice or in spleen, thymus, and BM B-lymphoid cells from either strain (Figure 1C). Primary OBs from Cre control mice expressed Ptch1, Smo, Hh ligands, and the Hh target Gli1; OBs from SmoOb-ko mice exhibited a 10-fold reduction in Smo transcripts and a similar reduction in the expression of Gli1 (supplemental Figure 3).
Osteoblastoid-specific ablation of Hh signaling impairs B-cell development. (A) The Smofl allele. Exon 1, neo cassette, and τ-lacZ are indicated. Blue arrowheads indicate loxP sites. GT5′ and GT3′, primers for amplification of wild-type and floxed alleles; rec3, reverse primer for detection of recombined allele. (B) Primary OBs examined under light-field (left) or immunostained with an osteocalcin-specific antibody (right). (C) Detection of Smo wild-type (wt), floxed, and recombined (rec.) alleles in spleen, thymus, CD19+ BM cells, and OB cells from Cre+/0, Smo+/+ and Cre+/0, Smofl/fl mice. Amplification was performed with genomic DNA templates. The Rag1 gene was amplified as a control. (D-E) Examination of B-lymphoid developmental subsets. BM was analyzed from 6- to 10-week-old mice of the indicated genotypes; the number of mice in each group is indicated in the inset (parentheses). Significant differences were determined by the Kruskal-Wallis test. (D) Percentages of pre-/pro-B (B220loCD19−CD43+), pro-B (B220loCD19+CD43+), and pre-B (B220loCD19+CD43−) subsets (mean and standard deviation [SD]). (E) Percentages of Rag1+, Rag1+ B220− (ELPs/CLPs), and Rag1+ B220+ subsets (mean and SD). (F) Impaired B lymphopoiesis from LSK progenitors maintained on Smo-depleted OB cells. LSK cells purified from mice of the genotypes indicated at top were maintained on Cre+/0, Smo+/+ or Cre+/0, Smofl/fl OB cells under conditions that promote B lymphopoiesis. Lymphoid and myeloid differentiation was assessed by flow cytometry.
Osteoblastoid-specific ablation of Hh signaling impairs B-cell development. (A) The Smofl allele. Exon 1, neo cassette, and τ-lacZ are indicated. Blue arrowheads indicate loxP sites. GT5′ and GT3′, primers for amplification of wild-type and floxed alleles; rec3, reverse primer for detection of recombined allele. (B) Primary OBs examined under light-field (left) or immunostained with an osteocalcin-specific antibody (right). (C) Detection of Smo wild-type (wt), floxed, and recombined (rec.) alleles in spleen, thymus, CD19+ BM cells, and OB cells from Cre+/0, Smo+/+ and Cre+/0, Smofl/fl mice. Amplification was performed with genomic DNA templates. The Rag1 gene was amplified as a control. (D-E) Examination of B-lymphoid developmental subsets. BM was analyzed from 6- to 10-week-old mice of the indicated genotypes; the number of mice in each group is indicated in the inset (parentheses). Significant differences were determined by the Kruskal-Wallis test. (D) Percentages of pre-/pro-B (B220loCD19−CD43+), pro-B (B220loCD19+CD43+), and pre-B (B220loCD19+CD43−) subsets (mean and standard deviation [SD]). (E) Percentages of Rag1+, Rag1+ B220− (ELPs/CLPs), and Rag1+ B220+ subsets (mean and SD). (F) Impaired B lymphopoiesis from LSK progenitors maintained on Smo-depleted OB cells. LSK cells purified from mice of the genotypes indicated at top were maintained on Cre+/0, Smo+/+ or Cre+/0, Smofl/fl OB cells under conditions that promote B lymphopoiesis. Lymphoid and myeloid differentiation was assessed by flow cytometry.
SmoOb-ko mice exhibited reduced body weight (supplemental Figure 4A). Tooth elongation was impaired (supplemental Figure 4B) and femurs were osteopenic, with thin growth plates and decreased trabecular bone (supplemental Figure 4C). In SmoOb-ko animals, splenic B-cell areas (the marginal zone and the periphery of the periarteriolar lymphoid sheath) were reduced, whereas the thymus showed a moderate decrease in cortical thymocytes (supplemental Figure 4D). Despite these abnormalities, the proportions of major lymphoid subsets in spleen and thymus of SmoOb-ko mice were normal (supplemental Figure 5A-B); in blood, the proportions of B and T cells or CD4+ and CD8+ T-cell subsets were likewise unaffected (supplemental Figure 5C-D). In contrast, the percentage and number of steady-state B progenitors at pre-B and later developmental stages (supplemental Figure 1D)13 were reduced in the BM of SmoOb-ko mice (supplemental Figure 5E-F), whereas the proportion of myeloid progenitors was unaltered (supplemental Figure 5G).
To identify the developmental stage at which B lymphopoiesis is impaired in SmoOb-ko animals, we examined B progenitors at steady state and during recovery from ablation. Early lymphoid progenitors (ELPs) and common lymphoid progenitors (CLPs) are marked by accumulation of Rag-1 transcripts before stable acquisition of lineage identity.14 We introduced a Rag-1 allele bearing a GFP cassette (Rag-1GFP) onto SmoOb-ko and Cre control backgrounds (supplemental Figure 1C). In SmoOb-ko, Rag-1GFP mice, BM pro-B and pre-B cells were significantly reduced (Figure 1D; supplemental Figure 6A), indicating an impairment of B lymphopoiesis at least as early as the pre-/pro-B to pro-B transition. The percentages and numbers of total GFP+ (Rag-1+) cells and GFP+, B220+ (Rag-1+ B220+) cells were also greatly diminished in SmoOb-ko, Rag-1GFP mice, whereas under these steady-state conditions, GFP+, B220− cells, which represent ELPs/CLPs, showed no obvious difference among genotypes (Figure 1E; supplemental Figures 6B and 7A).
Primary OB cells from Cre control and SmoOb-ko mice were tested for their ability to support B-lymphoid differentiation from LSK progenitors in vitro. Control OB cells robustly supported B lymphopoiesis from LSK cells of wild-type, Cre control, or SmoOb-ko origin (Figure 1F, left), whereas Smo-depleted OB cells were deficient in their support of B lymphopoiesis (Figure 1F, right). This indicated that Hh signaling in primary OB cells promotes B lymphopoiesis and suggested a causal relationship between the extinction of Hh signaling and the paucity of B-lymphoid progenitors in SmoOb-ko mice.
B-lymphoid repopulation after BM ablation reveals an early developmental delay in SmoOb-ko mice
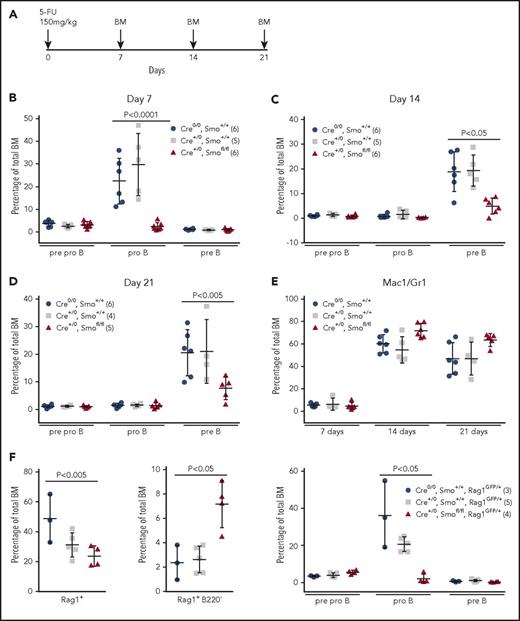
We examined recovery of BM B progenitors after chemical ablation by 5-FU (Figure 2A). At 7 days, pro-B cells were 20- to 30-fold less abundant in SmoOb-ko mice than in wild-type and Cre control animals (Figure 2B; supplemental Figure 6C). By day 14, pre-B cells had emerged but were markedly less numerous in SmoOb-ko mice than in controls (Figure 2C; supplemental Figure 6D). The relative sizes of B progenitor subsets at day 21 (Figure 2D; supplemental Figure 6E) were similar to those observed at day 14, suggesting that repopulation had reached steady state. In contrast, myeloid repopulation was unimpaired in SmoOb-ko mice (Figure 2E; supplemental Figure 6F).
Osteoblastoid-specific depletion of Smo impairs reappearance of early B-lymphoid progenitors after BM ablation. (A) BM ablation protocol. Mice were injected intraperitoneally with 5-FU, and BM was collected as indicated. (B-D) Pre-/pro-B (B220loCD19−CD43+), pro-B (B220loCD19+CD43+), and pre-B (B220loCD19+CD43−) compartments in mice of the indicated genotypes were examined 7 days (B), 14 days (C) and 21 days (D) after 5-FU treatment. The number of mice in each group is indicated in the insets (parentheses). Significant differences were determined by the Kruskal-Wallis test. (E) BM myeloid cells (Mac1+Gr1+) were assessed 7, 14, and 28 days after 5-FU treatment in the same mice. The number of mice in each group is indicated in the insets of panels B-D (parentheses). Significant differences were determined as in B-D. (F) The percentages of Rag1+ (left) and Rag1+B220− (ELPs/CLPs) (middle) BM cells 7 days after 5-FU treatment were determined in mice of genotypes indicated at right. The number of mice in each group is indicated in the insets (parentheses). Pre-/pro-B (B220loCD19−CD43+), pro-B (B220loCD19+CD43+), and pre-B cell (B220loCD19+CD43−) compartments were also examined 7 days after ablation (right). Mean and SD are indicated. Significant differences were determined as in panels B-D.
Osteoblastoid-specific depletion of Smo impairs reappearance of early B-lymphoid progenitors after BM ablation. (A) BM ablation protocol. Mice were injected intraperitoneally with 5-FU, and BM was collected as indicated. (B-D) Pre-/pro-B (B220loCD19−CD43+), pro-B (B220loCD19+CD43+), and pre-B (B220loCD19+CD43−) compartments in mice of the indicated genotypes were examined 7 days (B), 14 days (C) and 21 days (D) after 5-FU treatment. The number of mice in each group is indicated in the insets (parentheses). Significant differences were determined by the Kruskal-Wallis test. (E) BM myeloid cells (Mac1+Gr1+) were assessed 7, 14, and 28 days after 5-FU treatment in the same mice. The number of mice in each group is indicated in the insets of panels B-D (parentheses). Significant differences were determined as in B-D. (F) The percentages of Rag1+ (left) and Rag1+B220− (ELPs/CLPs) (middle) BM cells 7 days after 5-FU treatment were determined in mice of genotypes indicated at right. The number of mice in each group is indicated in the insets (parentheses). Pre-/pro-B (B220loCD19−CD43+), pro-B (B220loCD19+CD43+), and pre-B cell (B220loCD19+CD43−) compartments were also examined 7 days after ablation (right). Mean and SD are indicated. Significant differences were determined as in panels B-D.
At 7 days after BM ablation, the total GFP+ population was reduced in SmoOb-ko, Rag-1GFP mice relative to controls (Figure 2F, left; supplemental Figure 6G, left), consistent with a paucity of B progenitors (Figure 2B). Strikingly, ELPs/CLPs were overrepresented in SmoOb-ko animals (Figure 2F, middle; supplemental Figure 6G, middle; supplemental Figure 7B). The distributions of B progenitors at 7 days after ablation were similar to those in mice lacking the Rag-1GFP allele (Figure 2B,F; supplemental Figure 6C,G). These results confirm that extinction of Hh signaling in the osteoblastoid compartment impairs the emergence of pro-B cells. Moreover, the increase in accumulation of ELPs/CLPs in SmoOb-ko, Rag-1GFP mice at 7 days after ablation suggests that Hh signaling in OB cells promotes the exit of B-lymphoid progenitors from the CLP compartment.
Taken together, our results indicate that Hh signaling in osteoblastoid cells promotes B lymphopoiesis; this requirement may be enforced as early as the transition from the CLP to the pre-/pro-B cell stage. Deletion of Ptch1, an inhibitor of Smo, either nonspecifically or in stratified epithelia has been associated with depletion of B- and T-cell progenitors.15 Our study differs with respect to the lineage specificity of the deletion and the lymphopoietic defect, which was restricted to the B lineage. Although Osx-1-Cre–mediated deletion may occur in other BM cell types,16 previous studies uncovered a critical role for OBs in B-cell development,11,17 and we demonstrate that OBs from SmoOb-ko mice are defective in Hh signaling and in promoting B lymphopoiesis. Our findings indicate that the ability of osteoblastoid cells to promote B lymphopoiesis is conferred, at least in part, by Smo, whose sustained activity is likely to support expression of factors essential for B-lineage development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Kay Medina (Mayo) for the gift of Rag-1-GFP mice and members of the Department of Molecular Biology and Genetics for stimulating discussions.
This work was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK099188 (S.D.).
Authorship
Contribution: W.L. designed and executed experiments, interpreted results, and wrote the manuscript; D.D. designed and executed experiments and interpreted results; D.H. performed histopathologic analysis; and S.D. designed experiments, interpreted the results, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
David Huso died on 27 January 2016.
Correspondence: Stephen Desiderio, 733 North Broadway, Baltimore, MD 21205; e-mail: sdesider@jhmi.edu.

![Figure 1. Osteoblastoid-specific ablation of Hh signaling impairs B-cell development. (A) The Smofl allele. Exon 1, neo cassette, and τ-lacZ are indicated. Blue arrowheads indicate loxP sites. GT5′ and GT3′, primers for amplification of wild-type and floxed alleles; rec3, reverse primer for detection of recombined allele. (B) Primary OBs examined under light-field (left) or immunostained with an osteocalcin-specific antibody (right). (C) Detection of Smo wild-type (wt), floxed, and recombined (rec.) alleles in spleen, thymus, CD19+ BM cells, and OB cells from Cre+/0, Smo+/+ and Cre+/0, Smofl/fl mice. Amplification was performed with genomic DNA templates. The Rag1 gene was amplified as a control. (D-E) Examination of B-lymphoid developmental subsets. BM was analyzed from 6- to 10-week-old mice of the indicated genotypes; the number of mice in each group is indicated in the inset (parentheses). Significant differences were determined by the Kruskal-Wallis test. (D) Percentages of pre-/pro-B (B220loCD19−CD43+), pro-B (B220loCD19+CD43+), and pre-B (B220loCD19+CD43−) subsets (mean and standard deviation [SD]). (E) Percentages of Rag1+, Rag1+ B220− (ELPs/CLPs), and Rag1+ B220+ subsets (mean and SD). (F) Impaired B lymphopoiesis from LSK progenitors maintained on Smo-depleted OB cells. LSK cells purified from mice of the genotypes indicated at top were maintained on Cre+/0, Smo+/+ or Cre+/0, Smofl/fl OB cells under conditions that promote B lymphopoiesis. Lymphoid and myeloid differentiation was assessed by flow cytometry.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/131/3/10.1182_blood-2017-06-793539/4/m_blood793539f1.jpeg?Expires=1769308486&Signature=D~V60m8ljC~G-5yY3-2VFEW0S3wrxHVaQCEEhfR7bkbloTOwmmLPFkZXxrHPt1cmtqFF-QFVXm3f7IqZcU9ipLRa1AhQhPcHRt4MT49BO5esZPJE-djuGUiAguhOkPpsBwnwACtsDqfUQzOjsOEbbJcXmBWRK8RV5BL4Z~GEIT6fp0CqbWuDuHlFIuGbVw7VAgZavmexULEYOuCYQY9jFepi8fR2EEIaUVxi0qyDj5l17Nl99n7KB7gOf6VIggA8cz6DKUx8idr8oW~-zPL-xDPC4tWMUxdmndIWW80kGmrv4ct6mxEcynSBytjPaSmRHHd6sHITGyZ31H0OEzCNvg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal