To the editor:
Anti-programmed cell death protein 1 (anti-PD-1) antibodies demonstrated remarkable efficacy in patients with relapsed/refractory (R/R) Hodgkin lymphoma (HL). In the 2 largest prospective studies that evaluated the anti-PD-1 antibodies nivolumab (CHECKMATE-205)1 and pembrolizumab (KEYNOTE-087)2 in R/R HL, the overall response rate and complete response (CR) rate were around 70% and 20%, respectively. These results led to the approval of nivolumab and pembrolizumab in R/R HL by the US Food and Drug Administration and the European Medicines Agency. Importantly, a significant proportion of HL patients in CR seem to experience durable remissions with anti-PD-1 treatment.1 However, it is unclear whether these patients may be cured and how long they need to continue treatment with anti-PD-1 antibody. This is particularly important in HL patients because the remission rates are high, which raises questions about the need for continuing therapy. Most of these patients are young and may receive anti-PD-1 therapy indefinitely (or at least for a prolonged period), which could expose them to chronic or long-lasting toxicities and preclude procreation, and prolonged treatment has a high cost.
Recently, Robert et al3 reported the outcome of 67 patients with metastatic melanoma who discontinued pembrolizumab after reaching a CR. Interestingly, 89.9% of the patients maintained their CR after 2 years of anti-PD-1 discontinuation. Here, we report the outcome of patients with R/R HL who discontinued nivolumab treatment after reaching a CR and were observed with no subsequent therapy.
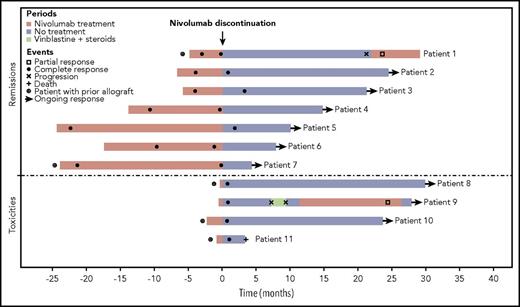
We retrospectively analyzed 78 patients with R/R HL who were treated with nivolumab in the French early access program between March and August 2015. Patients who did not respond to nivolumab and/or those who received subsequent therapy immediately after nivolumab discontinuation (eg, allogeneic hematopoietic stem cell transplantation [HSCT]) were excluded. Overall, nivolumab was discontinued in 11 patients who had prolonged remission (n = 7) or toxicity (n = 4). Decisions were made at the discretion of the physician. All patients were in CR at the time of nivolumab discontinuation. In the entire cohort, 7 of 28 patients in CR discontinued nivolumab because of prolonged remission after a median treatment duration of 13.8 months (range, 4.8-24.1 months). Four patients discontinued nivolumab because of toxicity, including 3 patients with prior allogeneic HSCT who experienced acute cerebellar syndrome and fatal gastrointestinal, liver, and cutaneous graft-versus-host disease (GVHD). Another limiting toxicity was laryngeal oppression. Exposure to nivolumab before discontinuation was shorter in patients who discontinued because of toxicity compared with patients who discontinued for remission (0.23 vs 13.8 months). Patient characteristics are summarized in Table 1. As expected, most of the patients were young (median age, 33 years) with advanced disease (64% Ann Arbor stage IV) and had been heavily pretreated (55% of the patients had previously received ≥7 lines of systemic therapy). Five patients (46%) had undergone prior allogeneic HSCT. The median duration of nivolumab treatment was 5.7 months (range, 0.5-24.2 months), and the median number of cycles was 10 (range, 1-51 cycles).
Patients’ characteristics and outcome after nivolumab discontinuation
| . | Remissions (n = 7) . | Toxicities (n = 4) . | All (N = 11) . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Characteristics at initiation of nivolumab | ||||||
| Median age (range), y | 33 (19-66) | 35.5 (26-53) | 33 (19-66) | |||
| Sex | ||||||
| Male | 4 | 43 | 3 | 75 | 7 | 64 |
| Female | 3 | 57 | 1 | 25 | 4 | 36 |
| Disease stage | ||||||
| I | 1 | 14 | 0 | 1 | 9 | |
| II | 2 | 29 | 0 | 2 | 18 | |
| IV | 4 | 57 | 3 | 75 | 7 | 64 |
| Unknown | 0 | 1 | 25 | 1 | 9 | |
| B symptoms | ||||||
| No | 6 | 86 | 4 | 100 | 10 | 91 |
| Missing | 1 | 0 | 1 | |||
| Prior lines of systemic therapy | ||||||
| 3 | 1 | 14 | 0 | 1 | 9 | |
| 4 | 2 | 29 | 0 | 2 | 18 | |
| 5 | 0 | 1 | 25 | 1 | 9 | |
| 6 | 1 | 14 | 0 | 1 | 9 | |
| ≥7 | 3 | 43 | 3 | 75 | 6 | 55 |
| Prior radiation therapy | 4 | 57 | 2 | 50 | 6 | 55 |
| Prior treatment with brentuximab vedotin | 7 | 100 | 4 | 100 | 11 | 100 |
| Prior autologous SCT | 4 | 57 | 3 | 75 | 7 | 64 |
| Prior allogeneic SCT | 2 | 29 | 3 | 75 | 5 | 46 |
| Anti-PD-1 treatment, discontinuation, and outcome | ||||||
| Median No. of nivolumab injections (range) | 28 (5-51) | 1.5 (1-5) | 10 (1-51) | |||
| Median duration of anti-PD-1 therapy (range), mo | 13.8 (4.8-24.1) | 0.2 (0-1.8) | 5.7 (0-24.1) | |||
| Concomitant radiotherapy | 2 | 29 | 0 | 2 | 18 | |
| Reason for nivolumab discontinuation | ||||||
| Prolonged remission | 7 | 100 | — | 7 | 64 | |
| Toxicity | — | 4 | 100 | 4 | 36 | |
| Acute GVHD | — | 2 | 2 | |||
| Cerebellar syndrome | — | 1 | 1 | |||
| Laryngeal oppression | — | 1 | 1 | |||
| CR at anti-PD-1 discontinuation4 | 7 | 100 | 4 | 100 | 11 | 100 |
| Median duration between first CR upon nivolumab and anti-PD-1 discontinuation (range), mo | 9.7 (3-22.4) | — | 9.7 (3-22.4) | |||
| Median follow-up from anti-PD-1 discontinuation (range), mo | 14.7 (4.2-29) | 25.6 (3.2-29.3) | 21.2 (3.2-29.3) | |||
| Patients alive at last follow-up | 7 | 100 | 3 | 75 | 10 | 91 |
| Patients in CR at last follow-up among patients alive | 6 | 86 | 2 | 67 | 8 | 80 |
| Retreatment with anti-PD-1 monotherapy among relapsed patients | 1 | 1 | 1 | 1 | 2 | 2 |
| Response to retreatment with anti-PD-1 in relapsed patients | ||||||
| CR | 0 | 0 | 0 | |||
| PR | 1 | 15 | 2 | |||
| . | Remissions (n = 7) . | Toxicities (n = 4) . | All (N = 11) . | |||
|---|---|---|---|---|---|---|
| No. . | % . | No. . | % . | No. . | % . | |
| Characteristics at initiation of nivolumab | ||||||
| Median age (range), y | 33 (19-66) | 35.5 (26-53) | 33 (19-66) | |||
| Sex | ||||||
| Male | 4 | 43 | 3 | 75 | 7 | 64 |
| Female | 3 | 57 | 1 | 25 | 4 | 36 |
| Disease stage | ||||||
| I | 1 | 14 | 0 | 1 | 9 | |
| II | 2 | 29 | 0 | 2 | 18 | |
| IV | 4 | 57 | 3 | 75 | 7 | 64 |
| Unknown | 0 | 1 | 25 | 1 | 9 | |
| B symptoms | ||||||
| No | 6 | 86 | 4 | 100 | 10 | 91 |
| Missing | 1 | 0 | 1 | |||
| Prior lines of systemic therapy | ||||||
| 3 | 1 | 14 | 0 | 1 | 9 | |
| 4 | 2 | 29 | 0 | 2 | 18 | |
| 5 | 0 | 1 | 25 | 1 | 9 | |
| 6 | 1 | 14 | 0 | 1 | 9 | |
| ≥7 | 3 | 43 | 3 | 75 | 6 | 55 |
| Prior radiation therapy | 4 | 57 | 2 | 50 | 6 | 55 |
| Prior treatment with brentuximab vedotin | 7 | 100 | 4 | 100 | 11 | 100 |
| Prior autologous SCT | 4 | 57 | 3 | 75 | 7 | 64 |
| Prior allogeneic SCT | 2 | 29 | 3 | 75 | 5 | 46 |
| Anti-PD-1 treatment, discontinuation, and outcome | ||||||
| Median No. of nivolumab injections (range) | 28 (5-51) | 1.5 (1-5) | 10 (1-51) | |||
| Median duration of anti-PD-1 therapy (range), mo | 13.8 (4.8-24.1) | 0.2 (0-1.8) | 5.7 (0-24.1) | |||
| Concomitant radiotherapy | 2 | 29 | 0 | 2 | 18 | |
| Reason for nivolumab discontinuation | ||||||
| Prolonged remission | 7 | 100 | — | 7 | 64 | |
| Toxicity | — | 4 | 100 | 4 | 36 | |
| Acute GVHD | — | 2 | 2 | |||
| Cerebellar syndrome | — | 1 | 1 | |||
| Laryngeal oppression | — | 1 | 1 | |||
| CR at anti-PD-1 discontinuation4 | 7 | 100 | 4 | 100 | 11 | 100 |
| Median duration between first CR upon nivolumab and anti-PD-1 discontinuation (range), mo | 9.7 (3-22.4) | — | 9.7 (3-22.4) | |||
| Median follow-up from anti-PD-1 discontinuation (range), mo | 14.7 (4.2-29) | 25.6 (3.2-29.3) | 21.2 (3.2-29.3) | |||
| Patients alive at last follow-up | 7 | 100 | 3 | 75 | 10 | 91 |
| Patients in CR at last follow-up among patients alive | 6 | 86 | 2 | 67 | 8 | 80 |
| Retreatment with anti-PD-1 monotherapy among relapsed patients | 1 | 1 | 1 | 1 | 2 | 2 |
| Response to retreatment with anti-PD-1 in relapsed patients | ||||||
| CR | 0 | 0 | 0 | |||
| PR | 1 | 15 | 2 | |||
SCT, stem cell transplantation.
Outcome for the entire cohort is summarized in Table 1 and Figure 1. The median follow-up was 28.6 months (range, 3.2-34.2 months) from nivolumab initiation and 21.2 months (range, 3.2-29.8 months) from nivolumab discontinuation. At the time of last follow-up, all patients remained alive, except 1 (patient 11). This patient had undergone prior allogeneic HSCT 19 months before anti-PD-1 initiation. He received only 1 injection of nivolumab because he developed acute gastrointestinal and liver GVHD, which occurred within a few days after anti-PD-1 infusion. The 18F-fluorodeoxyglucose positron emission tomography/computed tomography scan performed 1 month later showed a CR, but unfortunately the patient died of multiple organ failure 2 months later. Among the 10 remaining patients, 8 (80%) are still in CR after a median follow-up of 22.4 months (range, 4.2-29.8 months) from nivolumab discontinuation (patients 2-8 and 10). Four of them have been off therapy for more than 21 months and remain disease free. Two patients relapsed after nivolumab discontinuation (patients 1 and 9). Patient 1, who had previously undergone allogeneic HSCT, received 5 cycles of nivolumab, reached a CR, and then discontinued anti-PD-1 therapy because of the risk of GVHD. He remained in remission for 21 months without any additional treatment. The patient then relapsed and nivolumab therapy was resumed, which led to a partial response (PR) after 1 month. The patient is still receiving nivolumab therapy at 6 months. Patient 9 discontinued nivolumab prematurely (after 2 cycles) because of toxicity (grade 3 laryngeal oppression). Nevertheless, he was considered in CR at the time of the first evaluation. The patient relapsed 7 months later and was then treated with vinblastine. Because he was refractory to vinblastine, he was retreated with nivolumab, which led to a PR without significant toxicity that was assessed by computed tomography scan. Anti-PD-1 antibody was discontinued after 1 year, and the patient has been off therapy for 1 month.
To the best of our knowledge, our study is the first to report the outcome of HL patients after anti-PD-1 discontinuation. Although the median follow-up is still limited (21.2 months), almost all patients (91%) remain alive and 80% of them are still in CR after nivolumab discontinuation, some of them beyond 2 years. Interestingly, some of these prolonged remissions occurred in patients who had received only a few cycles of nivolumab, notably in patients with prior allogeneic HSCT. Longer follow-up and larger studies are warranted to confirm these results. Whether some of these patients may be cured remains to be determined. Clinical trials are also needed to prospectively evaluate the optimal duration of anti-PD-1 therapy in HL patients and identify patients to whom discontinuation can be safely proposed.
Authorship
Contribution: G.M. and R.H. designed the research, analyzed data, and wrote the paper; C.H., P.B., K.B., A.S., J.-M.S., H.G., and L.D. provided the data; and all authors reviewed and approved the final draft.
Conflict-of-interest disclosure: R.H., C.H., and P.B. have received consulting fees and/or honoraria from Bristol-Myers Squibb. The remaining authors declare no competing financial interests.
A complete list of the members of the Lymphoma Study Association appears in “Appendix.”
Correspondence: Roch Houot, Department of Hematology, Centre Hospitalier Universitaire Rennes, 2 rue Henri Le Guilloux, 35033 Rennes Cedex 9, France; e-mail: roch.houot@chu-rennes.fr.
Appendix: study group members
The members of the Lymphoma Study Association are: Benoît Bareau, Cécile Borel, Nadine Boullanger, Olivier Casasnovas, Adrien Chauchet, Bénédicte Deau-Fischer, Alain Derlmer, Rémy Duléry, Marjan Ertault, Luc-Matthieu Fornecker, Georges Garnier, Thomas Gastinne, Stéphanie Guidez, Julien Lazarovici, Katell Le Dû, Fabien Lebras, Sophie Lefort, Elena Loppinet, Jean-Pierre Marolleau, Marie-Pierre Moles-Moreau, Lysiane Molina, Nadine Morineau, Franck Morschhauser, Emmanuelle Nicolas-Virelizier, Frédéric Peyrade, Isabelle Roche-Lachaise, Anna Schmitt, Adrian Tempescul, Mohamed Touati, Olivier Tournihac, Elodie Gat, Laure Flament, and Florence Broussais.