Warfarin, an anticoagulant therapy used by millions worldwide, inhibits vitamin K epoxide reductase complex subunit 1 (VKORC1), thereby dampening the carboxylation and the procoagulant potential of vitamin K–dependent coagulation factors. However, the detailed molecular mechanism by which warfarin inhibits VKORC1 remains the subject of debate.1,2 In this issue of Blood, Rishavy et al3 puts this issue to rest by demonstrating that warfarin inhibits VKORC1 via a mechanism termed “the uncoupling of VKORC1.” The uncoupling of VKORC1 is significant because it highlights a potential cooperation of VKORC1 with a second warfarin-resistant vitamin K quinone reductase, thereby explaining how considerable carboxylation can proceed despite the presence of warfarin, and this may have direct implications for warfarin dosing in patients.
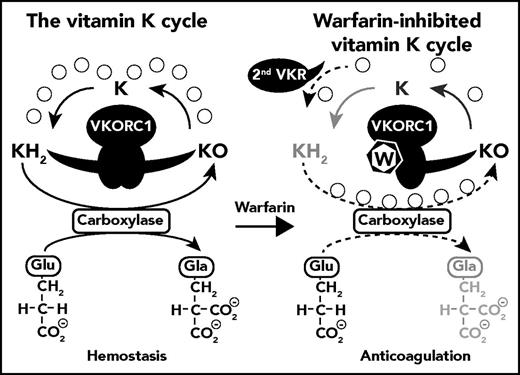
Inhibition of the vitamin K cycle by warfarin. KH2 is converted to KO during the carboxylation of Glu to Gla residues by γ-glutamyl carboxylase. Under normal physiologic conditions in the liver, VKORC1 efficiently recycles KO back to KH2 in a 2-step reaction, generating K as an intermediate product (left side). The uncoupling of the 2 VKORC1-mediated reactions (KO→K and K→KH2) in the presence of warfarin, as described by Rishavy et al, causes a loss in efficiency of VKORC1 for the full recycling of KO to KH2 and a loss in carboxylation. However, because warfarin uncouples the reaction rather than directly inhibits the reaction, VKORC1 can still generate considerable amounts of the K intermediary. The completion of the final step (K→KH2) then becomes dependent on the extent to which the second warfarin-resistant vitamin K quinone reductase (2nd VKR) is present and active. Thus, although VKORC1 is responsible for the full recycling of KO to KH2 in the absence of warfarin, the recycling pathway changes in the presence of warfarin and requires cooperation of VKORC1 and the 2nd VKR.3 The degree of carboxylation in the presence of warfarin is therefore dependent on the expression and activity of 2nd VKR (indicated by the dotted arrows), the identity of which remains unknown to date.
Inhibition of the vitamin K cycle by warfarin. KH2 is converted to KO during the carboxylation of Glu to Gla residues by γ-glutamyl carboxylase. Under normal physiologic conditions in the liver, VKORC1 efficiently recycles KO back to KH2 in a 2-step reaction, generating K as an intermediate product (left side). The uncoupling of the 2 VKORC1-mediated reactions (KO→K and K→KH2) in the presence of warfarin, as described by Rishavy et al, causes a loss in efficiency of VKORC1 for the full recycling of KO to KH2 and a loss in carboxylation. However, because warfarin uncouples the reaction rather than directly inhibits the reaction, VKORC1 can still generate considerable amounts of the K intermediary. The completion of the final step (K→KH2) then becomes dependent on the extent to which the second warfarin-resistant vitamin K quinone reductase (2nd VKR) is present and active. Thus, although VKORC1 is responsible for the full recycling of KO to KH2 in the absence of warfarin, the recycling pathway changes in the presence of warfarin and requires cooperation of VKORC1 and the 2nd VKR.3 The degree of carboxylation in the presence of warfarin is therefore dependent on the expression and activity of 2nd VKR (indicated by the dotted arrows), the identity of which remains unknown to date.
Some of the debate on the mechanism of VKORC1 inhibition by warfarin stems from the complexity of the 2-step vitamin K recycling reaction and the question of whether VKORC1 is responsible for both steps or just the first step in this reaction. The uncoupling of VKORC1 by warfarin is therefore an important mechanistic distinction from direct inhibition of VKORC1’s individual reactions and with its own consequences, which are illustrated by a juggler’s act. First, consider a juggler, on center stage, performing a left-handed ball toss while cascading with a right-handed club throw, culminating in a mesmerizing pattern of balls and clubs flying through the air at high speed, or VKORC1 under normal conditions. Now consider a 1-armed juggler or warfarin-inhibited VKORC1, the juggler with one arm still able to toss a ball or throw a club, but the lack of hand-to-hand cascading severely diminishing the flair of his act. The point is that a 1-armed juggler can still throw some balls compared with a no-arm juggler, or an uncoupled VKORC1 can generate considerably more intermediary product despite the presence of warfarin compared with directly inhibited VKORC1.
The posttranslational carboxylation of glutamic acid residues (Glu) to γ-carboxyglutamic acid (Gla) in vitamin K–dependent proteins and coagulation factors requires vitamin K hydroquinone (KH2) that is converted to vitamin K epoxide (KO) during carboxylation. VKORC1 recycles KO back to vitamin K quinone (K) and subsequently to KH2 in a 2-step process dubbed the vitamin K cycle (see figure).4 The mechanism by which warfarin inhibits these reactions remains largely unknown due in part to inconsistencies in the correlation between VKOR activity in wild-type and VKORC1 mutants and the inhibition of carboxylation. The studies of Rishavy et al reconcile these apparent inconsistencies by elucidating 3 steps in the molecular mechanism of VKORC1 and its inhibition by warfarin. First, VKORC1 effectively mediates both KO→K and K→KH2 reactions, and although warfarin inhibits both individual reactions to some extent, warfarin affects predominantly the overall reaction throughput by uncoupling the 2 reactions. Second, warfarin-resistant VKORC1 mutants (such as Y139H or Y139F) are similarly uncoupled by warfarin, albeit requiring higher concentrations. Third, analyses of carboxylation efficiency in cells are confounded by the expression of a second warfarin-resistant quinone reductase in some cells (eg, HEK293 cells) but not in other cells (eg, BHK cells), explaining why residual carboxylation in the presence of warfarin is found in some studies but not in others. The implication of these findings is that the inhibition by warfarin is not due to the inhibition of either of the individual reactions but instead that warfarin affects the overall reaction throughput. Consequently, VKORC1 can generate some intermediary K in the presence of warfarin, not sufficient to support carboxylation by itself, but sufficient to permit notable carboxylation when the second warfarin-resistant quinone reductase is present to complete the vitamin K recycling.
One of the major drawbacks of warfarin is its narrow therapeutic range and the relatively large interindividual susceptibility differences, which especially during the initial therapy may convey a considerable risk of serious bleeding. Genetic polymorphisms and mutations in VKORC15 that are associated with warfarin resistance determine ∼40% of the individual warfarin dose requirement, whereas CYP2C9 variants that affect the metabolism of warfarin explain another 7% to 10%.2,4,6 Additional factors such as age, sex, weight, and diet influence warfarin dose requirements, but it is intriguing to speculate that variations in expression or function of the second warfarin-resistant quinone reductase may provide the missing piece of the interindividual warfarin susceptibility puzzle.
The existence of a second warfarin-resistant quinone reductase in the liver mediating the specific recycling of K to KH2 has long been deduced from the observation that high-dose vitamin K administration can overcome the effects of warfarin overdose.6,7 In search of this antidotal enzyme, several candidates have been proposed but to no avail to date. For instance, the VKORC1 paralog, VKORC1 like-1, with a 30- to 50-fold reduced sensitivity to warfarin (and warfarin derivatives),8 can partially compensate for VKORC1 deficiency during development but not after birth, suggesting that this paralog is unlikely to be the much sought after second warfarin-resistant quinone reductase in the liver.9
Although these insights paint a clearer picture of the molecular mechanism of VKORC1 and its inhibition by warfarin, additional studies are needed to complete the picture, which include understanding the mechanism of VKORC1 uncoupling by warfarin, the role for VKORC1 dimers, and the domain topology of VKORC1.3,7 Direct oral anticoagulants are increasingly replacing warfarin as the anticoagulant therapy of choice due to their improved safety profiles and ease of use.10 The extent of continued use of warfarin as an anticoagulant in the future will likely depend on whether a better understanding of the molecular mechanism by which warfarin inhibits VKORC1 can be translated into improved and safer dosing algorithms. The study by Rishavy et al suggests that variations in expression or function of the second warfarin-resistant quinone reductase, which remains unidentified to date, may be a more important variable in the physiologic response to warfarin than was previously appreciated.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal