Abstract
Extranodal natural killer/T-cell lymphoma, nasal type (ENKL) is a subtype of mature T- and natural killer cell lymphomas characterized by its association with Epstein-Barr virus and extranodal involvement. Although there is geographic variance in the frequency of ENKL, its clinical features are similar between Western countries and endemic areas, such as East Asia. Anthracycline-containing chemotherapy is not recommended to treat ENKL. No standard treatment has been established based on the results of randomized controlled trials. In patients with localized disease, radiotherapy is a core component of the recommended first-line therapy. Radiotherapy administered at 50 to 54 Gy, extended involved-site radiotherapy considering tumor invasiveness, and the use of intensity modulated radiation therapy or volumetric modulated arc therapy are associated with efficacy of radiotherapy. Although the use of concurrent chemoradiotherapy has been supported by the results of clinical trials, accumulating evidence supports the use of sequential chemoradiotherapy with non–anthracycline-containing regimens that include l-asparaginase and/or platinum anticancer agents. l-asparaginase-containing chemotherapy is a key component of first-line treatments for systemic ENKL. Hematopoietic stem cell transplantation is recommended as a front-line consolidation therapy for newly diagnosed advanced-stage ENKL. Newer agents including immune checkpoint inhibitors are being investigated for treating ENKL. In this modern ENKL treatment era, multidisciplinary efforts are needed to identify the best timing and sequencing of radiotherapy, l-asparaginase, platinum, newer agents, and hematopoietic stem cell transplantation.
Introduction
Extranodal natural killer/T-cell lymphoma, nasal type (ENKL) is a well-characterized subtype of mature T- and natural killer (NK) cell lymphoma in the World Health Organization classification.1 ENKL is a predominantly extranodal lymphoma that is characterized by an association with Epstein-Barr virus (EBV).1 Although ENKL is more common in East Asia and Latin America than in other parts of the world, recent large retrospective studies performed in Western countries have demonstrated that the clinical features of ENKL are similar around the world.2,3
Cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy is not an optimal treatment of ENKL.4,5 No standard treatment has been established based on the results of randomized controlled trials because of the rarity of the disease. Recently, newer agents for ENKL, including immune checkpoint inhibitors, have been shown to exhibit promising efficacy.6 In this context, we summarize the treatment approaches for ENKL that have been developed since 2000 with the aim of providing clues that will support the development of better multidisciplinary treatment approaches for ENKL.
Key components in the treatment of ENKL
Chemotherapeutic agents
Previously, the 5-year overall survival (OS) rate in patients with localized nasal ENKL treated with CHOP-like chemotherapies followed by involved-field radiotherapy (RT) was lower than 50%.7,8 Later, in the mid-1990s, the multidrug resistance (MDR) 1/ABCB1 gene and its product, P-glycoprotein, were found to be expressed in ENKL tumor cells.9-11 Treatments that avoid the use of anthracycline have been actively developed, and several have become important concepts underlying current treatment approaches for ENKL.
l-asparaginase is an MDR-unrelated anticancer agent that exerts antitumor effects in both ENKL cell lines and primary ENKL cells.12 The dramatic response to l-asparaginase observed in patients with disseminated ENKL13,14 led to the development of l-asparaginase-containing regimens. Conventional Escherichia coli–derived l-asparaginase is the prototype. If an allergic reaction to conventional E coli–derived l-asparaginase occurs, Erwinia asparaginase can be used instead.15 Pegaspargase, a pegylated E coli–derived l-asparaginase, showed lower toxicity than E coli–derived l-asparaginase.
Platinum derivatives are also non–MDR-related and have been used as key agents in salvage chemotherapies to treat relapsed or refractory aggressive lymphomas. Cisplatin, carboplatin, and oxaliplatin have also been used to treat ENKL. The differences among these agents in the efficacy of treating ENKL have not been fully explored.
RT
RT has been used as a treatment of newly diagnosed localized ENKL since the 1990s, when ENKL was called angiocentric lymphoma. RT is the most important modality for treating ENKL, and the omission of RT has a negative impact on both local control and survival in patients with localized ENKL.16,17
The following factors should be taken into account when evaluating the efficacy of first-line therapies for localized ENKL.
Dose: Early retrospective studies suggested that the appropriate RT dose in localized ENKL is 50 Gy or more.7,18-27 In recent studies, the dose of RT has been determined in each treatment approach. In a large retrospective analysis in China, 50 Gy was recommended for RT alone in patients without primary tumor invasiveness (PTI; invasion to adjacent tissue and/or organs).28 In a retrospective study in Japan, 54 Gy or more was suggested for RT alone for patients with any size of localized ENKL.23 In a setting of concurrent chemoradiotherapy (CCRT), the RT dose differs (40-54 Gy) according to the intensity of the combined chemotherapeutic regimen.29-31 In sequential CRT, the dose of RT is usually the same as that in RT alone. A lower dose of RT (40-45 Gy) has also been explored in patients who obtained complete response (CR) after intensive chemotherapy.3
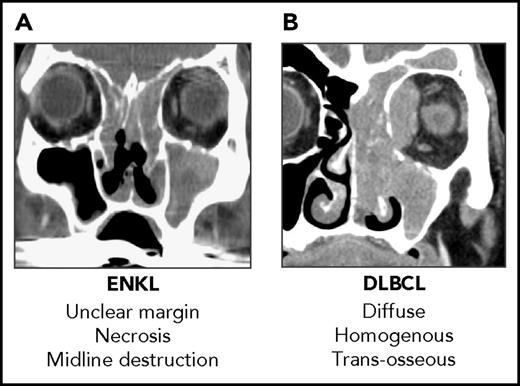
Clinical target volume (CTV): Using a small RT field reduced locoregional control and survival rates in ENKL.18,22 It is often difficult to diagnose the true extent of the tumor in ENKL even when precise radiological information is available from magnetic resonance imaging scans and 18-fluorodeoxyglucose-positron emission tomography computed tomography (FDG-PET/CT) scans because of secondary inflammation and the destructive nature of ENKL (Figure 1). In consideration of this obstacle, the concept of extended involved-site RT as proposed in the International Lymphoma Radiation Oncology Group guidelines.32 Using extended CTV with a margin covering the gross tumor volume (determined via endoscopy, CT scan, magnetic resonance imaging, and FDG-PET/CT) yielded high locoregional control rates.19,20,22
RT delivery: Intensity-modulated radiation therapy (IMRT) and volumetric modulated arc therapy (VMAT) are currently the standard treatments for both ENKL and head and neck neoplasms, although many patients in retrospective studies were treated with 3-dimensional conformal RT (3D-CRT).17,33 Accumulating evidence indicates that IMRT and VMAT provide excellent disease control without causing any severe toxicity.34,35
Radiological difference between ENKL and DLBCL. (A) Sagittal CT scan of a patient with ENKL. (B) Sagittal CT scan of a patient with DLBCL. It is difficult to diagnose the true extent of the tumor and contour the gross tumor volume in ENKL using only a CT scan. DLBCL, diffuse large B-cell lymphoma.
Radiological difference between ENKL and DLBCL. (A) Sagittal CT scan of a patient with ENKL. (B) Sagittal CT scan of a patient with DLBCL. It is difficult to diagnose the true extent of the tumor and contour the gross tumor volume in ENKL using only a CT scan. DLBCL, diffuse large B-cell lymphoma.
Because the induction of CR by first-line therapy is associated with prolonged survival in patients with localized ENKL, it is important to control the disease early using qualified upfront RT. Therefore, patients with localized ENKL should ideally be treated in a center with expert radiation oncologists and oncologists with experience in treating these patients in a multidisciplinary fashion.
The role of adjuvant local RT in systemic ENKL remains uncertain. Some data show that adjuvant local RT is not associated with OS in patients with newly diagnosed advanced ENKL.36 The role of RT in salvage settings should be investigated.
In patients with ENKL who received non–anthracycline-containing therapy, the circulating EBV-DNA load in peripheral blood is associated with response and survival37,38 and is used for disease monitoring. Although there is no firm consensus on the optimal blood sample type for the measurement of EBV-DNA, several studies suggest the advantage of cell-free (plasma) EBV-DNA than cellular EBV-DNA as a marker of EBV diseases.37,39 After first-line non–anthracycline-containing therapy, posttreatment FDG-PET/CT using the Deauville score and the presence of circulating EBV-DNA predicted treatment failure in a retrospective study including 140 patients with ENKL.40
Treatment approaches to newly diagnosed localized ENKL
Since the early 2000s, new approaches to treating localized ENKL have included CCRT and sequential CRT with non-anthracycline chemotherapy.
CCRT
CCRT is expected to achieve both local and systemic disease control. Because CCRT is not a common treatment approach, most CCRTs for newly diagnosed localized ENKL have been developed in multi-institutional clinical trials. There are 2 major types of CCRT to treat newly diagnosed localized ENKL (Table 1). One of these simultaneously initiates RT and chemotherapy. CCRT combined with RT and a two-thirds dose of dexamethasone, etoposide, ifosfamide, and carboplatin (DeVIC) chemotherapy (RT-2/3DeVIC)29 is a typical treatment protocol. CCRT with DEP (dexamethasone, etoposide, and cisplatin) followed by DVIP (dexamethasone, etoposide, ifosfamide, and cisplatin)41 and CCRT with ESHAP (etoposide, steroid, high-dose cytarabine, and cisplatin)42 were also developed. Another type of CCRT is typically initiated with RT administered with weekly cisplatin followed by non–anthracycline-containing chemotherapy. This approach is used to treat esophageal cancer and head and neck cancer. CCRT-VIPD (etoposide, ifosfamide, cisplatin, and dexamethasone),43 CCRT-etoposide, ifosfamide, dexamethasone, and l-asparaginase (VIDL),30 CCRT-methotrexate, ifosfamide, dexamethasone, l-asparaginase, and etoposide (MIDLE),44 and CCRT gemcitabine, dexamethasone, and cisplatin (GDP)45 are typical treatment protocols of this type. Moreover, there is a report of CCRT with intramaxillary arterial infusion chemotherapy.46
CCRT with non-anthracycline chemotherapy for newly diagnosed localized ENKL
| Reference . | Study design . | Treatment . | RT delivery Median dose (range) . | No. of patients . | CR, % . | Median follow-up, mo (range) . | OS, % . | PFS, % . | Leukopenia grade 3, %/grade 4, % . | Mucositis* grade 3, %/grade 4, % . | Febrile neutropenia grade 3, %/grade 4, % . | Treatment-related death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simultaneous initiation of RT and chemotherapy | ||||||||||||
| 29,47 | Phase 1/2 | RT-2/3DeVIC: | 3D-CRT | 27 | 77 | 67 (61-94) | 70 (5 y) | 63 (5 y) | 85/15† | 30/0 | 15/0* | None |
| RT+ 2/3DeVIC ×3 | 50 Gy (50-50.4) | |||||||||||
| 41 | Phase 2 | DEP-CCRT/DVIP: | NA | 33 | 63 | 59 (16-79) | 66 (5 y) | 60 (5 y) | 35/48† | 30/0 | 35/0† | Pancreatitis (n = 1), |
| RT + DEP ×2 | 50.4 Gy | Infection (n = 1) | ||||||||||
| → DVIP ×2 | ||||||||||||
| 42 | Retrospective | RT + modified ESHAP ×2 → modified ESHAP ×2 | 3D-CRT (n = 10), | 13 | 92 | 38 (NA) | 72 (2 y) | 90 (2 y, FFP) | 31/62‡ | 23/23 | 54/15 | None |
| IMRT (n = 3) | ||||||||||||
| 40 Gy (40-52.2) | ||||||||||||
| CCRT with weekly cisplatin followed by non-anthracycline chemotherapy | ||||||||||||
| 43 | Phase 2 | CCRT-VIPD: | 3D-CRT | 30 | 80 | 24 (17-37) | 86 (3 y) | 85 (3 y) | 20/27† | 0/0§ | 50/10† | Infection (n = 2) |
| RT + wCDDP | 40 Gy (40-52.8) | |||||||||||
| → VIPD ×3 | ||||||||||||
| 30 | Phase 2 | CCRT-VIDL: | NA | 30 | 87 | 44 (95% CI, 41-47) | 73 (5 y) | 60 (5 y) | 20/60† | 13/3§ | 17/0† | None |
| RT + wCDDP | 40 Gy (40-50) | |||||||||||
| → VIDL ×2 (→ HD-AHSCT if NK-PI score 2-3) | ||||||||||||
| 44 | Phase 2 | CCRT-MIDLE: | 3D-CRT or IMRT | 28 | 82 | 46 (95% CI, 39-47) | 82 (3 y) | 74 (3 y) | 9/83 (n = 23)†,‡ | 4/0§ | 43/0† | Acute kidney injury and pneumonia (n = 1) |
| RT + wCDDP + triweekly l-asp → MIDLE ×2 | NA (36-44) | |||||||||||
| 45 | Phase 2 | RT + wCDDP | IMRT | 32 | 84 | 38 (NA) | 88 (3 y) | 84 (3 y) | (n = 7)/(n = 6)† | 0/0§ | (n = 16)/(n = 2)† | Infection (n = 1) |
| → GDP ×3 | 56 Gy (NA) | |||||||||||
| Reference . | Study design . | Treatment . | RT delivery Median dose (range) . | No. of patients . | CR, % . | Median follow-up, mo (range) . | OS, % . | PFS, % . | Leukopenia grade 3, %/grade 4, % . | Mucositis* grade 3, %/grade 4, % . | Febrile neutropenia grade 3, %/grade 4, % . | Treatment-related death . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Simultaneous initiation of RT and chemotherapy | ||||||||||||
| 29,47 | Phase 1/2 | RT-2/3DeVIC: | 3D-CRT | 27 | 77 | 67 (61-94) | 70 (5 y) | 63 (5 y) | 85/15† | 30/0 | 15/0* | None |
| RT+ 2/3DeVIC ×3 | 50 Gy (50-50.4) | |||||||||||
| 41 | Phase 2 | DEP-CCRT/DVIP: | NA | 33 | 63 | 59 (16-79) | 66 (5 y) | 60 (5 y) | 35/48† | 30/0 | 35/0† | Pancreatitis (n = 1), |
| RT + DEP ×2 | 50.4 Gy | Infection (n = 1) | ||||||||||
| → DVIP ×2 | ||||||||||||
| 42 | Retrospective | RT + modified ESHAP ×2 → modified ESHAP ×2 | 3D-CRT (n = 10), | 13 | 92 | 38 (NA) | 72 (2 y) | 90 (2 y, FFP) | 31/62‡ | 23/23 | 54/15 | None |
| IMRT (n = 3) | ||||||||||||
| 40 Gy (40-52.2) | ||||||||||||
| CCRT with weekly cisplatin followed by non-anthracycline chemotherapy | ||||||||||||
| 43 | Phase 2 | CCRT-VIPD: | 3D-CRT | 30 | 80 | 24 (17-37) | 86 (3 y) | 85 (3 y) | 20/27† | 0/0§ | 50/10† | Infection (n = 2) |
| RT + wCDDP | 40 Gy (40-52.8) | |||||||||||
| → VIPD ×3 | ||||||||||||
| 30 | Phase 2 | CCRT-VIDL: | NA | 30 | 87 | 44 (95% CI, 41-47) | 73 (5 y) | 60 (5 y) | 20/60† | 13/3§ | 17/0† | None |
| RT + wCDDP | 40 Gy (40-50) | |||||||||||
| → VIDL ×2 (→ HD-AHSCT if NK-PI score 2-3) | ||||||||||||
| 44 | Phase 2 | CCRT-MIDLE: | 3D-CRT or IMRT | 28 | 82 | 46 (95% CI, 39-47) | 82 (3 y) | 74 (3 y) | 9/83 (n = 23)†,‡ | 4/0§ | 43/0† | Acute kidney injury and pneumonia (n = 1) |
| RT + wCDDP + triweekly l-asp → MIDLE ×2 | NA (36-44) | |||||||||||
| 45 | Phase 2 | RT + wCDDP | IMRT | 32 | 84 | 38 (NA) | 88 (3 y) | 84 (3 y) | (n = 7)/(n = 6)† | 0/0§ | (n = 16)/(n = 2)† | Infection (n = 1) |
| → GDP ×3 | 56 Gy (NA) | |||||||||||
CI, confidence interval; FFP, freedom from progression; l-asp, l-asparaginase; NA, data not available; NK-PI, NK-cell lymphoma prognostic index; wCDDP, weekly cisplatin.
During CCRT.
During chemotherapy.
Neutropenia.
Stomatitis.
RT-2/3DeVIC was explored in a phase 1/2 study of patients with newly diagnosed stage IE or contiguous stage IIE nasal ENKL (Japan Clinical Oncology Group [JCOG] 0211).29,47 RT with extended CTV was incorporated in the protocol treatment. Patients who received RT-2/3DeVIC had a 5-year OS rate of 70%, and this regimen is currently a standard of care for newly diagnosed localized nasal ENKL in Japan.48 The National Comprehensive Cancer Network (NCCN) guidelines listed RT-2/3DeVIC as a preferred regimen for newly diagnosed localized ENKL.49 A retrospective study of patients diagnosed between 2000 and 2013 in 31 institutes in Japan confirmed the efficacy and toxicity of RT-DeVIC that was documented in the JCOG0211 study.50
CCRT-VIPD was developed in a phase 2 study conducted by a group in Korea (CISL [Consortium for Improving Survival of Lymphoma]).43 It was also included in the NCCN guidelines as a suggested first-line treatment regimen.49 The most significant benefit of CCRT-VIPD was achieved at a low dose of RT (40 Gy). CCRT-VIDL was subsequently developed as a result of 2 treatment-related deaths that occurred during VIPD chemotherapy.30,43 High-dose chemotherapy with autologous hematopoietic stem cell transplantation (HD-AHSCT) was added after CCRT-VIDL in patients with 2 or 3 of the risk factors listed on the NK-cell lymphoma prognostic index.30,51 Toxicity was lower in CCRT-VIDL than in CCRT-VIPD, and efficacy was comparable between them (Table 1). A subsequent phase 2 study of CCRT-MIDLE indicated that concurrent use of l-asparaginase with CCRT and intensified consolidative chemotherapy did not reduce early progression and was not feasible as a result of toxicity.44
CCRT with GDP chemotherapy was tested in a single-institution phase 2 study in China.45 IMRT was used at 56 Gy in a treatment protocol for CCRT-GDP. Although the treatment was performed in an outpatient setting, febrile neutropenia and thrombocytopenia were frequently documented.
Sequential CRT
Table 2 lists sequential CRT with non-anthracycline chemotherapy as a therapy for newly diagnosed localized ENKL. There are 2 major types of sequential CRT for localized ENKL: (1) l-asparaginase-containing chemotherapy followed by RT and (2) sandwich CRT with l-asparaginase-containing chemotherapy.
Sequential CRT with non-anthracycline chemotherapy for newly diagnosed localized ENKL
| Reference . | Study design . | Treatment . | RT delivery median dose (range) . | No. of patients . | CR, % . | Median follow-up, mo (range) . | OS, % . | PFS, % . | Leukopenia grade 3, %/grade 4, % . | Mucositis grade 3, %/grade 4, % . | Febrile neutropenia grade 3, %/grade 4, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 53 | Prospective | SMILE (→ RT → SMILE) | NA | 17 (stage I/II) | 82 | NA | NA | NA | NA | NA | NA |
| 3 | Retrospective | Modified SMILE ×2-3 → RT | IMRT or 3D-CRT | 11 | NA | 24 (1-43) | 100 (2 y) | 83 (2 y) | NA | NA | NA |
| 45 Gy (45-54) | |||||||||||
| 54,55 | Phase 2 | LVP → RT → LVP | NA | 26 | 81 | 67 (4-78) (n = 25) | 64 (5 y) | 64 (5 y) | 8/0 | 23/0 | 0/0 |
| 56 Gy | |||||||||||
| 56 | Phase 2 | LVDP ×2 → RT + wCDDP → LVDP ×2 | IMRT or 3D-CRT | 66 | 83 | 24 (12-51) | 70 (3 y) | 67 (3 y) | 9/8 | 6/0 | 0/0 (NA) |
| NA | |||||||||||
| 31 | Phase 2 | IMRT → GDP ×4 | IMRT | 44 | 89 | 38 (6-90) | 85 (3 y) | 77 (3 y) | 30/7 | 25/0 | 0/0 (NA) |
| 51.5 Gy (50-56) |
| Reference . | Study design . | Treatment . | RT delivery median dose (range) . | No. of patients . | CR, % . | Median follow-up, mo (range) . | OS, % . | PFS, % . | Leukopenia grade 3, %/grade 4, % . | Mucositis grade 3, %/grade 4, % . | Febrile neutropenia grade 3, %/grade 4, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 53 | Prospective | SMILE (→ RT → SMILE) | NA | 17 (stage I/II) | 82 | NA | NA | NA | NA | NA | NA |
| 3 | Retrospective | Modified SMILE ×2-3 → RT | IMRT or 3D-CRT | 11 | NA | 24 (1-43) | 100 (2 y) | 83 (2 y) | NA | NA | NA |
| 45 Gy (45-54) | |||||||||||
| 54,55 | Phase 2 | LVP → RT → LVP | NA | 26 | 81 | 67 (4-78) (n = 25) | 64 (5 y) | 64 (5 y) | 8/0 | 23/0 | 0/0 |
| 56 Gy | |||||||||||
| 56 | Phase 2 | LVDP ×2 → RT + wCDDP → LVDP ×2 | IMRT or 3D-CRT | 66 | 83 | 24 (12-51) | 70 (3 y) | 67 (3 y) | 9/8 | 6/0 | 0/0 (NA) |
| NA | |||||||||||
| 31 | Phase 2 | IMRT → GDP ×4 | IMRT | 44 | 89 | 38 (6-90) | 85 (3 y) | 77 (3 y) | 30/7 | 25/0 | 0/0 (NA) |
| 51.5 Gy (50-56) |
Dexamethasone (steroid), methotrexate, ifosfamide, l-asparaginase, and etoposide (SMILE) chemotherapy,52 discussed in detail in later, has been used in clinical practice in East Asia since the mid-2000s. A prospective survey confirmed that SMILE followed by RT and sandwich CRT using SMILE were both feasible in clinical practice in East Asia.53 In the United States, a modified SMILE regimen in which conventional l-asparaginase in SMILE was switched to pegaspargase was tested in a phase 1 study and has been used in clinical practice.2,3 The RT dose was relatively low (45 Gy) in sequential CRT, particularly with modified SMILE, which is more intensive than other chemotherapeutic regimens for localized ENKL (Table 2).3 Based on these experiences, sequential CRT with modified SMILE was listed as a suggested regimen in the NCCN guidelines.49 In addition to SMILE chemotherapy, the use of sequential/sandwich CRT using l-asparaginase, vincristine, and prednisone or l-asparaginase, cisplatin, dexamethasone, and etoposide has also been reported (Table 2).54-56
Sequential CRT without l-asparaginase has also been evaluated. A phase 2 study in which IMRT was followed by 4 cycles of GDP in 44 patients reported a 3-year OS rate of 85% and a 3-year progression-free survival (PFS) rate of 77%.31 Although these results seem promising, it is possible that selection biases were present in studies including patients who could wait for IMRT planning.
Modern RT alone
Evidence supporting modern RT is based mostly on the results of retrospective studies. Early studies showed that RT alone was not sufficient to achieve a cure because of frequent systemic relapse.18 However, a recent large retrospective study of patients with newly diagnosed localized ENKL in China reported more favorable therapeutic outcomes, including improved 5-year OS (69.6%) and PFS (65.1%) rates, at a median follow-up of 53 months in 253 patients who received RT alone as a first-line therapy.28 Risk factors that affect OS were an age at diagnosis of more than 60 years old, performance status >1, stage II disease, elevated serum lactate dehydrogenase levels, and PTI. Combined modality therapy was recommended only for patients with risk factors based on the results of a propensity score–matched analysis.28 According to this model, almost one-half of the patients in the United States and Japan are classified as high risk because of their higher median age at diagnosis. Because PTI was evaluated in the study by radiologists in local institutes, the International Lymphoma Radiation Oncology Group is performing an ongoing validation study.
What is the best first-line treatment of localized ENKL?
No prospective study has compared the various treatment approaches available for newly diagnosed localized ENKL. A recent retrospective study compared CCRT (Korean protocol: CCRT-VIPD/VIDL/MIDLE; Japanese protocol: RT-DeVIC, n = 190) and sequential CRT (83% modern chemotherapy followed by RT, n = 54).57 Patients who received CCRT had lower scores on the prognostic index for NK lymphoma-E58 ), although many patients in both groups lacked data on circulating EBV-DNA. CCRT was associated with a better OS and a higher CR rate, but there was no significant difference in OS between the 2 groups.57 Considering geographic differences in the logistics of RT, both CCRT and sequential CRT should be regarded as treatment options for newly diagnosed localized ENKL.
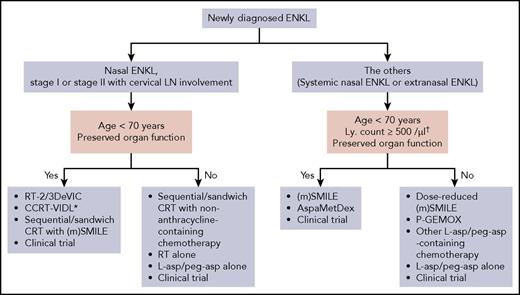
Figure 2 summarizes our recommended first-line therapy for ENKL. We recommend RT-2/3DeVIC, CCRT-VIDL, and CRT with (modified) SMILE based on the results of prospective studies (Tables 1 and 2).3,29,30,47,53 Because hematologic toxicity is observed frequently in these 3 regimens, they are suitable for young patients with preserved organ function. Intensive chemotherapeutic regimens such as SMILE and combined modality treatments should be given with cautious supports at experienced centers for profound myelosuppression.
Recommended first-line therapy for ENKL. Our recommended treatments are listed. There are no data to determine the best treatment among them. *HD-AHSCT is added if the NK-cell lymphoma prognostic index score is 2 or 3. †In case of SMILE chemotherapy. LN, lymph node; ly, lymphocyte; (m)SMILE, SMILE or modified SMILE; peg-asp, pegaspargase.
Recommended first-line therapy for ENKL. Our recommended treatments are listed. There are no data to determine the best treatment among them. *HD-AHSCT is added if the NK-cell lymphoma prognostic index score is 2 or 3. †In case of SMILE chemotherapy. LN, lymph node; ly, lymphocyte; (m)SMILE, SMILE or modified SMILE; peg-asp, pegaspargase.
Treatment approaches in advanced-stage or relapsed/refractory ENKL
The overall response rate (ORR) of conventional treatments, such as CHOP, was 36% for newly diagnosed stage IV ENKL but lower than 10% for relapsed or refractory ENKL.59 Non–anthracycline- and non–asparaginase-containing chemotherapies have shown moderate efficacy.60,61 Chemotherapeutic regimens for newly diagnosed and relapsed or refractory ENKL are listed in Table 3.
Chemotherapeutic regimens for newly diagnosed advanced-stage or relapsed/refractory ENKL
| Reference . | Study design . | Treatment . | Disease state . | No. of patients . | ORR, % . | CR, % . | Med. f/u, mo (range) . | OS, % . | PFS, % . | Leukopenia grade 3, %/grade 4, % . | Other adverse events grade 3, %/grade 4, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective clinical trials | |||||||||||
| 63 | Phase 2 | AspaMetDex ×3 | Relapsed (n = 11), refractory (n = 8) | 19 | 78 (n = 18) | 61 (n = 18) | 26 (17-49) | 12 mo* | 12 mo* | n = 8†,‡ | Liver, n = 3† Anemia, n = 4† Allergy, n = 1† |
| 52 | Phase 2 | SMILE ×2 | N-d stage IV (n = 20), first relapse (n = 14), first refractory (n = 4) | 38 | 79 (n = 38) 80 (N-d stage IV) | 45 (n = 38) 40 (N-d stage IV) | 24 (13-35) | 55 (1 y) | 53 (1 y) | 24/76 | Infection, 45/16 Anemia, 47/3 Thrombocytopenia, 24/40 |
| Retrospective studies | |||||||||||
| 69 | Multicenter | MEDA Med., 4 (range, 1-6) | Relapsed (n = 7) or refractory (n = 6) with advanced disease | 13 | 77 | 62 | 25 (20-30) | 69 (1 y) | 62 (1 y) | 46†,‡ | Infection, 15† |
| Thrombocytopenia, 31† | |||||||||||
| 70 | Single center | P-GEMOX Med., 5 (range, 2-8) → RT or HD-AHSCT | N-d advanced (n = 19), relapsed or refractory (n = 16) | 35 | 94§ | 26§ | 28 (9-50) | 65 (2 y) | 39 (2 y) | 31/9 | Anemia, 20/6 Thrombocytopenia, 14/17Hypertriglyceridemia, 9/11 |
| 73 | Single center | GDP Med., 6 (range, 2-8) | N-d stage IV (n = 15), relapsed or refractory (n = 26) | 41 | 83 | 42 | 16 (2-96) | 73 (1 y) | 55 (1 y) | 34†,‡ | Anemia, 15†Thrombocytopenia, 20† |
| Reference . | Study design . | Treatment . | Disease state . | No. of patients . | ORR, % . | CR, % . | Med. f/u, mo (range) . | OS, % . | PFS, % . | Leukopenia grade 3, %/grade 4, % . | Other adverse events grade 3, %/grade 4, % . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Prospective clinical trials | |||||||||||
| 63 | Phase 2 | AspaMetDex ×3 | Relapsed (n = 11), refractory (n = 8) | 19 | 78 (n = 18) | 61 (n = 18) | 26 (17-49) | 12 mo* | 12 mo* | n = 8†,‡ | Liver, n = 3† Anemia, n = 4† Allergy, n = 1† |
| 52 | Phase 2 | SMILE ×2 | N-d stage IV (n = 20), first relapse (n = 14), first refractory (n = 4) | 38 | 79 (n = 38) 80 (N-d stage IV) | 45 (n = 38) 40 (N-d stage IV) | 24 (13-35) | 55 (1 y) | 53 (1 y) | 24/76 | Infection, 45/16 Anemia, 47/3 Thrombocytopenia, 24/40 |
| Retrospective studies | |||||||||||
| 69 | Multicenter | MEDA Med., 4 (range, 1-6) | Relapsed (n = 7) or refractory (n = 6) with advanced disease | 13 | 77 | 62 | 25 (20-30) | 69 (1 y) | 62 (1 y) | 46†,‡ | Infection, 15† |
| Thrombocytopenia, 31† | |||||||||||
| 70 | Single center | P-GEMOX Med., 5 (range, 2-8) → RT or HD-AHSCT | N-d advanced (n = 19), relapsed or refractory (n = 16) | 35 | 94§ | 26§ | 28 (9-50) | 65 (2 y) | 39 (2 y) | 31/9 | Anemia, 20/6 Thrombocytopenia, 14/17Hypertriglyceridemia, 9/11 |
| 73 | Single center | GDP Med., 6 (range, 2-8) | N-d stage IV (n = 15), relapsed or refractory (n = 26) | 41 | 83 | 42 | 16 (2-96) | 73 (1 y) | 55 (1 y) | 34†,‡ | Anemia, 15†Thrombocytopenia, 20† |
f/u, follow-up; med., median; MEDA, high-dose methotrexate, etoposide, dexamethasone, and pegaspargase; N-d, newly diagnosed.
Median survival time.
Grades 3 and 4.
Neutropenia.
After 2 cycles of P-GEMOX.
A phase 2 study of l-asparaginase, methotrexate, and dexamethasone (AspaMetDex)62,63 for relapsed or refractory ENKL enrolled 19 patients and achieved a CR rate of 61% after 3 cycles in the 18 evaluable patients (Table 3). The major toxicities were neutropenia, elevated transaminase levels, and anemia. In this trial, allergic reactions were documented in 4 patients, and Erwinial-asparaginase was used in some of the patients.
SMILE chemotherapy was developed in cooperative clinical trials in East Asia to explore a more effective induction therapy before HSCT.64 This is the only chemotherapeutic regimen that has demonstrated efficacy in clinical trials in newly diagnosed stage IV ENKL (Table 3). A phase 2 study of SMILE was performed in 38 patients with newly diagnosed stage IV ENKL or relapsed/refractory ENKL after first-line therapy. After 2 cycles, the ORR and CR rates were 79% and 45%, respectively (Table 3).52 The 5-year OS rate of the patients with newly diagnosed stage IV disease was 45%.65
AspaMetDex and SMILE are listed as suggested treatment regimens in the NCCN guidelines.49 Although SMILE chemotherapy has been shown to achieve excellent efficacy, severe bone marrow suppression and infection have been reported.66,67 A triweek regimen of SMILE combined with daily administration of l-asparaginase resulted in unacceptable toxicity.68 To reduce the toxicity of SMILE, other SMILE-like regimens were developed.69 In Japan, a recent retrospective survey revealed that the incidence of severe infection was reduced by restricting the use of SMILE in fit patients.50 To avoid severe infection, SMILE chemotherapy is not recommended for patients with a low lymphocyte count (<500/μL) (Figure 2).52
P-GEMOX is a modification of the gemcitabine, l-asparaginase, and oxaliplatin (GELOX) regimen in which l-asparaginase is switched to pegaspargase.70 P-GEMOX was also included as a suggested treatment regimen in the NCCN guidelines.49 A single-institution retrospective study in China performed in 35 patients with newly diagnosed advanced stage or relapsed/refractory ENKL reported that this regimen achieved an excellent ORR of 94.3% after 2 cycles.70
l-asparaginase is regarded as the most important drug in treatments for advanced ENKL. A meta-analysis showed that the use of l-asparaginase was associated with better ORR and CR rates in both localized and systemic ENKL.71 No clinical trial has compared the efficacy of conventional E colil-asparaginase to that of pegaspargase, although a retrospective study of ifosfamide, methotrexate, etoposide, and prednisone administered with either pegaspargase or l-asparaginase reported that admissions were shorter for pegaspargase than l-asparaginase and that OS was comparable between the 2.72
A retrospective study of the GDP regimen in patients with disseminated ENKL reported that the ORR rate was 83% after a median of 6 cycles (Table 3). Bone marrow suppression was mild, and none of the patients experienced grade 3 or 4 febrile neutropenia.73 GDP is promising because it is a less toxic regimen; it therefore warrants further evaluation in a prospective trial.
HSCT in the new era
Evidence supporting the use of HSCT to treat ENKL is based on the results of phase 1 and 2 studies and retrospective studies. The current consensus is that consolidative HD-AHSCT is not necessary in patients with newly diagnosed localized ENKL who achieved a CR after treatment with a modern therapy.74 For example, the 5-year OS was ≥70% in patients who received RT-2/3DeVIC and no posttherapy following the first CR.47,50
However, there is a controversy over which type of HSCT is most appropriate in patients with newly diagnosed advanced-stage or relapsed ENKL. An early study published in the 2000s included 22 patients with ENKL who underwent allogeneic HSCT and reported that the 2-year OS rate for HSCT after a median follow-up of 34 months was 40%.75 There were no relapses later than 10 months after HSCT. This study demonstrated that HSCT produces long-term remission in a small group of patients with ENKL at the expense of nonrelapse mortality (NRM). The 1-year NRM for myeloablative conditioning and reduced-intensity conditioning was 30% and 20%, respectively. A retrospective study performed in East Asia that included 18 patients who underwent allogeneic HSCT, including 14 patients who received SMILE before allogeneic HSCT, reported that at a median follow-up of 20.5 months, the OS rate was >60% and the event-free survival rate was >50%.76 The survival curve reached a plateau at 2 years after HSCT. The use of SMILE chemotherapy before HSCT and the absence of acute graft-versus-host disease were significantly associated with better survival. Allogeneic HSCT was not recommended before the first CR because there was no significant difference in OS between patients who received allogeneic HSCT before the first and second CR. The largest retrospective analysis was performed by the Center for International Blood and Marrow Transplant Research and was reported in 2017.77 The study included 82 patients from 43 institutes and demonstrated that the 3-year OS, PFS, and NRM rates were 34%, 28%, and 30%, respectively, at a median follow-up of 36 months (range, 1-121 months).
In terms of the use of upfront HD-AHSCT, a retrospective analysis published by Consortium for Improving Survival of Lymphoma recruited 62 patients with ENKL, including 31 patients with advanced disease, and reported that the 3-year OS and PFS rates were 52.3% and 40.1%, respectively, at a median follow-up of 43.3 months.78 The transplant-related mortality rate was 3.2% (n = 1). The largest retrospective study in Western countries was performed by the European Society for Bone and Marrow Transplantation and included 28 patients.79 That study reported that the 1-year nontransplant-related mortality rate was 11%; the 2-year OS and PFS rates were 40% and 33%, respectively, in patients with advanced disease; and all documented relapses occurred within 1 year after transplant.
The guidelines by the American Society for Blood and Marrow Transplantation supported the use of both autologous (strong) and allogeneic (weak) HSCT for chemosensitive relapsed disease in localized ENKL or as a front-line consolidation therapy for disseminated ENKL.74 In contrast, no HSCT was recommended for use as a front-line consolidation therapy for localized ENKL. The European Society of Medical Oncology Clinical Practice guidelines listed only autologous HSCT in the algorithm used to determine which treatment was most appropriate for ENKL.80 However, experts in Asia have proposed that upfront allogeneic HSCT is beneficial when used in high-risk patients.81,82 Because all of these recommendations are based on retrospective analyses and may be influenced by expert opinions, further studies are needed to establish which HSCT is most appropriate for treating ENKL.
Immuno- and cellular therapy
Tumor cells in EBV-associated malignancies express latent membrane proteins (LMPs) that are good candidate targets for cytotoxic T-lymphocytes (CTLs). A clinical trial that evaluated the use of LMP-CTL as an adjuvant therapy for high-risk or multiple-relapse EBV-associated lymphoma in the United States enrolled 11 patients with ENKL.83 Among these patients, 8 survived for at least 2 years. Another clinical trial that evaluated the use of EBV-CTL as an adjuvant therapy following HD-AHSCT was conducted in South Korea and enrolled 13 patients with ENKL.84 Ten of 11 patients who received cell therapy achieved disease-free survival for more than 2 years. The results of these studies demonstrated that immunotherapy is a promising treatment strategy in ENKL.
Immune checkpoint inhibitors have produced promising results that challenge the current treatment paradigm for ENKL. Programmed death-ligand 1 (PD-L1) is frequently expressed by ENKL cells.85-88 Several studies have shown that serum PD-L1 levels are associated with a prognosis of ENKL.87,88 A pilot study of the use of a single agent, pembrolizumab (an anti–PD-1 antibody drug), was conducted by investigators in Asian countries.6 Seven patients who relapsed or were refractory after SMILE or SMILE plus platinum chemotherapy received a median of 7 cycles (range, 2-13) of pembrolizumab. Five of the patients achieved a CR (tissue EBER-positive, n = 2; circulating EBV-DNA-positive, n = 1), and 2 of the patients achieved a PR. The ORR was 100%. Two separate studies described 3 cases of relapsed or refractory ENKL that achieved a CR following therapy with pembrolizumab.89,90 In another study, low-dose nivolumab was used in 3 patients with relapsed or refractory ENKL after SMILE with or without platinum; this treatment achieved a clinical response.91 Because the follow-up period in these studies was ≤13 months, further long-term evaluations are warranted. Special precautions are needed in the use of immune checkpoint inhibitors before allogeneic HSCT because acute graft-versus-host disease occurred frequently in patients who received immune checkpoint inhibitors before allogeneic HSCT.92,93
Other new agents
There are few additional new candidate agents for treating ENKL. The number of patients is also small, with <20 patients tested using each of these new agents (Table 4). Because experience with the use of these newer agents in the treatment of ENKL is limited, use outside the context of clinical trials is not recommended.
Selected experiences with new agents in patients with ENKL
| Agent . | Study design . | Treatment . | No. of patients . | Disease state . | Outcome . | Reference . |
|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors | ||||||
| Pembrolizumab | Retrospective | Single agent | 7 | Relapsed or refractory after SMILE-like therapy | CR, n = 5; PR, n = 2; ORR, 100% | 6 |
| Retrospective | Single agent | 1 | Refractory | CR | 89 | |
| Retrospective | Single agent | 7 | Relapsed or refractory | CR, n = 2; PR, n = 2; ORR, 57% | 90 | |
| Nivolumab | Retrospective | Single agent, low dose | 3 | Relapsed or refractory after SMILE-like therapy | CR, n = 2; SD, n = 1 | 91 |
| Other “new” agents* | ||||||
| Alemtuzumab | Phase 2, multicenter | Combined with CHOP | 3 | Newly diagnosed | CR, n = 1; SD, n = 1; PD, n = 1 | 94 |
| Phase 2, multicenter | Combined with DHAP | 8 | Relapsed or refractory after first-line therapy | PR, n = 1; PD, n = 7 | 95 | |
| Thalidomide | Prospective, single center | Combined with CHOP and RT, GELOX, and others | 12 | Newly diagnosed (n = 9), relapsed (n = 3) | CR, n = 8; PR, n = 1; PD, n = 3 | 102 |
| Romidepsin | Phase 2 | Single agent | 1 | Relapsed or refractory | ORR, 0% | 107 |
| Pilot study | Single agent | 5 | Relapsed or refractory | NE, n = 4; SD, n = 1; EBV reactivation (n = 3) | 108 | |
| Agent . | Study design . | Treatment . | No. of patients . | Disease state . | Outcome . | Reference . |
|---|---|---|---|---|---|---|
| Immune checkpoint inhibitors | ||||||
| Pembrolizumab | Retrospective | Single agent | 7 | Relapsed or refractory after SMILE-like therapy | CR, n = 5; PR, n = 2; ORR, 100% | 6 |
| Retrospective | Single agent | 1 | Refractory | CR | 89 | |
| Retrospective | Single agent | 7 | Relapsed or refractory | CR, n = 2; PR, n = 2; ORR, 57% | 90 | |
| Nivolumab | Retrospective | Single agent, low dose | 3 | Relapsed or refractory after SMILE-like therapy | CR, n = 2; SD, n = 1 | 91 |
| Other “new” agents* | ||||||
| Alemtuzumab | Phase 2, multicenter | Combined with CHOP | 3 | Newly diagnosed | CR, n = 1; SD, n = 1; PD, n = 1 | 94 |
| Phase 2, multicenter | Combined with DHAP | 8 | Relapsed or refractory after first-line therapy | PR, n = 1; PD, n = 7 | 95 | |
| Thalidomide | Prospective, single center | Combined with CHOP and RT, GELOX, and others | 12 | Newly diagnosed (n = 9), relapsed (n = 3) | CR, n = 8; PR, n = 1; PD, n = 3 | 102 |
| Romidepsin | Phase 2 | Single agent | 1 | Relapsed or refractory | ORR, 0% | 107 |
| Pilot study | Single agent | 5 | Relapsed or refractory | NE, n = 4; SD, n = 1; EBV reactivation (n = 3) | 108 | |
DHAP, dexamethasone, high-dose cytarabine, and cisplatin; NE, not evaluated; PD, progressive disease; SD, stable disease.
Evaluated in ≥5 patients with ENKL.
Alemtuzumab, a humanized CD52 antibody, was used in combination with CHOP and produced 1 CR among 3 patients with newly diagnosed ENKL.94 The use of alemtuzumab with dexamethasone, high-dose cytarabine, and cisplatin in patients who relapsed or were refractory after first-line therapy produced a low ORR (1/8, 12.5%),95 suggesting that the efficacy of this antibody is limited in ENKL. A few patients with refractory ENKL achieved a CR after treatment with CD38 antibody (daratumumab) monotherapy96 or the antibody–drug conjugate brentuximab vedotin alone97 or in combination with bendamustine.98
Although bortezomib had an antitumor effect in ENKL cells in vitro,99,100 clinical experience with this agent is limited.101 In terms of immunomodulatory drugs, thalidomide showed moderate efficacy when used in combination with CHOP and RT or GELOX (Table 4).102 A report described the use of lenalidomide in a patient with relapsed ENKL after HD-AHSCT.103
Among histone deacetylase inhibitors, vorinostat had an inhibitory effect on the JAK-STAT pathway and inhibited proliferation in ENKL cell lines104 and a mouse xenograft model.105 A single case report described a case of relapsed pediatric ENKL in which the patient harbored a STAT3 mutation and obtained sustained remission for more than 2 years after treatment with vorinostat.106 Data regarding the efficacy of romidepsin are limited,107,108 and a fatal EBV reactivation was documented in a pilot study.108 However, EBV reactivation did not occur in 2 patients who were enrolled in a phase 2 study of panobinostat plus bortezomib and in a patient who received romidepsin plus bortezomib outside the context of a clinical trial.109
Conclusion and future perspectives
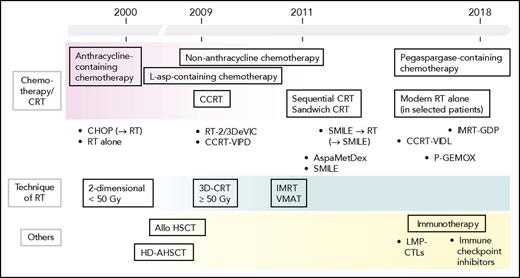
The treatments developed to treat ENKL are illustrated in Figure 3. The development of treatment approaches that use CCRT with platinum or l-asparaginase–containing chemotherapy and avoid the use of anthracyclines has contributed to improving the prognoses of patients with ENKL. However, nearly one-half of patients with newly diagnosed ENKL continue to experience disease progression, and the prognosis in patients with relapsed or refractory ENKL remains unsatisfactory.110 To explore a more effective treatment approach, multi-institutional, multinational, and multidisciplinary collaborations and clinical trials are needed in this era of revised treatments for ENKL.
Development of treatments for ENKL. Before 2000, patients with ENKL were treated with RT alone or with the same approaches, including CHOP-like chemotherapy with or without consolidative RT, used to treat other aggressive lymphomas. Clinical trials of CCRT were initiated in the early 2000s. In the mid-2000s, a study published achieving durable remission following the use of allogeneic HSCT in patients with disseminated ENKL. In 2009, the results of 2 clinical trials of CCRT with non-anthracycline chemotherapy were published. In 2011, a report showed that l-asparaginase–containing regimens achieved excellent efficacy. Then, reports described the use of pegaspargase-containing regimens and modern RT alone in selected patients. Furthermore, immunotherapy using LMP-CTL were developed. In 2017, immune checkpoint inhibitors produced promising responses. OS following the use of a first-line therapy could potentially be inferred from the efficacy of posttreatments, such as l-asparaginase–containing chemotherapy and allogeneic HSCT. Over time, RT delivery has changed from 2- or 3-dimensional CRT to modern techniques. The dose of RT has been increased from 40 to 50 Gy, and the RT volume was also increased. Allo, allogeneic.
Development of treatments for ENKL. Before 2000, patients with ENKL were treated with RT alone or with the same approaches, including CHOP-like chemotherapy with or without consolidative RT, used to treat other aggressive lymphomas. Clinical trials of CCRT were initiated in the early 2000s. In the mid-2000s, a study published achieving durable remission following the use of allogeneic HSCT in patients with disseminated ENKL. In 2009, the results of 2 clinical trials of CCRT with non-anthracycline chemotherapy were published. In 2011, a report showed that l-asparaginase–containing regimens achieved excellent efficacy. Then, reports described the use of pegaspargase-containing regimens and modern RT alone in selected patients. Furthermore, immunotherapy using LMP-CTL were developed. In 2017, immune checkpoint inhibitors produced promising responses. OS following the use of a first-line therapy could potentially be inferred from the efficacy of posttreatments, such as l-asparaginase–containing chemotherapy and allogeneic HSCT. Over time, RT delivery has changed from 2- or 3-dimensional CRT to modern techniques. The dose of RT has been increased from 40 to 50 Gy, and the RT volume was also increased. Allo, allogeneic.
Acknowledgments
This work was supported by the Japan Society for the Promotion of Science Grant-in-Aid for Scientific Research (JSPS KAKENHI, grants 26461418 and 17K09924) (M.Y. and R.S.).
Authorship
Contribution: M.Y., R.S., and M.O. wrote the paper.
Conflict-of-interest disclosure: M.Y. received honoraria from Bristol-Myers Squibb, Celgene, Chugai Pharmaceutical, Eisai, Kyowa Hakko Kirin, Meiji Seika Pharma, Nippon Shinyaku, Takeda Pharmaceutical, and Teijin Pharma; and served consulting or advisory role of Erytech Pharma. R.S. received honoraria from Kyowa Hakko Kirin, Chugai Pharmaceutical, Mochida Pharmaceutical, Novartis, Shionogi, Takeda Pharmaceuticals, Meiji Seika Pharma, Merck Sharpe & Dohme, Otsuka, Sawai Pharmaceutical, Celgene, Bristol-Myers Squibb, and Sumitomo Dainippon Pharma; and served consulting or advisory role of Gilead Sciences, Mundipharma, and Jazz Pharmaceuticals. M.O. declares no competing financial interests.
Correspondence: Motoko Yamaguchi, Department of Hematology and Oncology, Mie University Graduate School of Medicine, 2-174 Edobashi, Tsu, Mie 514-8507, Japan; e-mail: myamaguchi@clin.medic.mie-u.ac.jp.