Key Points
A model combining baseline metabolic tumor volume and EOI PET identify follicular lymphoma patients with a very high risk of progression.
Abstract
Both total metabolic tumor volume (TMTV), computed on baseline positron emission tomography (PET), and end of induction (EOI) PET are imaging biomarkers showing promise for early risk stratification in patients with high-tumor-burden follicular lymphoma. A model was built incorporating these 2 factors in 159 patients from three prospective trials: 2 Lymphoma Study Association (LYSA) studies and 1 Fondazione Italiana Linfomi (FIL) trial. Median follow up was 64 months. High TMTV (>510 cm3) and positive EOI PET were independent, significant risk factors for progression. Their combination stratified the population into 3 risk groups: patients with no risk factors (n = 102; 64%) had a 5-year progression-free survival (PFS) of 67% vs 33% (hazard ratio [HR], 2.9; 95% confidence interval [CI], 1.8-4.9) for patients with 1 risk factor (n = 44; 27%) and only 23% (HR, 4.6; 95% CI, 2.3-9.2) for patients with both risk factors (n = 13; 8%). 2-year PFS was respectively 90% vs 61% (HR, 4.8; 95% CI, 2.2-10.4) and 46% (HR, 8.1; 95%CI, 3.1-21.3). This model enhances the prognostic value of PET staging and response assessment, identifying a subset of patients with a very high risk of progression and early treatment failure at 2 years.
Introduction
Follicular lymphoma (FL) is the most frequent indolent lymphoma.1 Although outcomes have improved with immunochemotherapy and rituximab maintenance, ∼1 out of 5 patients experiences disease recurrence within 2 years after first-line therapy and has a 5-year survival of only 50%.2 The National Clinical Trials Network of the National Cancer Institute proposes a focused effort to better identify this group of high-risk patients, currently not adequately characterized by available clinical prognostic models, such as the Follicular Lymphoma International Prognostic Index (FLIPI) or FLIPI2.3 Therefore, models combining clinical and molecular data have been proposed,4 which require prospective validation in large cohorts.
Among promising prognostic factors, both disease burden and response to therapy can be mapped using positron emission tomography (PET) imaging. The value of PET for outcome prediction was already demonstrated in 2 studies performed on a pooled analysis of 3 prospective trials. First, a positive end of induction (EOI) PET identified a high-risk group with a 17-month median progression-free survival (PFS) and a significantly increased risk of death5 ; then, the total metabolic tumor volume (TMTV) measured on baseline PET, which reflects active tumor burden, was also strongly prognostic of PFS and overall survival (OS) and better identified patients progressing within 2 years than the FLIPI indices.6
This led us to determine whether a model combining both EOI PET and TMTV could better identify high-risk patients.
Study design
The follicular lymphoma collaboration population (FOLLCOLL) study pooled 1819 patients from 3 multicenter prospective studies.5 Patients with both an EOI PET and a baseline PET were included in this analysis. All the centers involved in the trials used same generation scanners and followed the rule of good practice defined for PET imaging in oncology.
EOI PET, performed within 3 months after the last rituximab administration, was centrally reported by 3 nuclear medicine physicians. Scans were defined as positive by a Deauville score of 4 (ie, fluorodeoxyglucose uptake moderately higher than uptake in the liver) or higher. TMTV was measured using the 41% thresholding method. This technique minimizes the impact on TMTV of technical variations in maximum standardized uptake values. A 510 cm3 cut point, determined by X tile and receiver operating characteristic analysis, validated in the population split into training and validation sets, was used for PFS and OS prediction.6
Survival functions were calculated using Kaplan-Meier estimates, and comparison between categories was made with the log-rank test. Univariate and multivariable analyses were performed using Cox proportional hazards models stratified on the variable “study” (ie, treatment regimen) and EOI PET included as time-dependent covariate. Differences between results of comparative tests were significant if the 2-sided P value was ≤.05. Statistical analyses used SAS 9.2 and X-tile 3.6.1 software (Yale University, New Haven, CT) and MedCalc software (MedCalc Software, Ostend, Belgium).
Results and discussion
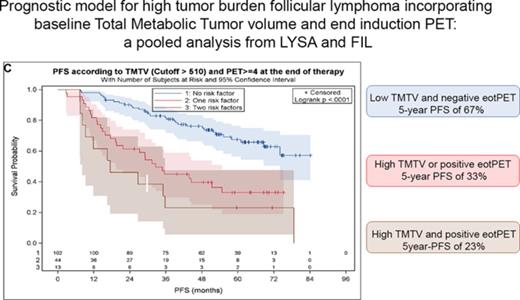
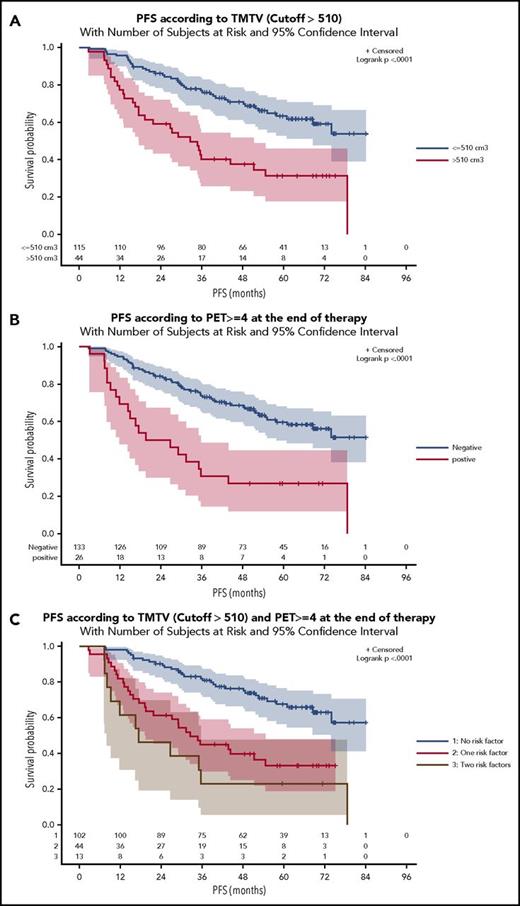
Of the 246 patients with centrally reviewed EOI PET and 185 with baseline PET, 159 had both PET examinations: 97 from the PET-Folliculaire (PET-FOL), 26 from the Primary Rituximab and Maintenance (PRIMA), and 36 from the FOLL05 study. Treatment regimens comprised rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisolone in 81% of patients plus cyclophosphamide, vincristine, and prednisolone in 14.5% or fludarabine and mitoxantrone in 4%. Only 10 patients received rituximab maintenance for 2 years. Only 2 patients were diagnosed with disease transformation during follow-up. Baseline characteristics of these 159 patients (Tables 1 and 2) did not differ significantly from the 1660 patients without PET scans except for number of extranodal sites >1 (35.2% vs 43.6%), lactate dehydrogenase above the upper limit of normal (22.6% vs 30.3%), and β2-microglobulin above the upper limit of normal (46.1% vs 35.8%). FLIPI2 calculation was missing in 20 patients (13% of the group). The median TMTV was 260 cm3 (interquartile range, 127-554 cm3). A high TMTV (>510 cm3) was present in 44 out of 159 (27.7%) patients, and 26 out of 159 (16.4%) had a positive EOI PET. Frequency of EOI PET positivity was significantly higher in patients with high TMTV (29.5% vs 11.3%, P = .008). However, half (13/26) of patients with a positive EOI PET had a low TMTV (≤510 cm3). With a median follow-up of 64 months, 71 patients progressed and 14 died, with 5-year PFS and OS estimates of 54.2% and 91.9%, which did not differ significantly from the patients not included in this PET study. As documented previously, patients with a high TMTV experienced an inferior (31.3%) 5-year PFS compared with patients with a low TMTV (63.2%) (hazard ratio [HR], 2.8; 95% confidence interval [CI], 1.7-4.5; P < .0001), and a 83.8% 5-year OS (vs 95%) (HR, 3.9; 95% CI, 1.3-11.9; P = .019; Figure 1A; supplemental Figure 1A, available at the Blood Web site). Patients with a positive EOI PET had an inferior (26.9%) 5-year PFS (vs 59.6% in PET-negative patients) (HR, 3.0; 95% CI, 1.7-5.2; P < .0001) and an 84.0% 5-year OS (vs 93.3%) (HR, 4.4; 95% CI, 1.50-12.62; P = .007; Figure 1B; supplemental Figure 1B). High TMTV and positive EOI PET were independent negative predictors of PFS and OS (Table 1). A prognostic model combining these 2 adverse factors identified 3 risk groups (Figure 1C). A low-risk reference group comprising the majority (n = 102) of patients, with a low TMTV and EOI PET–negative findings, had a 67.5% 5-year PFS. An intermediate-risk group (n = 44) comprising patients who were either EOI PET positive with a small TMTV (n = 13) or EOI PET negative with a high TMTV (n = 31) had a 33% 5-year PFS (P < .0001). A high-risk group (n = 13) comprising patients who were EOI PET positive and had a high TMTV had a 23.1% 5-year PFS (P < .0001; HR, 4.6; 95% CI, 2.3-9.2). The PFS was significantly different between the low- and high-risk groups, comprising 102 and 13 patients, respectively, with a power of 80.5%. There was also a significant difference in 5-year OS between low- and high-risk groups (96.3% and 83.3%, respectively) (P = .0016; HR, 11.33; 95% CI, 2.51-51.21; supplemental Figure 1C). The proportion of deaths was significantly higher in high-risk patients (30.8%) than in low-risk patients (2.9%) (P = .003). The 2-year PFS (progression of disease within 24 months) was 90.2% in the low-risk reference group, 61.4% in the intermediate-risk group (P < .0001; HR, 4.8; 95% CI, 2.2-10.4), and 46.2% in the high-risk group (P < .0001; HR, 8.1; 95% CI, 3.1-21.3). No difference in 2-year PFS was observed between the intermediate-risk and high-risk groups (P = .24).
Patient characteristics for the FOLLCOLL population, and univariate analysis of PFS and OS stratified according to study
| Characteristics . | Total population (N = 159) . | Univariate analysis of PFS . | Univariate analysis of OS . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Age (y), median (IQ range) | 56 (48-65) | ||||
| >60 | 59 (37) | 1.28 (0.78-2.08) | .33 | 1.47 (0.51-4.19) | .48 |
| Female | 79 (50) | 0.84 (0.52-1.35) | .47 | 0.41 (0.13-1.34) | .14 |
| Histologic grade | |||||
| 1 | 66 (44) | — | — | — | — |
| 2 | 52 (34) | 1 | 1 | ||
| 3a | 18 (12) | 1.07 (0.62-1.85) | .82 | 1.03 (0.29-3.68) | .96 |
| Unknown | 15 (10) | 0.70 (0.27-1.83) | .47 | 1.06 (0.13-9.00) | .96 |
| Missing | 8 | 1.12 (0.49-2.57) | .80 | 0.92 (0.11-7.82) | .94 |
| Ann Arbor stage III-IV | 144 (91) | 1.33 (0.54-3.32) | .54 | 1.37 (0.18-10.72) | .76 |
| ECOG 2-3 | 10 (6) | 2.35 (1.04-5.28) | .039 | — | — |
| Missing | 2 | — | — | — | — |
| Number of involved nodes >4 | 90 (57) | 1.22 (0.75-1.97) | .43 | 2.80 (0.76-10.34) | .12 |
| Number of extranodal sites >1 | 56 (35) | 1.71 (1.06-2.74) | .027 | 3.41 (1.13-10.33) | .03 |
| BMB+ | 85 (57) | 1.67 (0.99-2.82) | .057 | 3.86 (0.85-17.48) | .079 |
| Missing | 11 | — | — | — | — |
| LodLIN >6 cm | 69 (46) | 1.37 (0.84-2.22) | .21 | 2.19 (0.65-7.38) | .21 |
| Missing | 8 | — | — | — | — |
| Increased LDH | 36 (23) | 1.22 (0.69-2.15) | 0.49 | 1.18 (0.32-4.38) | 0.80 |
| Missing | 0 | — | — | — | — |
| Increased β2 microglobulin | 59 (46) | 1.97 (1.12-3.48) | 0.0188 | 1.95 (0.42-9.08) | 0.40 |
| Missing | 31 | — | — | — | — |
| FLIPI >3-5 | 58 (37) | 1.75 (1.09-2.82) | .021 | 3.09 (1.02-9.40) | .047 |
| Missing | 0 | — | — | — | — |
| FLIPI2 >3-5 | 43 (31) | 2.30 (1.38-3.85) | .0015 | 3.71 (0.88-15.70) | .075 |
| Missing* | 20 | — | — | — | — |
| High TMTV >510 cm3 | 44 (28) | 2.77 (1.71-4.51) | <.001 | 3.85 (1.25-11.85) | .019 |
| Positive EOI PET | 26 (16) | 3.01 (1.74-5.22) | <.001 | 4.35 (1.50-12.62) | .007 |
| Characteristics . | Total population (N = 159) . | Univariate analysis of PFS . | Univariate analysis of OS . | ||
|---|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | ||
| Age (y), median (IQ range) | 56 (48-65) | ||||
| >60 | 59 (37) | 1.28 (0.78-2.08) | .33 | 1.47 (0.51-4.19) | .48 |
| Female | 79 (50) | 0.84 (0.52-1.35) | .47 | 0.41 (0.13-1.34) | .14 |
| Histologic grade | |||||
| 1 | 66 (44) | — | — | — | — |
| 2 | 52 (34) | 1 | 1 | ||
| 3a | 18 (12) | 1.07 (0.62-1.85) | .82 | 1.03 (0.29-3.68) | .96 |
| Unknown | 15 (10) | 0.70 (0.27-1.83) | .47 | 1.06 (0.13-9.00) | .96 |
| Missing | 8 | 1.12 (0.49-2.57) | .80 | 0.92 (0.11-7.82) | .94 |
| Ann Arbor stage III-IV | 144 (91) | 1.33 (0.54-3.32) | .54 | 1.37 (0.18-10.72) | .76 |
| ECOG 2-3 | 10 (6) | 2.35 (1.04-5.28) | .039 | — | — |
| Missing | 2 | — | — | — | — |
| Number of involved nodes >4 | 90 (57) | 1.22 (0.75-1.97) | .43 | 2.80 (0.76-10.34) | .12 |
| Number of extranodal sites >1 | 56 (35) | 1.71 (1.06-2.74) | .027 | 3.41 (1.13-10.33) | .03 |
| BMB+ | 85 (57) | 1.67 (0.99-2.82) | .057 | 3.86 (0.85-17.48) | .079 |
| Missing | 11 | — | — | — | — |
| LodLIN >6 cm | 69 (46) | 1.37 (0.84-2.22) | .21 | 2.19 (0.65-7.38) | .21 |
| Missing | 8 | — | — | — | — |
| Increased LDH | 36 (23) | 1.22 (0.69-2.15) | 0.49 | 1.18 (0.32-4.38) | 0.80 |
| Missing | 0 | — | — | — | — |
| Increased β2 microglobulin | 59 (46) | 1.97 (1.12-3.48) | 0.0188 | 1.95 (0.42-9.08) | 0.40 |
| Missing | 31 | — | — | — | — |
| FLIPI >3-5 | 58 (37) | 1.75 (1.09-2.82) | .021 | 3.09 (1.02-9.40) | .047 |
| Missing | 0 | — | — | — | — |
| FLIPI2 >3-5 | 43 (31) | 2.30 (1.38-3.85) | .0015 | 3.71 (0.88-15.70) | .075 |
| Missing* | 20 | — | — | — | — |
| High TMTV >510 cm3 | 44 (28) | 2.77 (1.71-4.51) | <.001 | 3.85 (1.25-11.85) | .019 |
| Positive EOI PET | 26 (16) | 3.01 (1.74-5.22) | <.001 | 4.35 (1.50-12.62) | .007 |
Values represent n (%) of patients unless otherwise indicated.
BMB, bone marrow biopsy; IQ, interquartile; LDH, lactate dehydrogenase; LodLIN, longest diameter of the largest node.
LodLIN >6 cm and β2 microglobulin were not reported in all patients from PET-FOL and PRIMA, because these trials commenced and ended before FLIPI2 description.
Patient characteristics for the FOLLCOLL population, and multivariate analysis of PFS and OS stratified according to study
| Characteristics . | Multivariable analysis of PFS . | Multivariable analysis of OS . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| High TMTV >510 cm3 | 2.34 (1.4-3.9) | .0010 | 2.8 (0.9-9) | .080 |
| Positive EOI PET | 2.34 (1.3-4.4) | .0035 | 3.3 (1.1-9.7) | .036 |
| Characteristics . | Multivariable analysis of PFS . | Multivariable analysis of OS . | ||
|---|---|---|---|---|
| HR (95% CI) . | P . | HR (95% CI) . | P . | |
| High TMTV >510 cm3 | 2.34 (1.4-3.9) | .0010 | 2.8 (0.9-9) | .080 |
| Positive EOI PET | 2.34 (1.3-4.4) | .0035 | 3.3 (1.1-9.7) | .036 |
Prognostic model combining baseline and EOI PET. (A) PFS according to baseline TMTV with a cutoff of 510 cm3. (B) PFS according to end of induction PET (positive if 5-point scale (5PS) >3). (C) TMTV and EOI PET combined identify 3 risk categories.
Prognostic model combining baseline and EOI PET. (A) PFS according to baseline TMTV with a cutoff of 510 cm3. (B) PFS according to end of induction PET (positive if 5-point scale (5PS) >3). (C) TMTV and EOI PET combined identify 3 risk categories.
The incremental prognostic value of the combination of PET response with baseline PET quantitative parameters has been demonstrated in other lymphomas.7-11 In these studies, baseline TMTV better stratified the response to treatment assessed by EOI PET. Likewise, this integrated approach enables early identification of a subset of patients with FL with a very high risk of progression within 5 years of first-line treatment, better stratifying the risk of treatment failure than each parameter alone. Such high-risk patients could be considered for the evaluation of new agents.12 Importantly, this model clearly identified the majority low-risk population, lacking either risk factor, with a median PFS not reached after >5 years of follow-up. This population may likely benefit from the additional PFS advantage derived from rituximab maintenance, which was given to very few patients in this study. With validation of these results in other studies, including patients receiving maintenance, and standardization of both determination of TMTV and EOI PET status, this model could be applied in comparison or in combination with other biologically based prognostic indices4,13 or with minimal residual disease assessment at the end of therapy. Such composite models will likely provide a platform for both risk- and response-adapted therapy for FL in the future.
Presented at the 14th International Conference on Malignant Lymphoma, Lugano, Switzerland, 14-17 June 2017.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Lymphoma Academic Research Organisation and Romain Ricci for his help in handling the data.
Authorship
Contribution: A.S.C., M. Meignan, A.V., S.L., and J.T. contributed to study conception and design; M. Meignan, A.S.C., A.V., A.B.-R., R.-O.C., J.D., H.T., L.C., and J.T. collected and assembled data; A.S.C., M. Meignan, A.V., S.L., R.-O.C., M. Menga, V.T., H.T., C.H., G.S., and M.F. contributed to data analysis and interpretation; and all authors wrote the manuscript and approved the final draft.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Complete lists of the members of the Lymphoma Study Association and Fondazione Italiana Linfomi appear in “Appendix.”
Correspondence: Anne-Ségolène Cottereau, Nuclear Medicine Department, Cochin Hospital, René Descartes University, 27 rue du Faubourg Saint Jacques, 75014 Paris, France; e-mail: annesegolene.cottereau@gmail.com.
Appendix: study group members
The members of the Lymphoma Study Association are A.S.C., J.D., L.C., R.-O.C., A.B.-R., C.H., H.T., G.S., J.T., and M. Meignan. The members of the Fondazione Italiana Linfomi are A.V., S.L., M. Menga, V.T., and M.F.