Key Points
The coagulation protease FVIIa attenuates TNF-α- and LPS-induced inflammation both in vitro and in vivo via an EPCR-dependent mechanism.
FVIIa-EPCR-PAR1-mediated anti-inflammatory signaling transmits through the β-arrestin-1-dependent pathway.
Abstract
Recent studies show that endothelial cell protein C receptor (EPCR) interacts with diverse ligands, in addition to its known ligands protein C and activated protein C (APC). We showed in earlier studies that procoagulant clotting factor VIIa (FVIIa) binds EPCR and downregulates EPCR-mediated anticoagulation and induces an endothelial barrier protective effect. Here, we investigated the effect of FVIIa’s interaction with EPCR on endothelial cell inflammation and lipopolysaccharide (LPS)-induced inflammatory responses in vivo. Treatment of endothelial cells with FVIIa suppressed tumor necrosis factor α (TNF-α)- and LPS-induced expression of cellular adhesion molecules and adherence of monocytes to endothelial cells. Inhibition of EPCR or protease-activated receptor 1 (PAR1) by either specific antibodies or small interfering RNA abolished the FVIIa-induced suppression of TNF-α- and LPS-induced expression of cellular adhesion molecules and interleukin-6. β-Arrestin-1 silencing blocked the FVIIa-induced anti-inflammatory effect in endothelial cells. In vivo studies showed that FVIIa treatment markedly suppressed LPS-induced inflammatory cytokines and infiltration of innate immune cells into the lung in wild-type and EPCR-overexpressing mice, but not in EPCR-deficient mice. Mechanistic studies revealed that FVIIa treatment inhibited TNF-α-induced ERK1/2, p38 MAPK, JNK, NF-κB, and C-Jun activation indicating that FVIIa-mediated signaling blocks an upstream signaling event in TNFα-induced signaling cascade. FVIIa treatment impaired the recruitment of TNF-receptor-associated factor 2 into the TNF receptor 1 signaling complex. Overall, our present data provide convincing evidence that FVIIa binding to EPCR elicits anti-inflammatory signaling via a PAR1- and β-arrestin-1 dependent pathway. The present study suggests new therapeutic potentials for FVIIa, which is currently in clinical use for treating bleeding disorders.
Introduction
Endothelial cell protein C receptor (EPCR) is a key cellular receptor for protein C and activated protein C (APC). EPCR plays a critical role in the anticoagulation pathway by promoting protein C activation by the thrombin-thrombomodulin complex.1 Recent studies have established that EPCR plays a pivotal role in supporting APC-induced cytoprotective signaling through activation of protease-activated receptors (PARs).2-5 In addition to protein C and APC, other ligands such as Plasmodium falciparum erythrocyte membrane protein, a specific variant of the T-cell receptor, and factor VIIa (FVIIa) also bind EPCR.5 These observations indicate that EPCR may play a broader role in influencing various pathophysiological processes by interacting with different ligands in different milieus.
FVIIa’s primary function is to bind tissue factor (TF) after vascular injury and initiate the coagulation cascade by activating clotting factors IX and X. FVIIa-TF has also been shown to influence various cellular processes through the activation of PAR-mediated cell signaling.6,7 FVIIa-TF mediates a broad spectrum of signaling mechanisms, mostly inducing proinflammatory and proangiogenic cytokines and growth factors.7-10 Presently, it is not entirely clear whether FVIIa-EPCR, similar to FVIIa-TF or APC-EPCR, activates the PAR-mediated cell signaling. Initial studies employing a heterologous cell model system expressing EPCR and PAR1 or PAR2 reporter constructs showed no evidence that FVIIa-EPCR was capable of activating PARs or PAR-mediated cell signaling.11 Disse et al12 showed that EPCR is a functional component of the TF-FVIIa-FXa ternary complex and that EPCR induces more efficient cleavage of PAR1 and PAR2 by TF-FVIIa-FXa. Our studies with endothelial cells that constitutively express EPCR and PAR1 showed that FVIIa cleaves endogenous PAR1 in an EPCR-dependent fashion and that FVIIa binding to EPCR provides the barrier-protective effect in endothelial cells.13 In vivo studies in mice showed that the administration of FVIIa attenuated lipopolysaccharide (LPS)-induced vascular leakage in the lung and kidney.13 A subsequent study showed that FVIIa administration reduced LPS- and vascular endothelial growth factor (VEGF)-induced vascular permeability in wild-type (WT), but not EPCR-deficient, mice.13,14 These studies also showed that the FVIIa-induced barrier protective effect involves the activation of PAR1.14 Overall, our published data indicate that FVIIa-EPCR-PAR1 activates a barrier-protective signaling pathway in endothelial cells. However, studies conducted in EA.hy26 cells failed to show that FVIIa could prevent thrombin-induced enhanced permeability.15 Recent studies by Gleeson et al16 showed that an APC chimeric with an FVIIa-gla domain failed to mediate the EPCR- and PAR1-dependent barrier protective effect, indicating that amino acid residues other than the EPCR binding site in APC were necessary for cytoprotective PAR1 signaling. It is unclear at present the reason for these differences in FVIIa’s ability to provide a barrier-protective effect in the above studies, but it may reflect differences in endothelial cell types or reagents used in these studies.
Because FVIIa has been used widely to treat hemophilia patients with inhibitors and other bleeding disorders,17 it is important to find out with certainty whether FVIIa binding to EPCR mediates cell signaling and the consequences of such signaling. The goals of the present study were to investigate whether FVIIa binding to EPCR is capable of activating cytoprotective signaling beyond the barrier-protective effect and, if so, to elucidate the mechanisms by which FVIIa-EPCR exerts this cytoprotective effect. The data presented here are the first to show that FVIIa-EPCR attenuates tumor necrosis factor α (TNF-α)- and LPS-induced inflammation in both in vitro and in vivo models.
Materials and methods
See supplemental Materials and methods (available on the Blood Web site) for further information.
Endothelial cells
Primary human umbilical vein endothelial cells (HUVECs) were purchased from Lonza (Walkersville, MD).
Mice
Small interfering RNA (siRNA)
HUVECs were transfected with either control/scrambled oligonucleotide (scRNA) or siRNA specific for EPCR, PAR1, PAR2, β-arrestin 1, β-arrestin 2, or ApoER2 using Lipofectamine RNAiMax transfection reagent (ThermoFisher, Waltham, MA).
Cell treatments, RNA extraction, and RT-PCR
Monolayers of HUVECs were treated with a control vehicle, FVIIa, or other agonist with or without TNF-α or LPS. Messenger RNA (mRNA) and protein expression levels of adhesion molecules and interleukin-6 (IL-6) were measured by reverse transcription polymerase chain reaction (RT-PCR), western blot analysis, or enzyme-linked immunosorbent assay (ELISA).
Immunoprecipitation and immunoblotting
Cells were lysed in cell lysis buffer containing protease and phosphatase inhibitors. Cell lysates were incubated with Dynabeads coupled with a specific antibody overnight at 4°C to capture the immunocomplexes. The bound material was eluted by adding sodium dodecyl sulfate polyacrylamide gel electrophoresis sample buffer and subjected to immunoblot analysis. The band intensities were quantified by densitometric analysis using the Bio-Rad Chemi XRS system and ImageJ software.
Immunofluorescence microscopy, image acquisition, scoring, and colocalization
Animal studies
WT, EPCR-deficient, and EPCR-overexpressing (Tie2-EPCR) mice were injected with saline, human rFVIIa, or APC (25-250 μg/kg body weight in 100 μL saline) IV via the tail vein. One hour later, LPS (Escherichia coli O111: B4, 5 mg/kg; Sigma) was administered to mice via intraperitoneal injection. Animals were killed by exsanguination after 6 hours of LPS challenge. Lungs and kidneys were removed and processed for histology/immunohistochemistry or to measure cytokines. In some studies, control and FVIIa-treated WT mice were subjected to cecal ligation and puncture (CLP; 40% ligation) to induce polymicrobial sepsis, and plasma was collected 6 hours after CLP.
Measurement of mouse cytokines
Frozen tissues of lungs and kidneys were pulverized into powder and suspended in RIPA buffer (Cell Signaling Technology, MA) containing protease inhibitors. TNF-α, IL-6, IL-1β, and CCL2 levels in tissue extracts or plasma were estimated using ELISA kits (eBioscience).
Tissue sectioning, histology, and immunohistochemistry
The inflated lung tissues were fixed in Excel fixative (American Master Tech Scientific) and processed for embedding in paraffin. Thin tissue sections (5 μm) were cut and stained with hematoxylin and eosin to assess extravasation of blood cells and edema or processed for immunohistochemistry to identify the type of infiltrated blood cells.
Results
FVIIa attenuates TNF-α-induced inflammatory mediators
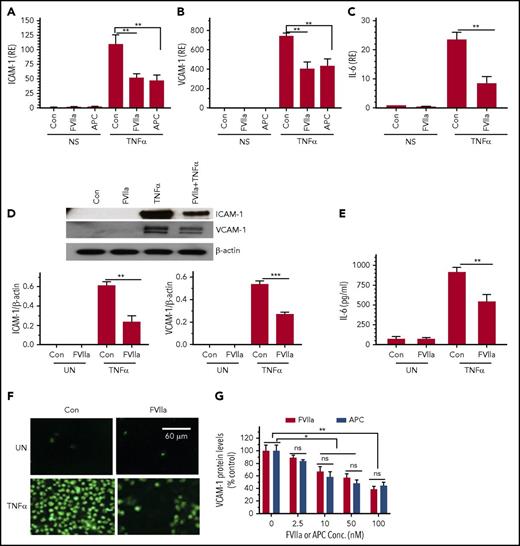
To investigate the role of FVIIa-EPCR in suppressing endothelial inflammation, endothelial cells were pretreated with a control vehicle or FVIIa and exposed to inflammatory cytokine TNF-α for 6 hours, and TNF-α-induced expression of cell adhesion molecules (ICAM-1 and VCAM-1) and IL-6 expression was measured. As shown in Figure 1A-C, FVIIa treatment markedly reduced TNF-α-induced ICAM-1, VCAM-1, and IL-6 gene expression. Analysis of ICAM-1 and VCAM-1 expression by western blot analysis (Figure 1D) and measurement of IL-6 protein levels in cell supernatants in an ELISA (Figure 1E) further confirmed that FVIIa reduces TNF-α-induced inflammatory mediators in endothelial cells. Confirming the functional importance of FVIIa suppression of TNF-α-induced cell adhesion molecules, exposure of endothelial cells to FVIIa prior to TNF-α stimulation reduced the adhesion of THP-1 monocytic cells to TNF-α-activated endothelial cells (Figure 1F). Analysis of FVIIa dose dependency showed that FVIIa at doses as low as 2.5 nM was sufficient to elicit detectable inhibitory effect on TNF-α-induced inflammatory gene expression; 10 to 100 nM of FVIIa was required to show a significant inhibition of TNF-α-induced inflammation as evaluated in the expression of VCAM-1 protein (Figure 1G), ICAM-1, and IL-6 (data not shown). A comparison of FVIIa and APC dose–response studies showed that FVIIa is as effective as APC in suppressing the TNF-α-induced inflammatory gene expression (Figure 1G).
FVIIa downregulates TNF-α-induced inflammatory gene expression in endothelial cells. HUVECs were serum-starved for 12 hours and stabilized for 1 hour in the serum-free medium. Cells were exposed to FVIIa or APC (100 nM) for 1 hour in serum-free medium and then treated with TNF-α (5 ng/mL) for 6 hours. (A-C) Total RNA was extracted from cells, and levels of mRNA were analyzed by quantitative RT-PCR for ICAM-1 (A), VCAM-1 (B), and IL-6 (C). mRNA levels present in nonstimulated (NS) cells treated with a control vehicle (Con) were taken as 1, and values in the experimental treatments were expressed relative to this value (RE). (D-E) FVIIa suppression of TNF-α-induced ICAM-1, VCAM-1, and IL-6 protein expression. The experimental conditions were the same as described above. ICAM-1 and VCAM-1 protein expression in cell lysates was analyzed by western blot analysis, and the signals were quantified by densitometric analysis using ImageJ software (D); IL-6 protein levels in the supernatants were measured by an ELISA (E). (F) HUVECs grown on the glass coverslip were treated with FVIIa and TNF-α (5 ng/ml) for 4 hours as described above. THP-1 cells labeled with a green fluorescent cell linker (PKH67) were added to the endothelial cell monolayer and allowed to adhere for 30 minutes in serum-rich RPMI medium. The nonadherent THP-1 cells were washed with serum-free medium, and the cells were fixed with 4% paraformaldehyde. The images of the adherent cells were captured by a fluorescent microscope at 100× magnification. UN, unstimulated cells. (G). Dose-dependent effect of FVIIa and APC on attenuation of TNF-α-induced VCAM-1 expression. HUVEC were treated with varying concentrations of FVIIa or APC (0 to 100 nM) for 1 hour and then stimulated with TNF-α (5 ng/ml) for 6 hours. Cell extracts were subjected to immunoblot analysis to determine VCAM-1 protein expression and actin (as a loading control). Band intensities were quantitated by densitometric analysis and normalized to the loading control. VCAM-1 expression levels in cells treated with TNFα alone was taken as 100%. Data shown in the bar graphs represent the mean ± SEM of 3 to 6 independent experiments. *P < .05; **P < .01; ***P < .001; ns, not statistically significant.
FVIIa downregulates TNF-α-induced inflammatory gene expression in endothelial cells. HUVECs were serum-starved for 12 hours and stabilized for 1 hour in the serum-free medium. Cells were exposed to FVIIa or APC (100 nM) for 1 hour in serum-free medium and then treated with TNF-α (5 ng/mL) for 6 hours. (A-C) Total RNA was extracted from cells, and levels of mRNA were analyzed by quantitative RT-PCR for ICAM-1 (A), VCAM-1 (B), and IL-6 (C). mRNA levels present in nonstimulated (NS) cells treated with a control vehicle (Con) were taken as 1, and values in the experimental treatments were expressed relative to this value (RE). (D-E) FVIIa suppression of TNF-α-induced ICAM-1, VCAM-1, and IL-6 protein expression. The experimental conditions were the same as described above. ICAM-1 and VCAM-1 protein expression in cell lysates was analyzed by western blot analysis, and the signals were quantified by densitometric analysis using ImageJ software (D); IL-6 protein levels in the supernatants were measured by an ELISA (E). (F) HUVECs grown on the glass coverslip were treated with FVIIa and TNF-α (5 ng/ml) for 4 hours as described above. THP-1 cells labeled with a green fluorescent cell linker (PKH67) were added to the endothelial cell monolayer and allowed to adhere for 30 minutes in serum-rich RPMI medium. The nonadherent THP-1 cells were washed with serum-free medium, and the cells were fixed with 4% paraformaldehyde. The images of the adherent cells were captured by a fluorescent microscope at 100× magnification. UN, unstimulated cells. (G). Dose-dependent effect of FVIIa and APC on attenuation of TNF-α-induced VCAM-1 expression. HUVEC were treated with varying concentrations of FVIIa or APC (0 to 100 nM) for 1 hour and then stimulated with TNF-α (5 ng/ml) for 6 hours. Cell extracts were subjected to immunoblot analysis to determine VCAM-1 protein expression and actin (as a loading control). Band intensities were quantitated by densitometric analysis and normalized to the loading control. VCAM-1 expression levels in cells treated with TNFα alone was taken as 100%. Data shown in the bar graphs represent the mean ± SEM of 3 to 6 independent experiments. *P < .05; **P < .01; ***P < .001; ns, not statistically significant.
The role of EPCR, PAR1, and β-arrestin in FVIIa-induced anti-inflammatory effect
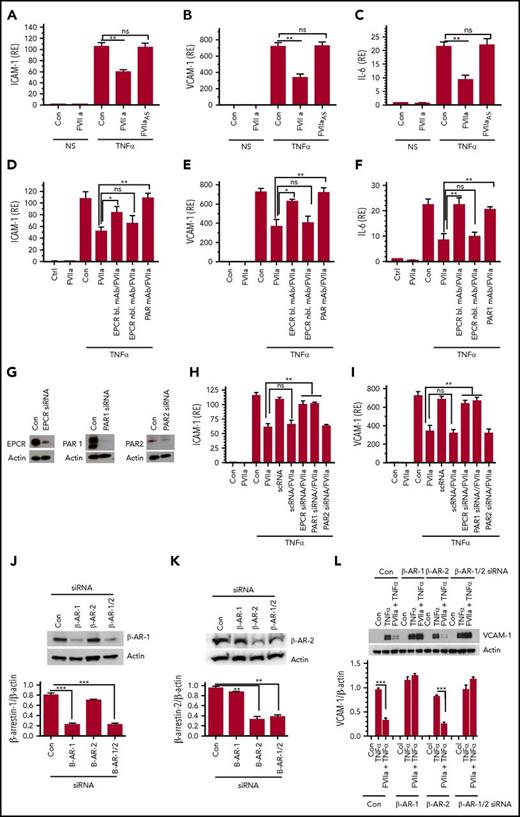
FVIIa-mediated suppression of TNF-α-induced inflammatory mediators requires FVIIa protease activity, as an active-site-inhibited FVIIa failed to block TNF-α-induced ICAM-1, VCAM-1, and IL-6 gene expression (Figure 2A-C). Pretreatment of endothelial cells with EPCR-blocking antibodies or PAR1 antibodies attenuated the FVIIa-induced anti-inflammatory effect, indicating the involvement of both EPCR and PAR1 in the FVIIa-mediated anti-inflammatory effect (Figure 2D-F). Consistent with the above data, suppression of EPCR or PAR1 expression by siRNA (Figure 2G) also blocked the anti-inflammatory effect of FVIIa (Figure 2H-I). In contrast, PAR2 silencing did not affect the FVIIa-mediated anti-inflammatory effect (Figure 2H-I). In additional experiments, we investigated the potential contribution TF in the FVIIa-induced anti-inflammatory effect. Treatment of endothelial cells with TF function-neutralizing monoclonal antibody (mAb) did not affect FVIIa suppression of TNF-α-induced ICAM-1, VCAM-1, and IL-6 expression (data not shown). In additional studies, endothelial cells were treated with either Rivaroxaban or hirudin, specific inhibitors of FXa and thrombin, respectively, prior to the addition of FVIIa and TNF-α. These inhibitors did not affect FVIIa-mediated inhibition of TNF-α-induced VCAM-1 expression (supplemental Figure 1). These data rule out that traces of FXa or thrombin that could be conceivably generated by FVIIa were responsible for the observed anti-inflammatory effect of FVIIa.
The FVIIa-mediated anti-inflammatory effect requires its protease activity, EPCR, PAR1, and β-arrestin-1. (A-C) FVIIa protease activity requirement. Serum-starved HUVECs were treated with FVIIa or active-site–inhibited FVIIa or FVIIaAS (100 nM) for 1 hour and then left unperturbed (NS) or stimulated with TNF-α (5 ng/ml) for 6 hours. ICAM-1 (A), VCAM-1 (B), and IL-6 (C) mRNA levels were measured by quantitative RT-PCR. (D-F) EPCR-blocking or PAR1 antibodies attenuate the anti-inflammatory effect of FVIIa. HUVECs were incubated with EPCR blocking (bl.) or nonblocking (nbl.) mAb (25 µg/mL) or a mixture of PAR1 mAb (ATAP-2, 25 µg/mL, and WEDE15, 20 µg/mL) for 1 hour prior to the addition of FVIIa (100 nM). Following FVIIa treatment for 1 hour, cells were stimulated with TNF-α (5 ng/ml) for 6 hours and the relative levels of ICAM-1 (D), VCAM-1 (E), and IL-6 (F) mRNA were measured by quantitative RT-PCR. (G). Silencing of EPCR, PAR1, or PAR2. HUVECs were transfected with control or EPCR-, PAR1-, or PAR2-specific siRNAs (200 nM) using Lipofectamine RNA Max reagent in serum-free medium. After 72 hours, cell lysates were made and analyzed for the expression of EPCR, PAR1, or PAR2 by western blot analysis using specific antibodies against these proteins. (H-I). HUVECs transfected with scrambled siRNA (scRNA), EPCR, PAR1, or PAR2 siRNA as described in panel G were treated with FVIIa (100 nM) for 1 hour and then stimulated with TNF-α (5 ng/ml) for 6 hours. ICAM-1 (H) and VCAM-1 (I) mRNA levels were measured in quantitative RT-PCR. (K-L) HUVECs were transfected with control siRNA or siRNA specific for β-arrestin-1 (β-AR-1), β-arrestin-2 (β-AR-2), or an equimolar mixture of both β-arrestin isoforms (β-AR-1/2). Forty-eight hours after transfection, cell lysates were made from the transfected cells probed for the expression of β-arrestin-1 (J) or β-arrestin-2 (K) by western blot analysis. (L) HUVECs transfected with control siRNA or siRNA specific for β-arrestin-1, β-arrestin-2, or β-arrestin-1/2 were treated with FVIIa (100 nM) and then stimulated with TNF-α (5 ng/ml). Cell lysates were probed for VCAM-1 levels by immunoblot analysis. Data are the mean ± SEM of 3 to 4 independent experiments. *P < .05; **P < .01; ***P < .001; ns, not statistically significant.
The FVIIa-mediated anti-inflammatory effect requires its protease activity, EPCR, PAR1, and β-arrestin-1. (A-C) FVIIa protease activity requirement. Serum-starved HUVECs were treated with FVIIa or active-site–inhibited FVIIa or FVIIaAS (100 nM) for 1 hour and then left unperturbed (NS) or stimulated with TNF-α (5 ng/ml) for 6 hours. ICAM-1 (A), VCAM-1 (B), and IL-6 (C) mRNA levels were measured by quantitative RT-PCR. (D-F) EPCR-blocking or PAR1 antibodies attenuate the anti-inflammatory effect of FVIIa. HUVECs were incubated with EPCR blocking (bl.) or nonblocking (nbl.) mAb (25 µg/mL) or a mixture of PAR1 mAb (ATAP-2, 25 µg/mL, and WEDE15, 20 µg/mL) for 1 hour prior to the addition of FVIIa (100 nM). Following FVIIa treatment for 1 hour, cells were stimulated with TNF-α (5 ng/ml) for 6 hours and the relative levels of ICAM-1 (D), VCAM-1 (E), and IL-6 (F) mRNA were measured by quantitative RT-PCR. (G). Silencing of EPCR, PAR1, or PAR2. HUVECs were transfected with control or EPCR-, PAR1-, or PAR2-specific siRNAs (200 nM) using Lipofectamine RNA Max reagent in serum-free medium. After 72 hours, cell lysates were made and analyzed for the expression of EPCR, PAR1, or PAR2 by western blot analysis using specific antibodies against these proteins. (H-I). HUVECs transfected with scrambled siRNA (scRNA), EPCR, PAR1, or PAR2 siRNA as described in panel G were treated with FVIIa (100 nM) for 1 hour and then stimulated with TNF-α (5 ng/ml) for 6 hours. ICAM-1 (H) and VCAM-1 (I) mRNA levels were measured in quantitative RT-PCR. (K-L) HUVECs were transfected with control siRNA or siRNA specific for β-arrestin-1 (β-AR-1), β-arrestin-2 (β-AR-2), or an equimolar mixture of both β-arrestin isoforms (β-AR-1/2). Forty-eight hours after transfection, cell lysates were made from the transfected cells probed for the expression of β-arrestin-1 (J) or β-arrestin-2 (K) by western blot analysis. (L) HUVECs transfected with control siRNA or siRNA specific for β-arrestin-1, β-arrestin-2, or β-arrestin-1/2 were treated with FVIIa (100 nM) and then stimulated with TNF-α (5 ng/ml). Cell lysates were probed for VCAM-1 levels by immunoblot analysis. Data are the mean ± SEM of 3 to 4 independent experiments. *P < .05; **P < .01; ***P < .001; ns, not statistically significant.
Recent studies showed that ApoER2 serves as a cell receptor or coreceptor for some of the APC’s cell signaling activities.22,23 To examine the role of ApoER2 in FVIIa suppression of TNF-α signaling in endothelial cells, endothelial cells were incubated with receptor-associated protein, which was used to block ApoER2-dependent APC cell signaling in an earlier study.22 Receptor-associated protein did not affect FVIIa-mediated suppression of TNF-α signaling (supplemental Figure 2A-B). Additional studies showed that ApoER2 silencing (supplemental Figure 2C) did not affect FVIIa suppression of TNF-α-induced VCAM-1 expression, whereas it partly reversed APC-mediated suppression of TNF-α-induced VCAM-1 expression (supplemental Figure 2D-E).
Earlier studies showed that APC-activated PAR1 cytoprotective signaling was mediated via a β-arrestin-dependent pathway.24 To assess the involvement of β-arrestins in mediating the FVIIa-induced anti-inflammatory effect, we specifically knocked down the expression of β-arrestin-1, β-arrestin-2, or both isoforms using specific siRNAs, and the effect on FVIIa-mediated suppression of TNF-α-induced VCAM-1 expression was investigated. β-Arrestin-1/2 siRNA knocked down the expression of both the isoforms, whereas β-arrestin-1 and β-arrestin-2 siRNAs selectively suppressed the expression of β-arrestin-1 or β-arrestin-2 (Figure 2J-K). β-Arrestin-1 knockdown completely blocked the FVIIa-mediated suppression of TNF-α-induced VCAM-1 expression (Figure 2L). In contrast, β-arrestin-2 knockdown had no significant effect on FVIIa suppression of TNF-α-induced VCAM-1 expression.
FVIIa inhibits LPS-induced endothelial activation
Endotoxemia is the primary cause of endothelial activation during bacterial infection. Therefore, we examined the effect of FVIIa on LPS-induced endothelial cell activation. Similar to TNF-α, LPS markedly increased the expression of inflammatory genes ICAM-1, VCAM-1, and IL-6 (supplemental Figure 3). FVIIa treatment suppressed LPS-induced ICAM-1, VCAM-1, and IL-6 expression in endothelial cells. Silencing the expression of either EPCR or PAR1 abolished the FVIIa-mediated suppressive effect on LPS-induced inflammation (supplemental Figure 3).
FVIIa attenuates LPS-induced inflammatory cytokines in vivo in the lung and kidney
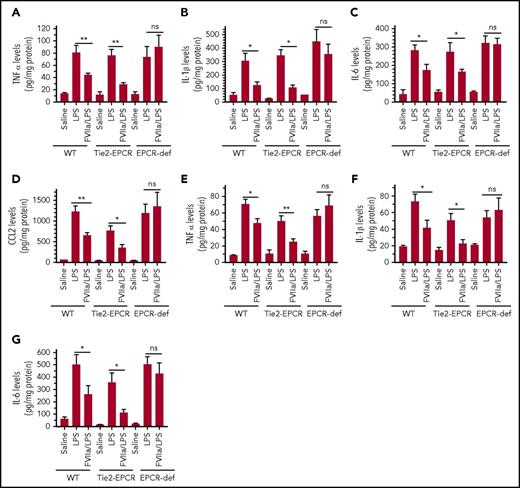
To determine whether the FVIIa-mediated protective effect on LPS-induced endothelial cell inflammation observed in vitro was also present in vivo, WT, EPCR-deficient, and EPCR-overexpressing mice were given control saline or FVIIa (250 µg/kg) and then challenged with LPS. The extent of inflammatory insult caused by endotoxin in the lung and kidney tissues of mice was assessed by measuring cytokines such as TNF-α, IL-1β, and IL-6. LPS treatment markedly increased expression of TNF-α, IL-1β, and IL-6 in both the lungs and kidneys of all genotypes (Figure 3A-C,E-G). There were no significant differences among genotypes in the extent of the increase in TNF-α (Figure 3A), IL-1β (Figure 3B), and IL-6 (Figure 3C) in lung tissues in LPS-administered mice. In the kidney, the extent of the cytokine increase in response to LPS appeared to be slightly lower in EPCR-overexpressing mice than in WT and EPCR-deficient mice (Figure 3E-G). FVIIa treatment markedly attenuated the LPS-induced increase in cytokine levels in WT and EPCR-overexpressing mice in both the lung and kidney (Figure 3A-G). In contrast, FVIIa administration did not suppress the LPS-induced increase in cytokine levels in EPCR-deficient mice. As with cytokines, LPS administration markedly increased the expression of the chemokine CCL2, a chemoattractant for neutrophils and monocytes, in the lung (Figure 3D). FVIIa treatment markedly reduced LPS-induced CCL2 expression in the lungs of WT and EPCR-overexpressing mice, but not in lungs of EPCR-deficient mice (Figure 3D). In additional studies, we compared the effectiveness of FVIIa and APC in attenuating LPS-induced inflammatory cytokines in dose–response studies. At 25 and 100 µg/kg doses, both FVIIa and APC exhibited a small but statistically insignificant protective effect (supplemental Figure 4). At 250 µg/kg, both FVIIa and APC treatments reduced LPS-induced inflammatory cytokines significantly compared with control treatment, and the difference between them in terms of the extent of reduction in inflammatory cytokines was not statistically significant (supplemental Figure 4). In further studies, we investigated whether FVIIa exerts an anti-inflammatory effect in bacterial infection models, such as polymicrobial sepsis induced by CLP. As observed in LPS-induced endotoxemia, FVIIa treatment reduced the elaboration of IL-6, TNF-α, and MCP-1 in this model as well (supplemental Figure 5).
FVIIa attenuates LPS-induced inflammatory cytokine elaboration in the lung and kidney tissues of the mice in EPCR-dependent fashion. WT C57 BL/6J, EPCR-overexpressing (Tie2-EPCR), or EPCR-deficient (EPCR-def) mice were administered with saline or human FVIIa (250 µg/kg body weight) IV via the tail vein. After 2 hours, the mice receiving saline were left either unchallenged (saline) or administered with LPS (5 mg/kg; LPS) intraperitoneally. The same dose of LPS was administered in parallel to mice injected with FVIIa. Six hours following LPS administration, the mice were killed by exsanguination, perfused with saline to remove blood, and the lung and kidney tissues were collected. The tissues were homogenized in the RIPA buffer containing protease inhibitors and centrifuged at 10 000g at 4°C to remove tissue debris. The supernatants were used to measure the cytokines by ELISA. The cytokines measured were TNF-α (A,E), IL-1β (B,F), IL-6 (C,G), and CCL2 (D) in lung tissue (A-D) and kidney tissue (E-G). Data represent mean ± SEM of 3 independent experiments, a total of 6 to 9 animals in a group. *P < .05; **P < .01; ns, not statistically significant.
FVIIa attenuates LPS-induced inflammatory cytokine elaboration in the lung and kidney tissues of the mice in EPCR-dependent fashion. WT C57 BL/6J, EPCR-overexpressing (Tie2-EPCR), or EPCR-deficient (EPCR-def) mice were administered with saline or human FVIIa (250 µg/kg body weight) IV via the tail vein. After 2 hours, the mice receiving saline were left either unchallenged (saline) or administered with LPS (5 mg/kg; LPS) intraperitoneally. The same dose of LPS was administered in parallel to mice injected with FVIIa. Six hours following LPS administration, the mice were killed by exsanguination, perfused with saline to remove blood, and the lung and kidney tissues were collected. The tissues were homogenized in the RIPA buffer containing protease inhibitors and centrifuged at 10 000g at 4°C to remove tissue debris. The supernatants were used to measure the cytokines by ELISA. The cytokines measured were TNF-α (A,E), IL-1β (B,F), IL-6 (C,G), and CCL2 (D) in lung tissue (A-D) and kidney tissue (E-G). Data represent mean ± SEM of 3 independent experiments, a total of 6 to 9 animals in a group. *P < .05; **P < .01; ns, not statistically significant.
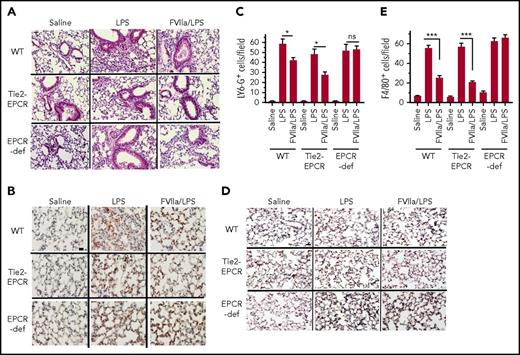
FVIIa ameliorates LPS-induced immune cell infiltration into the lung
As shown in hematoxylin and eosin–stained lung tissue sections (Figure 4A), LPS administration markedly increased the infiltration of immune cells into the lungs of all 3 genotypes (ie, WT, EPCR-overexpressing, and EPCR-deficient mice). The extent of infiltration of immune cells appears to be slightly lower in EPCR-overexpressing mice. FVIIa administration markedly reduced the infiltration of immune cells in both WT and EPCR-overexpressing mice. In contrast, FVIIa administration had no noticeable effect on immune cell infiltration in EPCR-deficient mice in response to LPS. Next, lung tissue sections were stained with neutrophil- and macrophage-specific markers to evaluate the extent of neutrophil and macrophage infiltration into the lungs and the effect of FVIIa on these innate immune cells. Staining with LY-6G, a marker of neutrophil, revealed a marked increase in infiltration of neutrophils into the lung of LPS-treated mice irrespective of the genetic background of mice used in the study (Figure 4B-C). FVIIa treatment showed a significant decrease in neutrophil recruitment in the lungs of WT and EPCR-overexpressing mice challenged with LPS (Figure 4C). FVIIa failed to abrogate the LPS-induced neutrophil recruitment in the EPCR-deficient mice (Figure 4C). Analysis of monocyte recruitment by staining tissue sections with a monocyte/macrophage marker (F4/80) showed that, as observed with neutrophil recruitment, FVIIa administration significantly lowered the migration of these cells in both the WT and EPCR-overexpressing mice (Figure 4D-E). FVIIa had no significant effect on monocyte/macrophage recruitment in the lung in EPCR-deficient mice in response to LPS. Overall, the above data indicate that the FVIIa-induced anti-inflammatory effect in vivo requires the presence of EPCR.
FVIIa inhibits LPS-induced leukocyte infiltration into lungs. WT C57 BL/6J (WT), EPCR-overexpressing (Tie2-EPCR) or EPCR-deficient mice were administered with FVIIa and LPS as described in Figure 4. The lung tissues were fixed, sectioned and stained with hematoxylin and eosin (A), neutrophil marker LY-6G (B) or monocyte/macrophage marker F4/80 (D). The images were captured at 40X magnification. The scale bar indicates 100 µm. The number of LY-6G- (C) and F4/80- positive (E) cells were counted at 3 randomly chosen areas covering the entire section from tissue sections prepared 3 animals in a group. Data are the Mean ± SEM. *P < .05; ***P < .001; ns, not statistically significant.
FVIIa inhibits LPS-induced leukocyte infiltration into lungs. WT C57 BL/6J (WT), EPCR-overexpressing (Tie2-EPCR) or EPCR-deficient mice were administered with FVIIa and LPS as described in Figure 4. The lung tissues were fixed, sectioned and stained with hematoxylin and eosin (A), neutrophil marker LY-6G (B) or monocyte/macrophage marker F4/80 (D). The images were captured at 40X magnification. The scale bar indicates 100 µm. The number of LY-6G- (C) and F4/80- positive (E) cells were counted at 3 randomly chosen areas covering the entire section from tissue sections prepared 3 animals in a group. Data are the Mean ± SEM. *P < .05; ***P < .001; ns, not statistically significant.
Potential mechanisms involved in the FVIIa-mediated anti-inflammatory effect
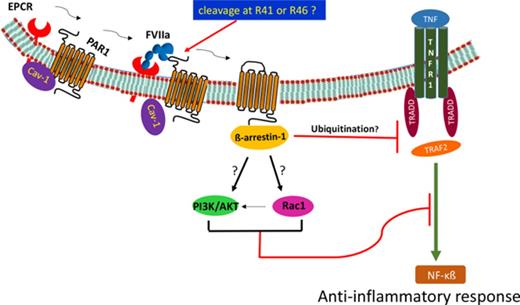
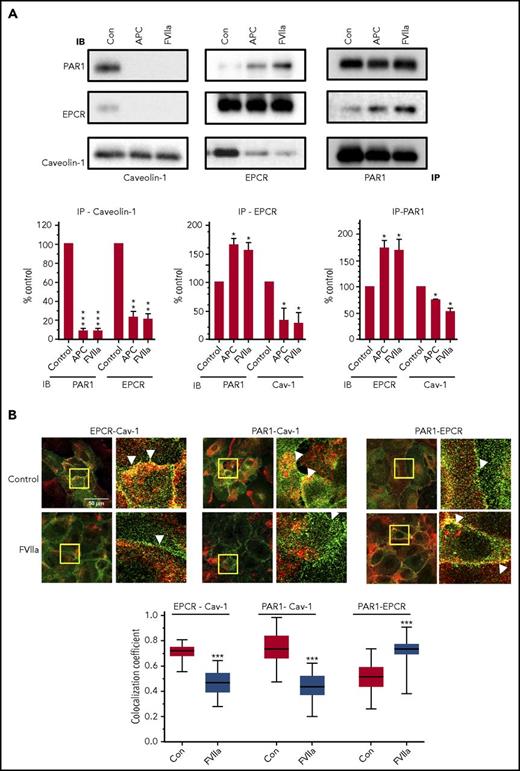
Elucidation of APC-EPCR-PAR1–mediated signaling suggested that cleavage of PAR1 at the noncanonical site (Arg46) by APC was responsible for the cytoprotective signaling of APC.25 If the EPCR is occupied by its ligand (protein C) or APC, then thrombin cleavage of PAR1 at the canonical site (Arg 41) was also shown to induce cytoprotective signaling.15 To examine FVIIa-EPCR cleavage of PAR1, endothelial cells transfected to express PAR1 reporter were treated with FVIIa (100 nM for 1 h). We found no detectable cleavage of the PAR1 reporter by FVIIa. In contrast, PAR1 reporter cleavage was readily observed by thrombin and APC (supplemental Figure 6). These data were consistent with our earlier findings and did not permit us to identify the potential FVIIa-EPCR cleavage site in PAR1. Next, we investigated the effect of FVIIa binding to EPCR on caveolin-1’s association with EPCR and PAR1 in coimmunoprecipitation and colocalization studies. As shown in Figure 5A, immunoprecipitation of caveolin-1 with caveolin-1–specific antibodies coimmunoprecipitated both EPCR and PAR1 in control endothelial cells. Exposure of endothelial cells to FVIIa reduced the coimmunoprecipitation of EPCR and PAR1 with caveolin-1. Coimmunoprecipitation studies with EPCR and PAR1 antibodies revealed that only traces of PAR1 were associated with EPCR in control cells and that FVIIa treatment significantly promoted the association between EPCR and PAR1. Similar results were noted with APC treatment (Figure 5A). Analysis of immunofluorescence colocalization of EPCR, PAR1, and caveolin-1 in endothelial cells treated with a control vehicle or FVIIa provided elegant and visual evidence to the above finding. Both EPCR and PAR1 were found to be colocalized with caveolin-1 to a greater extent in control endothelial cells; FVIIa treatment markedly reduced their colocalization (Figure 5B). As observed in coimmunoprecipitation studies, FVIIa enhanced the colocalization of EPCR and PAR1 (Figure 5B). Overall, the above data indicate that in unstimulated cells, both EPCR and PAR1 are associated with caveolin-1, and FVIIa binding to EPCR uncouples caveolin-1 from both EPCR and PAR1 and promotes the interaction between EPCR and PAR1.
FVIIa treatment disrupts caveolin-1 interaction with EPCR and PAR1 and promotes the interaction between EPCR and PAR1. (A) HUVECs were treated with a control vehicle (con), APC (80 nM) or FVIIa (20 nM) for 1 h in serum-free medium and then lysed in RIPA buffer. Total cell lysates were immunoprecipitated with anti-caveolin-1, anti-EPCR, or anti-PAR1 antibodies. The immunoprecipitates were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted with anti-caveolin-1, anti-EPCR, or anti-PAR1 antibodies raised in a different animal species than that were used for immunoprecipitation (see “Materials and methods”). Each experiment was repeated at least 3 times, and the band intensities were quantified by FIJI software ImageJ2. IP, immunoprecipitation; IB, immunoblotting. (B) HUVECs cultured on a glass coverslip were treated with FVIIa as described in panel A. Cells were fixed and permeabilized and processed for double immunostaining to stain EPCR (green), caveolin-1 (red, left), PAR1 (green), caveolin-1 (red, center), or PAR1 (green), and EPCR (red, right). The images shown are merged. A small portion of the merged image (bordered with the yellow box) was digitally enlarged to illustrate colocalization. Scale bar indicates 50 µm. The colocalization coefficient between the 2 signals was analyzed using Zen 2009 software (Carl Zeiss; n = 30 cells). A Mann-Whitney U test was used to determine the significance between control and FVIIa treatment. ***P < .001.
FVIIa treatment disrupts caveolin-1 interaction with EPCR and PAR1 and promotes the interaction between EPCR and PAR1. (A) HUVECs were treated with a control vehicle (con), APC (80 nM) or FVIIa (20 nM) for 1 h in serum-free medium and then lysed in RIPA buffer. Total cell lysates were immunoprecipitated with anti-caveolin-1, anti-EPCR, or anti-PAR1 antibodies. The immunoprecipitates were separated on sodium dodecyl sulfate polyacrylamide gel electrophoresis and immunoblotted with anti-caveolin-1, anti-EPCR, or anti-PAR1 antibodies raised in a different animal species than that were used for immunoprecipitation (see “Materials and methods”). Each experiment was repeated at least 3 times, and the band intensities were quantified by FIJI software ImageJ2. IP, immunoprecipitation; IB, immunoblotting. (B) HUVECs cultured on a glass coverslip were treated with FVIIa as described in panel A. Cells were fixed and permeabilized and processed for double immunostaining to stain EPCR (green), caveolin-1 (red, left), PAR1 (green), caveolin-1 (red, center), or PAR1 (green), and EPCR (red, right). The images shown are merged. A small portion of the merged image (bordered with the yellow box) was digitally enlarged to illustrate colocalization. Scale bar indicates 50 µm. The colocalization coefficient between the 2 signals was analyzed using Zen 2009 software (Carl Zeiss; n = 30 cells). A Mann-Whitney U test was used to determine the significance between control and FVIIa treatment. ***P < .001.
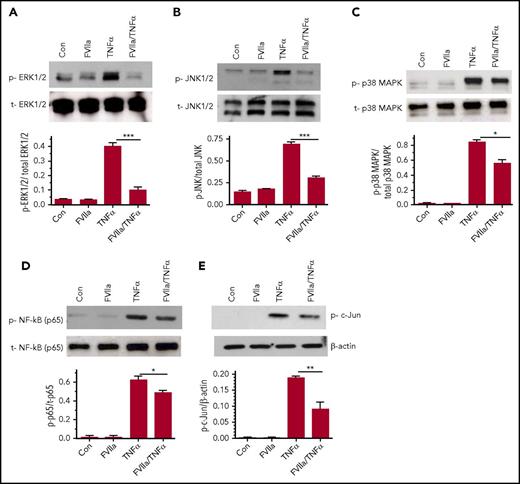
The role of MAPKs such as p38, ERK1/2, and JNK is well documented in the TNF-α-induced inflammation in endothelial cells. Therefore, we examined the effect of FVIIa in modulating these signaling pathways. As shown in Figure 6A-B, FVIIa pretreatment markedly attenuated TNF-α-induced ERK1/2 and JNK activation. FVIIa also inhibited TNF-α-induced p38 activation (Figure 6C), but the inhibitory effect was not as robust as that of FVIIa inhibition of TNF-α-induced ERK1/2 and JNK activation (Figure 6A-B). Additional studies revealed that FVIIa treatment significantly reduced TNFα-induced activation of NF-κB and c-Jun as shown in decreased phosphorylation of p65 and c-Jun, respectively (Figure 6C-D). These data indicate that FVIIa-EPCR mediated signaling blocks TNFα-induced inflammatory signaling by affecting upstream events in TNFR1 signaling, probably impairing translocation of TNFR1 to lipid rafts following TNFα engagement with the receptor or the assembly of TNFR1 signaling initiating complex inside the cell.
Effect of FVIIa on TNF-α-induced MAPK signaling and NF-κB and AP-1 activation in endothelial cells. HUVEC, serum-deprived for 12 h, were treated with FVIIa (100 nM) for 1 hour and then with TNF-α (5 ng/mL) for 30 minutes in serum-free medium. The cell lysates were subjected to immunoblotting with specific antibodies to analyze activation of ERK1/2 (A), JNK1/2 (B), p38 MAPK (C), NF-κB (p65 phosphorylation; D) and AP-1 (c-Jun phosphorylation; E). p, phospho; t, total. Bar graphs below immunoblots depict the quantification of the phosphorylated signal normalized to the total protein by densitometric analysis of the bands using ImageJ software. Data represent the mean ± SEM of 3 independent experiments. *P < .05; **P < .01; ***P < .001.
Effect of FVIIa on TNF-α-induced MAPK signaling and NF-κB and AP-1 activation in endothelial cells. HUVEC, serum-deprived for 12 h, were treated with FVIIa (100 nM) for 1 hour and then with TNF-α (5 ng/mL) for 30 minutes in serum-free medium. The cell lysates were subjected to immunoblotting with specific antibodies to analyze activation of ERK1/2 (A), JNK1/2 (B), p38 MAPK (C), NF-κB (p65 phosphorylation; D) and AP-1 (c-Jun phosphorylation; E). p, phospho; t, total. Bar graphs below immunoblots depict the quantification of the phosphorylated signal normalized to the total protein by densitometric analysis of the bands using ImageJ software. Data represent the mean ± SEM of 3 independent experiments. *P < .05; **P < .01; ***P < .001.
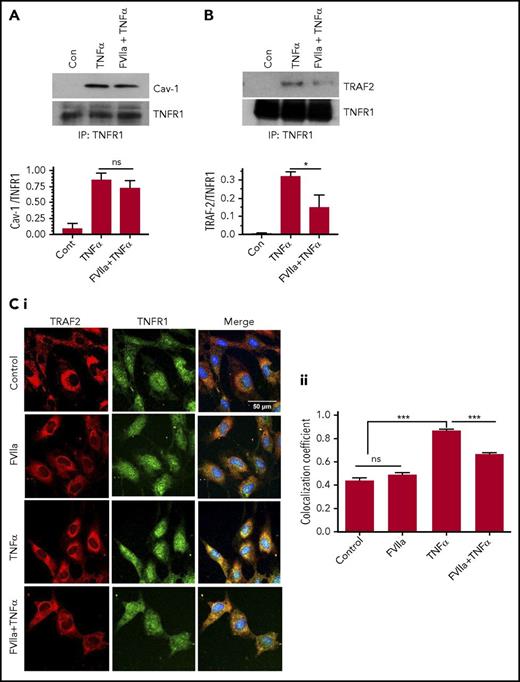
Translocation of TNFR1 to lipid rafts and the subsequent recruitment of TRAF2 to TNFR1 complex were shown to be essential for TNF-α-mediated NF-κB activation.26 Therefore, we next examined the effect of FVIIa binding to EPCR on translocation of TNFR1 to lipid rafts and the recruitment of TRAF2 to TNFR1 complex. Coimmunoprecipitation studies showed that upon engagement by TNF-α, caveolin-1 was coimmunoprecipitated with TNFR1 (Figure 7A). FVIIa treatment had no noticeable effect on the association between TNFR1 and caveolin-1 (Figure 7A). These data suggest that FVIIa treatment does not affect TNFR1 translocation to lipid rafts or its interaction with caveolin-1. Coimmunoprecipitation of TNFR1 and TRAF2 showed that TRAF2 was recruited to TNFR1 following the treatment of endothelial cells with TNF-α, and pretreatment of cells with FVIIa substantially reduced the TNF-α-induced recruitment of TRAF2 to TNFR1 (Figure 7B). Analysis of colocalization of TNFR1 and TRAF2 by immunofluorescence confocal microscopy and quantification of colocalization coefficient confirmed the above findings; ie, FVIIa treatment significantly reduced the association of TRAF2 with TNFR1 following TNF-α stimulation (Figure 7C).
FVIIa-mediated signaling impairs TNF-α-induced TRAF2 recruitment to TNFR1 and not colocalization of TNFR1 with caveolin-1. HUVECs were treated with a control vehicle or FVIIa (100 nM) for 1 hour and then stimulated with TNF-α (5 ng/mL) for 5 minutes in serum-free medium. (A-B) Cell lysates were immunoprecipitated with TNFR1 antibodies, and the immunoprecipitates were probed for the presence of caveolin-1 (A) or TRAF2 (B) using western blotting with specific antibodies. The bottom panels show quantitated data from densitometric analysis of immunoblots from 3 to 4 independent experiments (ns, not significant, *P < .05). (C). HUVECs cultured on a glass coverslip were treated as described above and subjected immunofluorescence confocal microscopy to probe for localization of TNFR1 (green) or TRAF2 (red). Panel Ci shows representative immunofluorescence micrographs, and panel Cii shows colocalization coefficient calculated from the analysis of ≥30 cells (***P < .001). Please note that because of differences in the intensity of red and green fluorescences, colocalization does not necessarily give a yellow color signal in merged images and therefore it can be difficult to discern differences visually among the images. Scale bar indicates 100 µm.
FVIIa-mediated signaling impairs TNF-α-induced TRAF2 recruitment to TNFR1 and not colocalization of TNFR1 with caveolin-1. HUVECs were treated with a control vehicle or FVIIa (100 nM) for 1 hour and then stimulated with TNF-α (5 ng/mL) for 5 minutes in serum-free medium. (A-B) Cell lysates were immunoprecipitated with TNFR1 antibodies, and the immunoprecipitates were probed for the presence of caveolin-1 (A) or TRAF2 (B) using western blotting with specific antibodies. The bottom panels show quantitated data from densitometric analysis of immunoblots from 3 to 4 independent experiments (ns, not significant, *P < .05). (C). HUVECs cultured on a glass coverslip were treated as described above and subjected immunofluorescence confocal microscopy to probe for localization of TNFR1 (green) or TRAF2 (red). Panel Ci shows representative immunofluorescence micrographs, and panel Cii shows colocalization coefficient calculated from the analysis of ≥30 cells (***P < .001). Please note that because of differences in the intensity of red and green fluorescences, colocalization does not necessarily give a yellow color signal in merged images and therefore it can be difficult to discern differences visually among the images. Scale bar indicates 100 µm.
Discussion
Recent studies have established that APC binding to EPCR initiates PAR1-mediated cytoprotective signaling that includes endothelial cell barrier protection, cell survival, and anti-inflammatory properties.2,5,27 Recent studies from us and others showed that FVIIa binds EPCR with an affinity similar to that of protein C/APC, and both FVIIa and APC bind to the same binding motif of EPCR.11,28,29 However, little is known about the relevance of FVIIa binding to EPCR in vivo and whether FVIIa-EPCR, like APC-EPCR, can activate cytoprotective signaling. Initial studies that failed to show FVIIa-EPCR cleavage of PAR1 in a heterologous expression system11 and FVIIa's barrier-protective effect in the EA.hy26 endothelial cell model system15 dampened interest in further examining FVIIa-EPCR’s ability to mediate PAR-dependent signaling. However, recent studies from our laboratory showed that FVIIa bound to EPCR could activate endogenous PAR113 and induce a barrier-protective effect both in vitro and in vivo.13,14 The data presented here show that FVIIa treatment of endothelial cells suppresses TNF-α- and LPS-induced expression of the inflammatory proteins ICAM-1, VCAM-1, and IL-6 via an EPCR- and PAR-1 dependent mechanism. FVIIa treatment also reduced the adherence of monocytic cells to endothelial cells. In vivo, low doses of FVIIa attenuated LPS-induced elaboration of inflammatory cytokines and chemokines, as well as infiltration of neutrophils and monocytes into lungs of WT and EPCR-overexpressing mice, but not EPCR-deficient mice.
Recent studies on APC-EPCR-mediated cytoprotective signaling have provided insights into how APC activation of PAR1 mediates cytoprotective signaling. They include localization of PAR1 and EPCR in caveolae,14,30,31 the occupancy of EPCR by its ligands modulating PAR1 signaling specificity,15 the cleavage of PAR1 by APC at the noncanonical Arg46 cleavage site,25,32 and the involvement of β-arrestin-2 in the APC-induced cytoprotective effects.24,33 AP-PAR1 fusion reporter constructs and cleavage site-specific mutants were routinely used to investigate the cleavage of PAR1 by thrombin, APC, or other proteases.11,15,25,34 Unfortunately, this approach was not useful in determining the cleavage site of PAR1 by FVIIa on endothelial cells, because in contrast to thrombin or APC, FVIIa treatment failed to show a detectable cleavage of AP-PAR1 expressed in endothelial cells. These data were consistent with our earlier findings observed in CHO cells transfected to express EPCR and AP-PAR1.11 Our earlier studies on FVIIa activation of endogenous PAR1 in endothelial cells showed that only a small fraction of PAR1 was accessible and cleaved by FVIIa bound to EPCR.13 This, together with the absence of cleavage-specific antibodies, suggests it is not feasible to determine at present whether FVIIa cleaved endogenous PAR1 at the Arg41 or Arg46 site.
It was suggested earlier that the ligand occupancy of EPCR recruits PAR1 to a protective pathway by coupling the receptor to the Gi protein in lipid rafts.15 It was also shown earlier that EPCR resides in caveolae/lipid rafts30,35 and associates with caveolin-1.15 The occupancy of EPCR by its ligands dissociates caveolin-1 from EPCR, probably moving the EPCR from caveolae, and this process couples PAR-1 to Gi-protein–mediated protective signaling.15 The EPCR-dependent switch in the specificity of PAR1 signaling was thought to be unique to protein C/APC.15 Our present data confirm the association of EPCR with caveolin-1 in endothelial cells and the dissociation of EPCR from caveolin-1 upon the EPCR occupancy by APC. More important and relevant to the present investigation is that EPCR occupancy by FVIIa also leads to dissociation of EPCR from caveolin-1. Our studies provide additional new information suggesting that similar to EPCR, PAR1 also associates with caveolin-1 in endothelial cells and that FVIIa treatment results in dissociation of PAR1 from caveolin-1. Furthermore, FVIIa treatment not only disrupts the association of caveolin-1 from EPCR and PAR1 but also promotes the association between PAR1 and EPCR. These observations suggest that the occupancy of EPCR by FVIIa, as observed with the occupancy of EPCR by protein C/APC, may switch the PAR1 specificity to a protective signaling pathway.
Recent studies indicated that β-arrestins play a key role in mediating APC-induced PAR1 cytoprotective signaling.24,33 Soh and Trejo24 showed that both β-arrestin-1 and 2 isoforms are enriched in caveolar fractions (along with PAR1) and form a preassembled complex with PAR1. They also showed that depletion of β-arrestin expression (particularly β-arrestin-2) by RNA silencing resulted in the loss of APC-induced Rac1 activation and protection against thrombin-induced endothelial barrier permeability. Roy et al33 showed that occupancy of EPCR by protein C was responsible for recruitment of β-arrestin-2 to the cytoplasmic domain of PAR1. Our present data demonstrate that, in contrast to APC-mediated signaling via β-arrestin-2, the FVIIa-induced anti-inflammatory effect is mediated via β-arrestin-1 and not β-arrestin-2. The reduction of β-arrestin-1 and not β-arrestin-2 abolished the FVIIa suppression of TNF-α-induced inflammation. Studies by Lefkowitz and colleagues demonstrated that β-arrestins (particularly β-arrestin-1) regulate NF-κB activity.36 It had been shown that β-arrestin-mediated cytosolic retention of phospho ERK induced by PAR2 activation prevents ERK-dependent transcription.37 Similar to this mode, it is possible that FVIIa-EPCR-PAR1 recruitment of β-arrestin-1 may retain phospho ERK and NF-κB in the cytosol and thus prevent TNF-α-induced NF-κB-dependent transcription of inflammatory genes. In-depth studies are needed to examine the role of β-arrestin-1 in facilitating FVIIa-EPCR-induced anti-inflammatory effect.
At present, it is not entirely clear how FVIIa-EPCR–mediated signaling attenuates LPS- and TNF-α-induced inflammation. Our data suggest that FVIIa-EPCR–mediated signaling probably blocks the TNFα-induced signaling by impairing an early step in TNFR1-mediated signaling since FVIIa treatment attenuated not only the NF-κB activation/NF-κB–dependent inflammatory gene expression but also other TNF-α-induced signaling, such as activation of ERK1/2, p38 MAPK, and JNK. It was shown that upon binding to TNF-α, TNFR1 translocates to caveolae/lipid rafts in the plasma membrane and that this is crucial for NF-κB activation.38 The binding of TNF-α to the TNFR1 also induces a conformational change in the cytoplasmic domain receptor that enables the recruitment of TNFR1-associated death domain protein and TNFR-associated factor 2 (TRAF2) to TNFR1 signaling initiating complex.39 TRAF2 is a key player in TNFR1 signaling as it mediates multiple receptors specific functions, including NF-κB activation and MAPK and JNK activation.40 Our studies indicate that FVIIa-EPCR-mediated signaling appears to inhibit TNFα-induced signaling by impairing the recruitment of TRAF2 to the TNFR1 signaling complex. Currently, it is unknown how FVIIa-EPCR-mediated signaling impairs TRAF2 recruitment to the TNFR1 signaling complex. It is possible that FVIIa activation of phosphatidylinositol 3-kinase (PI3K)/Akt pathway might be responsible for FVIIa suppression of LPS-induced inflammation. A number of studies showed that PI3K/Akt signaling pathway negatively regulates LPS-induced acute inflammatory responses by blocking the activation of transcriptional factors NF-κB, AP-1, and Egr-1 that drive the expression inflammatory genes.26,41-43 Angiopoietin 1 activation of PI3K/Akt pathway in endothelial cells was also shown to suppress the transcriptional activation of NF-κB and AP1-mediated gene expression.44 Our preliminary data showing that FVIIa induces the activation of Akt (supplemental Figure 7) support the above possibility, but further experiments are needed to confirm the role of the PI3K/Akt pathway in FVIIa suppression of LPS-induced inflammatory signaling.
Recent studies by Weiler and colleagues45 revealed a novel mechanism by which APC provides an anti-inflammatory effect in the context of endotoxemia. In this mechanism, APC, following its association with cofactors FV and protein S, is thought to displace FVIIa or FXa from the EPCR, resulting in the uncoupling of inflammatory PAR2 signaling initiated by the TF-FVIIa-FXa-EPCR complex. It is unlikely that a similar mechanism is responsible for FVIIa’s anti-inflammatory effect in our LPS-induced endotoxemia model. FVIIa competition for the EPCR in the complex of TF-FVIIa-FXa-EPCR may replace endogenous FVIIa with exogenously administered FVIIa, but it does not uncouple the complex. Furthermore, FVIIa, unlike APC, does not interact with FV and protein S, the cofactors that are critical for the inhibition of TF-FVIIa-FXa-EPCR signaling by APC.
Our present data raise the importance of using FVIIa in hemophilia therapy. Inflammatory responses induced by iron deposited in the joint from bleeding appears to play a central role in arthropathy in hemophilia.46 Inflammatory mediators TNF-α, IL-1β, IL-6, and growth factors VEGF and HIF were shown to increase in blood-induced joint damage in a murine model of hemophilia.47 If FVIIa binding to EPCR provides a barrier-protective effect and suppresses inflammation in joints, then use of FVIIa for prophylaxis may be far superior in preventing the hemarthrosis and joint damage of not only hemophilia patients with inhibitors but also all hemophilia patients. In this context, it may be pertinent to note that the dose of FVIIa administered to mice in the present study (250 µg/kg) that showed significant inhibition of LPS-induced inflammatory gene expression was much lower than doses of FVIIa required to restore hemostasis in hemophilia mice (∼4 to 10 mg/kg).48-50 Because human rFVIIa was used in all of these studies, it is unlikely that species specificity of FVIIa or potential differences in pharmacokinetics between mouse and human FVIIa is responsible for the observed differences in the FVIIa doses required to produce an anti-inflammatory effect vs restoration of hemostasis in hemophilia mice. The dose of 250 µg/kg rFVIIa in mice translates into a human equivalent dose of 20 µg/kg.51 This suggests that subtherapeutic concentrations of rFVIIa may be sufficient to protect against inflammation that may result from infection or disease conditions.
Lastly, recent studies of well-documented cytoprotective effects of APC in a variety of model systems has renewed interest in developing APC or APC variants as a therapeutic agent to treat multiple indications, including sepsis, acute lung injury, wound healing, retinal injury, and ischemic stroke.52-57 FVIIa has been used widely with minimal risk for more than 2 decades to treat patients with bleeding disorders associated with not only hemophilia with inhibitors but also other conditions, including intracerebral hemorrhage, sepsis, and trauma.58-60 If FVIIa provides many of the same cytoprotective effects as APC, then FVIIa could become an option to treat some of the above indications for which the use of APC has been under consideration. However, the increased risk of thrombosis associated with FVIIa administration61 needs to be taken into consideration when developing FVIIa as a therapeutic agent to treat inflammatory disorders. Further detailed studies are needed to investigate the cytoprotective effects of FVIIa in various experimental model systems to determine the therapeutic potential of FVIIa in treating various disorders associated with inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Varun Lella for proofreading the manuscript and minor English editing.
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grants HL107483 (L.V.M.R.) and UM1 HL120877 (C.T.E.).
Authorship
Contribution: V.K., J.W., and S.K. performed experiments and analyzed data; U.R.P. contributed to study design, provided technical expertise in performing the study, and participated in data analysis; C.T.E. provided breeding pairs of EPCR-deficient and Tie2-EPCR mice and EPCR antibodies; L.V.M.R. conceived and designed the research, analyzed the data, and wrote the manuscript; and all authors contributed to the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: L. Vijaya Mohan Rao, Department of Cellular and Molecular Biology, The University of Texas Health Science Center at Tyler, Tyler, TX 75708-3154; e-mail: vijay.rao@uthct.edu; and Usha R. Pendurthi, Department of Cellular and Molecular Biology, The University of Texas Health Science Center at Tyler, Tyler, TX 75708-3154; e-mail: usha.pendurthi@uthct.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal