Key Points
The same type of APA on 2 occasions or >1 type of APA on the same or different occasions is associated with recurrent VTE.
APA and D-dimer levels seem to be independently associated with recurrence after a first unprovoked VTE.
Abstract
It is uncertain whether antiphospholipid antibodies (APAs) increase the risk of recurrence after a first unprovoked venous thromboembolism (VTE). We tested for anticardiolipin antibodies, anti–β2 glycoprotein 1 antibodies, and lupus anticoagulant on 2 occasions ∼6 months apart in 307 patients with a first unprovoked VTE who were part of a prospective cohort study. We then determined if APAs were associated with recurrent thrombosis in the 290 patients who stopped anticoagulant therapy in response to negative D-dimer results. Compared with those without an APA, the hazard ratios for recurrent VTE were 1.8 (95% confidence interval [CI], 0.9-3.7; P = .09) in the 25.9% of patients with an APA on ≥1 occasions, 2.7 (95% CI, 1.1-.7; P = .03) in the 9.0% of patients with the same APA on 2 occasions, and 4.5 (95% CI, 1.5-13.0; P = .006) in the 3.8% of patients with 2 or 3 different APA types on either the same or different occasions. There was no association between having an APA and D-dimer levels. We conclude that having the same type of APA on 2 occasions or having >1 type of APA on the same or different occasions is associated with recurrent thrombosis in patients with a first unprovoked VTE who stop anticoagulant therapy in response to negative D-dimer tests. APA and D-dimer levels seem to be independent predictors of recurrence in patients with an unprovoked VTE. This trial was registered at www.clinicaltrials.gov as #NCT00720915.
Introduction
Antiphospholipid antibodies (APAs) bind to cardiolipins or phospholipid-associated plasma proteins, such as β2 glycoprotein 1, prothrombin, or annexin V.1-4 APAs are usually detected as antibodies against cardiolipin (ACAs) or β2 glycoprotein 1 (anti-β2GP1s) or as lupus anticoagulants (LAs). APAs, particularly if persistent, are thought to be an important risk factor for recurrent venous thromboembolism (VTE), and the presence of an APA is often considered an indication for indefinite anticoagulant therapy.5 However, a recent systematic review concluded that there was only weak evidence that an APA was associated with an increased risk of recurrence in patients with a first VTE.2 Consequently, it is uncertain if patients with a VTE, including those with a first unprovoked event, should be routinely tested for APAs and whether APA results should influence decisions about the duration of treatment.
In the recent D-dimer Optimal Duration Study (DODS), we tested the hypothesis that patients with a first unprovoked VTE and a negative D-dimer test receiving anticoagulant therapy, along with a second negative test 1 month after stopping treatment, have a low risk of recurrence.6 We failed to confirm this hypothesis; the overall rate of recurrent VTE in DODS patients who met these criteria was higher than that predefined as acceptable. During the course of the study, we collected plasma samples that provided us with an opportunity to address a number of questions about the role of APAs in patients with an unprovoked VTE. The most important of these questions was whether an APA was associated with a higher risk of recurrent VTE in patients who stopped anticoagulant therapy in response to negative D-dimer testing. A secondary question was whether APAs were associated with a prothrombotic state, as reflected by higher D-dimer levels.
Methods
Study patients and clinical management
The design and main results of DODS, and a subsequent analysis of quantitative D-dimer levels in DODS patients, were described previously.6,7 In brief, patients 18 to 75 years of age with a first unprovoked proximal deep vein thrombosis or pulmonary embolism who had received anticoagulant treatment for 3 to 7 months were eligible for DODS if they did not have a strong contraindication to, or need for, ongoing anticoagulant therapy. The presence of an APA was not a study exclusion criterion. Although it was discouraged, clinical centers were allowed to test potentially eligible patients for an APA and exclude those with a positive result if they considered them to have a high risk of recurrence. The decision to test potentially eligible patients for an APA and exclude those with a positive result had to have been made before D-dimer testing. We did not monitor how often potentially eligible patients were tested for an APA.
At enrollment, when all patients were receiving anticoagulant therapy, D-dimer was measured using a point-of-care, slide-based test that yields a positive or negative D-dimer result.6,7 Patients with a positive D-dimer test remained on anticoagulant therapy indefinitely and did not undergo a second D-dimer test. Patients with a negative D-dimer test stopped anticoagulant therapy, and a second test with the same qualitative assay was performed 1 month later. If the second D-dimer test had converted to positive, anticoagulant therapy was restarted. If the second D-dimer was also negative, patients remained off anticoagulants indefinitely. All patients were observed to detect recurrent VTE, which was evaluated in a standardized way. Suspected events were assessed by a central adjudication committee, the members of which were blinded to all D-dimer and APA testing results and as to whether patients were receiving anticoagulant therapy.
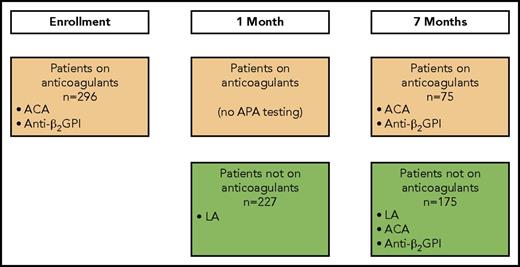
Twelve of the 13 clinical centers in DODS collected blood samples for the current analysis. When possible, these samples were collected at 3 time points: enrollment, 1 month after enrollment, and 7 months after enrollment (Figure 1). At each time point, 4.5 mL of blood was collected into a 5-mL vacutainer tube containing 3.2% sodium citrate. After centrifugation at 1700 ×g for 15 minutes at 23°C, platelet-poor plasma was harvested and subjected to centrifugation for an additional 5 minutes to ensure removal of platelets. Plasma was stored in aliquots at −80°C, and a fresh aliquot was used for these analyses. DODS and the current analyses were approved by the institutional review boards of all participating clinical centers, and all patients provided written informed consent.
Patient flow and when APA testing was performed. At enrollment, all patients were receiving anticoagulants. At 1 month, patients with a positive D-dimer at enrollment remained on anticoagulants. At 7 months, patients with a positive D-dimer at enrollment or 1 month were receiving anticoagulants, and patients with a negative D-dimer at enrollment and at 1 month were not receiving anticoagulants.
Patient flow and when APA testing was performed. At enrollment, all patients were receiving anticoagulants. At 1 month, patients with a positive D-dimer at enrollment remained on anticoagulants. At 7 months, patients with a positive D-dimer at enrollment or 1 month were receiving anticoagulants, and patients with a negative D-dimer at enrollment and at 1 month were not receiving anticoagulants.
Laboratory assays
D-dimer assays.
The Clearview Simplify assay (Alere Inc., San Diego, CA) was used by the clinical centers to qualitatively classify D-dimer levels as negative or positive, and these results determined how patients were managed clinically.8 With this test, appearance of a purple line in the test zone of the reading window of the point-of-care testing cartridge indicates a positive test, and absence of any line indicates a negative test.
After the study was completed, quantitative D-dimer levels were measured using the STA-Liatest (Diagnostica Stago, Asnières, France) latex agglutination assay. This assay has a lower limit of detection of 220 μg/L and no upper limit.6 The STA-Liatest measurements were performed in a central laboratory on an STA Compact analyzer by technologists who were unaware of the results of the Clearview Simplify D-dimer assay or APA testing or clinical outcomes. The relationship between the quantitative and qualitative D-dimer results and recurrent VTE in DODS was previously described.7 The quantitative results could not influence management decisions.
APAs.
The presence of an APA was assessed as ≥1 of the following: an ACA, an anti-β2GP1, or an LA (Figure 1). Testing for each APA type was attempted on 2 occasions. Criteria for a positive APA test were predefined (ie, not influenced by knowledge of whether patients had recurrent VTE) and based on manufacturers’ suggestions.
ACAs, either immunoglobulin G (IgG) or IgM antibodies, were identified using enzyme immunoassays (Corogenix, Med-Ox Diagnostics, Nepean, ON, Canada) as described by Harris et al.9 An ACA test was considered positive if either antibody titer (IgG or IgM) was ≥23 units. Testing for an ACA was performed in patients both receiving and not receiving anticoagulant therapy at enrollment, and again at 7 months after enrollment (Figure 1).
Anti-β2GP1 antibodies of the IgG subtype were identified using the REAADS anti-β2GP1 enzyme-linked immunosorbent assay kit (Alere Inc., Ottawa, ON, Canada), and the test was considered positive with a titer of ≥20 G units. Testing for anti-β2GP1 antibodies was performed in patients both receiving and not receiving anticoagulant therapy at enrollment, and again at 7 months after enrollment (Figure 1).
An LA was identified using 2 assays, each combined with a confirmatory test if the initial screening test result was positive. The first assay uses an LA-sensitive activated partial thromboplastin time reagent (Diagnostica Stago; Abbott, Mississauga, ON, Canada); an abnormal test was confirmed using a hexagonal phospholipid assay (Diagnostica Stago).10 The second assay measures the clotting time after addition of dilute Russell viper venom; an abnormal test was confirmed by normalization of results after addition of a high concentration of phospholipid (Diagnostica Stago).11 If either of the assays was abnormal and the confirmatory test was positive, patients were considered to have an LA. Testing for an LA was only performed in patients not receiving anticoagulant therapy at 1 month (patients who had a negative D-dimer at enrollment and stopped anticoagulants) and at 7 months (patients who had a negative D-dimer at enrollment, stopped anticoagulants, and had a negative D-dimer at 1 month and remained off anticoagulants; Figure 1).
Statistical analysis
We tabulated the number of samples that had an APA at enrollment (ACA-IgG, ACA-IgM, and anti-β2GP1 in all patients), at 1 month (LA in patients not receiving anticoagulants), and at 7 months (ACA-IgG, ACA-IgM, and anti-β2GP1 in all patients and LA in patients not receiving anticoagulants).
Association between APAs and recurrent VTE.
We estimated the rate of recurrent VTE in patients with different APA findings, as the number of VTE events during the observation period divided by the total number of person-years of observation, with corresponding 95% confidence intervals (CIs) for these rate estimates. We compared recurrent VTE rates between patient groups with different APA findings and calculated hazard ratios (HRs) using the Cox regression model.
We also summarized rates of recurrent VTE in patients who were APA− on all occasions, APA+ on ≥1 occasions, APA+ with the same APA type on 2 occasions, and APA+ with 2 or 3 different APA types on either the same or different occasions. The observation period for recurrent VTE was confined to when patients were not receiving anticoagulants (ie, in response to negative qualitative D-dimer testing) and included time before and after blood sampling for APA testing. Therefore, the observation period for APA+ patients could include time before the blood draw that yielded the first positive APA result. The observation period for recurrent VTE in individual patients could be as short as 1 month (negative D-dimer at enrollment and positive D-dimer at 1 month) or for the whole duration of the study (negative D-dimer at both enrollment and 1 month). Analyses were performed in all study patients and then repeated after excluding women with estrogen-associated VTE (VTEs in these women are often considered to have been provoked, and none of them had recurrent VTE in DODS6,12 ).
Association between APAs and D-dimer levels.
At enrollment, when all patients were receiving anticoagulants, we compared the prevalence of a positive qualitative D-dimer test in patients who were APA+ (on any occasion) and in patients who were APA− (on all occasions) and compared quantitative D-dimer levels in patients who were APA+ (on any occasion) and APA− (on all occasions). For these analyses, the D-dimer level was set at 110 μg/L if the level was below the detection limit of 220 μg/L (ie, half of the detection limit of the quantitative test).
At 1 month, among patients who had stopped anticoagulants at enrollment in response to a negative qualitative D-dimer test, we compared the prevalence of D-dimer conversion from negative to positive in patients who were APA+ (on any occasion) and APA− (on all occasions), compared D-dimer levels in patients who were APA+ (on any occasion) and APA− (on all occasions), and compared D-dimer levels in patients with and without a specific APA (on any occasion).
At 7 months, among patients who had stopped anticoagulants at enrollment in response to a negative qualitative D-dimer test and had remained off anticoagulants at 1 month in response to a second negative qualitative D-dimer test, we compared D-dimer levels in patients who were APA+ (on any occasion) and APA− (on all occasions) and compared D-dimer levels in patients with and without a specific APA (on any occasion).
We compared the prevalence of a positive qualitative D-dimer at enrollment or at 1 month (or both) in patients with and without an APA (on any occasion) and in patients with and without a specific APA (on any occasion). For these comparisons, the χ2 test was used to compare frequencies and the Mann-Whitney nonparametric test was used to compare D-dimer levels.
Results
Of the 410 patients in DODS, 369 were enrolled in the 12 clinical centers that took part in this substudy, and 307 of these patients had at least 1 APA test performed (Tables 1 and 2). Compared with patients who were not included, those included in the current analysis were somewhat older, and a higher proportion presented with pulmonary embolism rather than deep vein thrombosis, had risk factors for bleeding, were receiving a statin, and had signs of postthrombotic syndrome (Table 1).
Baseline characteristics of patients included and not included in the analysis
| Characteristic . | Patients included (n = 307) . | Patients not included (n = 103) . | P . |
|---|---|---|---|
| Subgroup, N (%) | .58 | ||
| Men | 175 (57) | 56 (54) | |
| Women not taking estrogen | 83 (27) | 26 (25) | |
| Women taking estrogen | 49 (16) | 21 (21) | |
| Mean (SD) age, years | 52 (13) | 48 (16) | .01 |
| Mean (SD) weight, kg | 94 (24) | 90 (23) | .16 |
| Mean (SD) BMI, kg/m2 | 31 (7) | 30 (7) | .52 |
| Mean (SD) months on anticoagulation | 5 (1) | 5 (1) | .70 |
| Qualifying thrombotic event, N (%) | .003 | ||
| Proximal DVT only | 124 (40) | 59 (57) | |
| PE (with or without DVT) | 183 (60) | 44 (43) | |
| Risk factors for bleeding,*N (%) | .05 | ||
| None | 260 (85) | 95 (92) | |
| ≥1 | 47 (15) | 8 (8) | |
| Diabetes,† N (%) | 22 (7) | 8 (8) | .84 |
| Antiplatelet therapy,‡ N (%) | 22 (7) | 6 (6) | .65 |
| Statin therapy, N (%) | 54 (18) | 8 (8) | .02 |
| Family history of VTE,§ N (%) | 72 (23) | 16 (16) | .09 |
| Hyperpigmentation/edema of either leg, N (%) | 83 (27) | 17 (17) | .03 |
| Performance status,‖N (%) | .54 | ||
| Fully active | 277 (90) | 95 (92) | |
| Less than fully active | 30 (10) | 8 (8) |
| Characteristic . | Patients included (n = 307) . | Patients not included (n = 103) . | P . |
|---|---|---|---|
| Subgroup, N (%) | .58 | ||
| Men | 175 (57) | 56 (54) | |
| Women not taking estrogen | 83 (27) | 26 (25) | |
| Women taking estrogen | 49 (16) | 21 (21) | |
| Mean (SD) age, years | 52 (13) | 48 (16) | .01 |
| Mean (SD) weight, kg | 94 (24) | 90 (23) | .16 |
| Mean (SD) BMI, kg/m2 | 31 (7) | 30 (7) | .52 |
| Mean (SD) months on anticoagulation | 5 (1) | 5 (1) | .70 |
| Qualifying thrombotic event, N (%) | .003 | ||
| Proximal DVT only | 124 (40) | 59 (57) | |
| PE (with or without DVT) | 183 (60) | 44 (43) | |
| Risk factors for bleeding,*N (%) | .05 | ||
| None | 260 (85) | 95 (92) | |
| ≥1 | 47 (15) | 8 (8) | |
| Diabetes,† N (%) | 22 (7) | 8 (8) | .84 |
| Antiplatelet therapy,‡ N (%) | 22 (7) | 6 (6) | .65 |
| Statin therapy, N (%) | 54 (18) | 8 (8) | .02 |
| Family history of VTE,§ N (%) | 72 (23) | 16 (16) | .09 |
| Hyperpigmentation/edema of either leg, N (%) | 83 (27) | 17 (17) | .03 |
| Performance status,‖N (%) | .54 | ||
| Fully active | 277 (90) | 95 (92) | |
| Less than fully active | 30 (10) | 8 (8) |
BMI, body mass index; DVT, deep vein thrombosis; PE, pulmonary embolism; SD, standard deviation.
Risk factors include: previous stroke, previous peptic ulcer disease, previous gastrointestinal bleeding, previous genitourinary bleeding, known gastrointestinal abnormality associated with bleeding, known genitourinary abnormality associated with bleeding, diabetes requiring medication, and antiplatelet therapy.
Requiring medication.
Aspirin in all patients.
One or more parents, siblings, or children.
Using Eastern Cooperative Oncology Group performance status and dichotomized as fully active (score, 0) vs less than fully active (score, 1-4).
Prevalence of APA at enrollment and 1 and 7 months after enrollment
| . | Enrollment . | 1 month . | 7 month . | Twice . | Once (tested twice) . | Once (tested once) . |
|---|---|---|---|---|---|---|
| ACA | 22 of 296 | — | 21 of 250 | 16 of 242 | 6 of 242 | 5 of 62 |
| IgG | 12 of 296 | — | 10 of 250 | 8 of 242 | 2 of 242 | 4 of 62 |
| IgM | 11 of 296 | — | 11 of 249 | 8 of 241 | 2 of 241 | 2 of 62 |
| IgG and IgM | 1 of 296 | — | 0 of 249 | 0 of 241 | 0 of 241 | 1 of 62 |
| Anti-β2GP1 | 3 of 296 | — | 2 of 250 | 2 of 242 | 1 of 242 | 0 of 62 |
| LA | — | 41 of 227 | 39 of 175 | 17 of 162 | 27 of 162 | 19 of 78 |
| APTT | — | 12 of 226 | 13 of 175 | 6 of 162 | 10 of 162 | 3 of 77 |
| DRVV | — | 35 of 226 | 33 of 173 | 14 of 159 | 21 of 159 | 18 of 78 |
| APTT and DRVV | — | 6 of 225 | 7 of 173 | 3 of 159 | 5 of 159 | 2 of 77 |
| . | Enrollment . | 1 month . | 7 month . | Twice . | Once (tested twice) . | Once (tested once) . |
|---|---|---|---|---|---|---|
| ACA | 22 of 296 | — | 21 of 250 | 16 of 242 | 6 of 242 | 5 of 62 |
| IgG | 12 of 296 | — | 10 of 250 | 8 of 242 | 2 of 242 | 4 of 62 |
| IgM | 11 of 296 | — | 11 of 249 | 8 of 241 | 2 of 241 | 2 of 62 |
| IgG and IgM | 1 of 296 | — | 0 of 249 | 0 of 241 | 0 of 241 | 1 of 62 |
| Anti-β2GP1 | 3 of 296 | — | 2 of 250 | 2 of 242 | 1 of 242 | 0 of 62 |
| LA | — | 41 of 227 | 39 of 175 | 17 of 162 | 27 of 162 | 19 of 78 |
| APTT | — | 12 of 226 | 13 of 175 | 6 of 162 | 10 of 162 | 3 of 77 |
| DRVV | — | 35 of 226 | 33 of 173 | 14 of 159 | 21 of 159 | 18 of 78 |
| APTT and DRVV | — | 6 of 225 | 7 of 173 | 3 of 159 | 5 of 159 | 2 of 77 |
| . | Enrollment . | 1 month . | 7 month . | Any time* . | . | . |
|---|---|---|---|---|---|---|
| APA+(≥1 tests) | 23 of 296 | 41 of 227 | 54 of 250 | 80 of 307 | ||
| 1 assay only† | 21 of 296 | NA | 47 of 250 | 69 of 307 | ||
| ACA + anti-β2GP1 | 2 of 296 | NA | 0 of 250 | 0 of 307 | ||
| ACA + LA | NA | NA | 6 of 250 | 9 of 307 | ||
| Anti-β2GP1 + LA | NA | NA | 0 of 250 | 0 of 307 | ||
| ACA + anti-β2GP1 + LA | NA | NA | 1 of 250 | 2 of 307 |
| . | Enrollment . | 1 month . | 7 month . | Any time* . | . | . |
|---|---|---|---|---|---|---|
| APA+(≥1 tests) | 23 of 296 | 41 of 227 | 54 of 250 | 80 of 307 | ||
| 1 assay only† | 21 of 296 | NA | 47 of 250 | 69 of 307 | ||
| ACA + anti-β2GP1 | 2 of 296 | NA | 0 of 250 | 0 of 307 | ||
| ACA + LA | NA | NA | 6 of 250 | 9 of 307 | ||
| Anti-β2GP1 + LA | NA | NA | 0 of 250 | 0 of 307 | ||
| ACA + anti-β2GP1 + LA | NA | NA | 1 of 250 | 2 of 307 |
APTT, activated partial thromboplastin time; DRVV, dilute Russell viper venom; NA, not applicable.
The number of each APA assay performed at each of the 3 time points is shown in the upper part of this table.
IgG and IgM subgroups of ACA and APTT and DRVV subgroups of LA were not counted as separate assays.
The numbers of patients who provided blood samples for the current analyses were 296 at enrollment (ACA-IgG, ACA-IgM, and anti-β2GP1 in all patients, all of whom were receiving anticoagulants), 227 at 1 month (LA in patients who had a negative qualitative D-dimer at enrollment and stopped anticoagulants), and 250 at 7 months (ACA-IgG, ACA-IgM, and anti-β2GP1 in all patients and LA in 175 patients not receiving anticoagulants; Table 2).
On ≥1 occasions, results were positive for an APA in 26.1%, an ACA (IgM or IgG) in 8.9%, an anti-β2GP1 in 1.0%, and an LA in 26.2% of tested patients (Table 2; supplemental Table 1; Figure 2). The same APA test was performed twice in the same patient 646 times (ie, total of 1292 tests). When the same test was performed twice, for all types of APA tests combined, the test was positive once in 5.3% and positive twice in 5.4% of patients. Of these, ACA testing was positive once in 2.5% and twice in 6.6%, anti-β2GP1 was positive once in 0.4% and twice in 0.8%, and LA was positive once in 16.7% and twice in 10.5% (Table 2). Of the 276 patients who were tested for at least 2 different types of APAs (on the same or different occasions), 25.0% were positive for 1 type of APA and 3.3% were positive for 2 types of APAs. Of the 191 patients who were tested for the 3 types of APA, 1.0% (2 patients) were positive for all 3 APA types (Table 2). The prevalence of any APA did not differ according to whether patients were men, women whose index VTE was not associated with estrogen therapy, or women whose index VTE was associated with estrogen therapy (P = .38; supplemental Table 1).
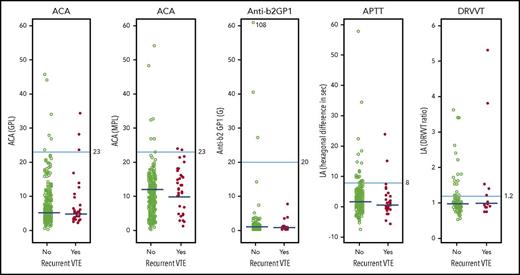
Association between quantitative APA assay results and recurrent VTE after stopping anticoagulant therapy. Results of APA assays in individual patients according to whether they did or did not have recurrent VTE during follow-up after stopping anticoagulant therapy. The cutoff values used to define whether a result was within the normal range are shown for each type of APA assay. When an APA was measured on 2 occasions in the same patient, the higher of the 2 values was used in these figures. The median values in patients with and without recurrent VTE are shown; the P values (2-sided median test) associated with each of these differences were: ACA IgG, P = .15; ACA IgM, P = .041; anti-β2GP1, P = .70; LA APTT, P = .17; and LA dilute Russell viper venom time (DRVVT), P = .70. GPL, IgG phospholipid units; MPL, IgM phospholipid units.
Association between quantitative APA assay results and recurrent VTE after stopping anticoagulant therapy. Results of APA assays in individual patients according to whether they did or did not have recurrent VTE during follow-up after stopping anticoagulant therapy. The cutoff values used to define whether a result was within the normal range are shown for each type of APA assay. When an APA was measured on 2 occasions in the same patient, the higher of the 2 values was used in these figures. The median values in patients with and without recurrent VTE are shown; the P values (2-sided median test) associated with each of these differences were: ACA IgG, P = .15; ACA IgM, P = .041; anti-β2GP1, P = .70; LA APTT, P = .17; and LA dilute Russell viper venom time (DRVVT), P = .70. GPL, IgG phospholipid units; MPL, IgM phospholipid units.
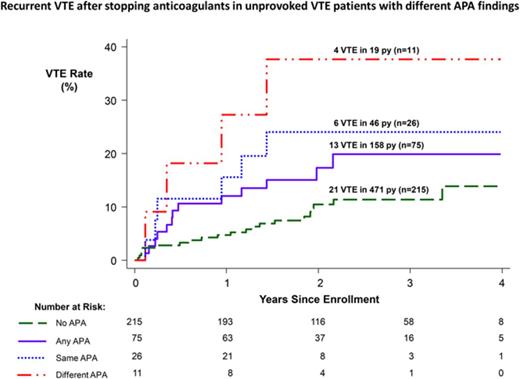
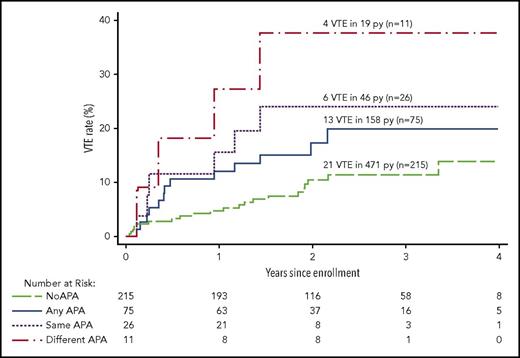
Association between APAs and recurrent VTE
Overall, the rate of recurrent VTE was 5.4% per person-years in patients not receiving anticoagulants (Table 3). The rate of recurrent VTE was 8.2% per person-years in patients with any APA and 4.5% per person-years in patients with no APA (HR, 1.8; 95% CI, 0.9-3.7; P = .09; Figure 3). After adjusting for the 3 sex/estrogen categories, the HR for recurrence with any APA was unchanged (HR, 1.8; 95% CI, 0.9-3.6; P = .09). When women with estrogen-associated VTE were excluded, the rate of recurrent VTE was 10.5% per person-years in patients with any APA and 5.4% per person-years in patients with no APA (HR, 1.9; 95% CI, 0.9-3.7; P = .08; Table 4). Rates of recurrent VTE in men and in women whose index VTE was not associated with estrogen therapy, according to whether there was no APA, any APA, an APA once, or the same APA twice, are listed in Table 4. The association between APAs and recurrent VTE did not seem to be strongly influenced by the cutoff value that was used to classify an APA result as positive (Figure 2).
Recurrent VTE rates during follow-up in patients with different APA findings
| Group . | N of patients . | N of VTE events . | Person-years . | Event rate per 100 person-years (95% CI) . |
|---|---|---|---|---|
| All patients | 290* | 34 | 627 | 5.4 (3.8-7.6) |
| No APA | 215 | 21 | 471 | 4.5 (2.8-6.8) |
| Any APA | 75 | 13 | 158 | 8.2 (4.4-14.1) |
| APA once† | 49 | 7 | 110 | 6.4 (2.6-13.1) |
| APA twice‡ | 26 | 6 | 46 | 13.0 (4.8-28.4) |
| ACA only§ | 11 | 1 | 28 | 3.6 (0.1-19.9) |
| IgG only‖ | 6 | 1 | 13 | 7.7 (0.2-42.9) |
| IgM only¶ | 5 | 0 | 14 | 0.0 (0.0-26.3) |
| Anti-β2GP1 only§ | 1 | 0 | 2 | 0.0 (0.0-184.4) |
| LA only§ | 52 | 8 | 108 | 7.4 (3.2-14.6) |
| 1 APA type# | 64 | 9 | 137 | 6.6 (3.0-12.5) |
| 2 APA types# | 9 | 4 | 12 | 33.1 (9.1-85.3) |
| 3 APA types# | 2 | 0 | 7 | 0.0 (0.0-52.7) |
| Group . | N of patients . | N of VTE events . | Person-years . | Event rate per 100 person-years (95% CI) . |
|---|---|---|---|---|
| All patients | 290* | 34 | 627 | 5.4 (3.8-7.6) |
| No APA | 215 | 21 | 471 | 4.5 (2.8-6.8) |
| Any APA | 75 | 13 | 158 | 8.2 (4.4-14.1) |
| APA once† | 49 | 7 | 110 | 6.4 (2.6-13.1) |
| APA twice‡ | 26 | 6 | 46 | 13.0 (4.8-28.4) |
| ACA only§ | 11 | 1 | 28 | 3.6 (0.1-19.9) |
| IgG only‖ | 6 | 1 | 13 | 7.7 (0.2-42.9) |
| IgM only¶ | 5 | 0 | 14 | 0.0 (0.0-26.3) |
| Anti-β2GP1 only§ | 1 | 0 | 2 | 0.0 (0.0-184.4) |
| LA only§ | 52 | 8 | 108 | 7.4 (3.2-14.6) |
| 1 APA type# | 64 | 9 | 137 | 6.6 (3.0-12.5) |
| 2 APA types# | 9 | 4 | 12 | 33.1 (9.1-85.3) |
| 3 APA types# | 2 | 0 | 7 | 0.0 (0.0-52.7) |
Does not include the 12 patients who had a positive qualitative D-dimer at enrollment and therefore did not stop anticoagulants.
Patients may have tested positive for ≥1 types of APAs, but no type of APA was present on 2 occasions. Includes patients who were tested for any APA once (n = 25), twice (n = 74), or 3 times (n = 191); the same APA test was not performed more than twice.
Only includes patients who had the same type of APA on 2 occasions (consistent with the Sapporo-Sydney criteria for diagnosis of antiphospholipid syndrome).
Test may have been positive on 1 or 2 occasions.
Highest anticardiolipin IgG antibody level in each patient: 23, 31, 28, 24, 24, and 24 units (recurrent VTE).
Highest anticardiolipin IgM antibody level in each patient: 27, 35, 32, 54, and 48 units.
Tests may have been positive on the same or different occasions. Event rate for patients with either 2 or 3 APA types was 21.1 (95% CI, 5.7-53.9) per 100 person-years.
Recurrent VTE after stopping anticoagulant therapy in patients with different APA findings. The cumulative proportion for recurrent VTE during follow-up after stopping anticoagulant therapy according to the APA findings in different groups of patients. No APA means no positive APA test on all occasions tested, and any APA means ≥1 positive APA tests on ≥1 occasions; these 2 groups are mutually exclusive. Same APA means that the same type of APA was positive on 2 occasions; these patients are a subgroup of the any APA group. Different APA means that at least 2 different types of APAs were positive, either on the same or different occasions; these patients are a subgroup of the any APA group.
Recurrent VTE after stopping anticoagulant therapy in patients with different APA findings. The cumulative proportion for recurrent VTE during follow-up after stopping anticoagulant therapy according to the APA findings in different groups of patients. No APA means no positive APA test on all occasions tested, and any APA means ≥1 positive APA tests on ≥1 occasions; these 2 groups are mutually exclusive. Same APA means that the same type of APA was positive on 2 occasions; these patients are a subgroup of the any APA group. Different APA means that at least 2 different types of APAs were positive, either on the same or different occasions; these patients are a subgroup of the any APA group.
Recurrent VTE rates during follow-up in patients with different APA findings (women with estrogen-associated VTE excluded)
| Group/subgroup . | N of patients . | N of VTE events . | Person-years . | Event rate (95% CI) . |
|---|---|---|---|---|
| All patients | 241* | 34 | 510 | 6.7 (4.6-9.3) |
| No APA | 179 | 21 | 386 | 5.4 (3.4-8.3) |
| Men | 119 | 15 | 255 | 5.9 (3.3-9.7) |
| Women | 60 | 6 | 131 | 4.6 (1.7-10.0) |
| Any APA | 62 | 13 | 124 | 10.5 (5.6-17.9) |
| Men | 47 | 11 | 91 | 12.1 (6.0-21.6) |
| Women | 15 | 2 | 32 | 6.3 (0.8-22.6) |
| APA once† | 40 | 7 | 87 | 8.0 (3.2-16.6) |
| Men | 29 | 6 | 62 | 9.7 (3.6-21.1) |
| Women | 11 | 1 | 25 | 4.0 (0.1-22.3) |
| APA twice‡ | 22 | 6 | 37 | 16.2 (6.0-35.5) |
| Men | 18 | 5 | 30 | 16.7 (5.4-38.9) |
| Women | 4 | 1 | 7 | 14.3 (0.4-79.6) |
| ACA only§ | 10 | 1 | 24 | 4.2 (0.1-23.2) |
| IgG only‖ | 6 | 1 | 13 | 7.7 (0.2-42.9) |
| IgM only¶ | 4 | 0 | 10 | 0.0 (0.0-36.9) |
| Anti-β2GP1 only§ | 0 | 0 | 0 | — |
| LA only§ | 41 | 8 | 82 | 9.8 (4.2-19.2) |
| 1 APA type# | 51 | 9 | 105 | 8.6 (3.9-16.3) |
| 2 APA types# | 9 | 4 | 12 | 33.3 (9.1-85.3) |
| 3 APA types# | 2 | 0 | 7 | 0.0 (0.0-52.7) |
| Group/subgroup . | N of patients . | N of VTE events . | Person-years . | Event rate (95% CI) . |
|---|---|---|---|---|
| All patients | 241* | 34 | 510 | 6.7 (4.6-9.3) |
| No APA | 179 | 21 | 386 | 5.4 (3.4-8.3) |
| Men | 119 | 15 | 255 | 5.9 (3.3-9.7) |
| Women | 60 | 6 | 131 | 4.6 (1.7-10.0) |
| Any APA | 62 | 13 | 124 | 10.5 (5.6-17.9) |
| Men | 47 | 11 | 91 | 12.1 (6.0-21.6) |
| Women | 15 | 2 | 32 | 6.3 (0.8-22.6) |
| APA once† | 40 | 7 | 87 | 8.0 (3.2-16.6) |
| Men | 29 | 6 | 62 | 9.7 (3.6-21.1) |
| Women | 11 | 1 | 25 | 4.0 (0.1-22.3) |
| APA twice‡ | 22 | 6 | 37 | 16.2 (6.0-35.5) |
| Men | 18 | 5 | 30 | 16.7 (5.4-38.9) |
| Women | 4 | 1 | 7 | 14.3 (0.4-79.6) |
| ACA only§ | 10 | 1 | 24 | 4.2 (0.1-23.2) |
| IgG only‖ | 6 | 1 | 13 | 7.7 (0.2-42.9) |
| IgM only¶ | 4 | 0 | 10 | 0.0 (0.0-36.9) |
| Anti-β2GP1 only§ | 0 | 0 | 0 | — |
| LA only§ | 41 | 8 | 82 | 9.8 (4.2-19.2) |
| 1 APA type# | 51 | 9 | 105 | 8.6 (3.9-16.3) |
| 2 APA types# | 9 | 4 | 12 | 33.3 (9.1-85.3) |
| 3 APA types# | 2 | 0 | 7 | 0.0 (0.0-52.7) |
Does not include the 12 patients who had a positive qualitative D-dimer at enrollment and therefore did not stop anticoagulants.
Includes patients who were tested for any APA once (n = 25), twice (n = 66), or 3 times (n = 154); the same test was not performed more than twice.
Only includes patients who had the same APA on 2 occasions (consistent with the Sapporo-Sydney criteria for diagnosis of antiphospholipid syndrome).
Test may have been positive on 1 or 2 occasions.
Highest anticardiolipin IgG antibody in each patient: 23, 31, 28, 24, 24, and 24 units (recurrent VTE).
Highest anticardiolipin IgM antibody in each patient: 35, 32, 54, and 48 units.
Tests may have been positive on the same or different occasions. Event rate for patients with either 2 or 3 APA types was 21.1 (95% CI, 5.7-53.9) per 100 person-years.
Same type of APA on the 2 occasions tested (consistent with the Sapporo-Sydney criteria for diagnosis of antiphospholipid syndrome).
The rate of recurrent VTE was 13.0% per person-years in patients with ≥1 of the same type of APA on the 2 occasions tested and 6.4% per person-years in patients with ≥1 type of the same type of APA on 1 occasion (HR, 1.8; 95% CI, 0.6-5.5; P = .28; Table 3; Figure 3). The rate of recurrence was higher in patients with ≥1 of the same type of APA on the 2 occasions tested than in patients who had no APA (HR, 2.7; 95% CI, 1.1-6.7; P = .03). When women with estrogen-associated VTE were excluded, the rate of recurrent VTE was 16.2% per person-years in patients with ≥1 of the same type of APA on the 2 occasions tested and 8.0% per person-years in patients with ≥1 of the same type of APA on 1 occasion (HR, 1.8; 95% CI, 0.6-5.3; P = .31), and the rate of recurrence was higher in patients with ≥1 of the same type of APA on the 2 occasions tested than in patients with no APA (HR, 2.7; 95% CI, 1.1-6.7; P = .03; Table 4).
Different type of APA in the same patient on the same or different occasions.
The rate of recurrent VTE was 21.1% per person-years in patients with 2 or 3 types of APAs on the same or different occasions and 6.6% per person-years in patients with only 1 type of APA on ≥1 occasions (HR, 3.1; 95% CI, 0.9-10.0; P = .06; Tables 3; Figure 3). The rate of recurrence was higher in patients with 2 or 3 types of APAs on the same or different occasions than in patients with no APA (HR, 4.5; 95% CI, 1.5-13.0; P = .006). When women with estrogen-associated VTE were excluded, the rate of recurrent VTE was 21.1% per person-years in patients with 2 or 3 types of APAs on ≥1 occasions and 8.6% per person-years in patients with only 1 type of APA (HR, 2.4; 95% CI, 0.7-7.8; P = .15), and the rate of recurrence was higher in patients with 2 or 3 types of APAs on ≥1 occasions than in patients with no APA (HR, 3.7; 95% CI, 1.3-10.7; P = .02; Table 4).
Association between APAs and qualitative D-dimer levels
At enrollment, when all patients were receiving anticoagulants, 6.3% (5 of 80) of APA+ and 3.1% (7 of 227) of APA− patients had a positive qualitative D-dimer test (P = .21; supplemental Table 1). At 1 month, among patients who had stopped anticoagulants at enrollment in response to a negative qualitative D-dimer test, the qualitative D-dimer test converted to positive in 9.3% (7 of 75) of APA+ and 19.2% (39 of 204) of APA− patients (P = .05; supplemental Table 2). Of APA+ patients, 15.0% (12 of 80) had a positive D-dimer at enrollment or at 1 month, whereas 20% (46 of 227) of APA− patients had a positive D-dimer at enrollment or at 1 month (P = .30; supplemental Table 2).
Association between APAs and quantitative D-dimer levels
At enrollment when all patients were receiving anticoagulants, at 1 month among patients who had stopped anticoagulants at enrollment in response to a negative qualitative D-dimer test, and at 7 months among patients who had stopped anticoagulants at enrollment in response to a negative qualitative D-dimer test and remained off anticoagulants in response to a negative qualitative D-dimer test, there was no difference in D-dimer levels between patients with and without an APA (Table 5).
D-dimer levels in patients with and without APAs
| . | Median (IQR) . | P . | |
|---|---|---|---|
| APA− . | APA+ . | ||
| Enrollment; all patients on anticoagulants | |||
| Any APA vs no APA | 110 (110-275) | 110 (110-285) | .69 |
| ACA vs no ACA | 110 (110-270) | 110 (110-320) | .69 |
| Anti-β2GP1 vs no anti-β2GP1 | 110 (110-280) | 110 (110-110) | .20 |
| LA vs no LA | 110 (110-240) | 110 (110-260) | .32 |
| 1 month; negative qualitative D-dimer at enrollment and stopped anticoagulants | |||
| Any APA vs no APA | 310 (110-440) | 325 (240-430) | .37 |
| ACA vs no ACA | 310 (110-430) | 360 (240-900) | .13 |
| Anti-β2GP1 vs no anti-β2GP1 | 310 (110-430) | 440 (240-640) | .63 |
| LA vs no LA | 310 (110-440) | 310 (230-390) | .88 |
| 7 months; negative qualitative D-dimer at enrollment and stopped anticoagulants, and negative qualitative D-dimer at 1 month and remained off anticoagulants | |||
| Any APA vs no APA | 320 (110-540) | 340 (245-535) | .38 |
| ACA vs no ACA | 330 (110-540) | 340 (290-520) | .63 |
| Anti-β2GP1 vs no anti-β2GP1 | 330 (220-540) | 230 (110-350) | .42 |
| LA vs no LA | 330 (110-550) | 330 (230-450) | .82 |
| . | Median (IQR) . | P . | |
|---|---|---|---|
| APA− . | APA+ . | ||
| Enrollment; all patients on anticoagulants | |||
| Any APA vs no APA | 110 (110-275) | 110 (110-285) | .69 |
| ACA vs no ACA | 110 (110-270) | 110 (110-320) | .69 |
| Anti-β2GP1 vs no anti-β2GP1 | 110 (110-280) | 110 (110-110) | .20 |
| LA vs no LA | 110 (110-240) | 110 (110-260) | .32 |
| 1 month; negative qualitative D-dimer at enrollment and stopped anticoagulants | |||
| Any APA vs no APA | 310 (110-440) | 325 (240-430) | .37 |
| ACA vs no ACA | 310 (110-430) | 360 (240-900) | .13 |
| Anti-β2GP1 vs no anti-β2GP1 | 310 (110-430) | 440 (240-640) | .63 |
| LA vs no LA | 310 (110-440) | 310 (230-390) | .88 |
| 7 months; negative qualitative D-dimer at enrollment and stopped anticoagulants, and negative qualitative D-dimer at 1 month and remained off anticoagulants | |||
| Any APA vs no APA | 320 (110-540) | 340 (245-535) | .38 |
| ACA vs no ACA | 330 (110-540) | 340 (290-520) | .63 |
| Anti-β2GP1 vs no anti-β2GP1 | 330 (220-540) | 230 (110-350) | .42 |
| LA vs no LA | 330 (110-550) | 330 (230-450) | .82 |
IQR, interquartile range.
Discussion
Of patients with a first unprovoked VTE who took part in this substudy, ∼25% were APA+ (ACA, anti-β2GP1, LA) on ≥1 occasions. The prevalence of an APA did not differ according to whether the index VTE was in a man, a woman who was not taking estrogen, or a woman who was taking estrogen. Among patients who stopped anticoagulants in response to a negative qualitative D-dimer test and remained off anticoagulants because the D-dimer test was still negative 1 month later, there was a suggestion that the rate of recurrence was higher in APA+ compared with APA− patients (P = .09, both with and without adjustment for sex category). Patients who had the same APA test positive on 2 occasions (P = .03; consistent with the Sapporo-Sydney criteria for diagnosis of antiphospholipid syndrome) or who had >1 type of APA positive on the same or different occasions (P = .006) had an approximately three-fold higher risk of recurrence than patients who were APA−. Therefore, there is a stronger argument for indefinite anticoagulant therapy in patients with either of these APA findings, who collectively accounted for ∼10% of patients who stopped anticoagulant therapy in response to negative D-dimer results.
The prevalence of an APA was not associated with sex/estrogen group, suggesting that sex/estrogen group and APAs independently predict recurrence. Similarly, APAs predicting recurrence in patients with negative qualitative D-dimer results and APAs not being associated with quantitative D-dimer levels suggest that D-dimer levels and APAs independently predict recurrence. There was no convincing evidence that the risk of recurrent VTE differed according to the type of APA that was present, although the number of patients in this study with each type of APA was too small to be able to exclude important differences in these rates.
A recent meta-analysis suggested that an APA might be associated with recurrent VTE in patients with a first VTE (risk ratio, 1.41; 95% CI, 0.99-2.00) and in the subgroup of those with a first unprovoked VTE (risk ratio, 1.94; 95% CI, 0.84-4.46).2 However, the overall quality of the evidence in that analysis was judged to be low because of major methodological weaknesses in the studies that were included and inconsistency of findings among these studies. The results of the current analysis are consistent with the meta-analysis and suggest that APAs, in a dose-dependent way, are associated with a moderate increase in recurrence risk after a first VTE. Importantly, as noted, our study does not suffer from many of the methodological weaknesses that were present in earlier studies evaluating an association between APAs and recurrent VTE. Our findings are also consistent with retrospective studies reporting a higher risk of recurrent VTE in patients with either an APA that was positive on >1 occasion (compared with on only 1 occasion) or with >1 type of APA (compared with only 1 type).4,13-15
Our analysis has both strengths and limitations.2,16 Strengths include: prospective enrollment; a well-defined patient population; few losses to follow-up; standardized evaluation of recurrent VTE by an adjudication committee; blinding of patients, health care providers, and adjudicators to APA status; central laboratory testing for an APA; APA testing on 2 occasions 6 or 7 months apart; and follow-up that was confined to when patients had stopped anticoagulant therapy. Limitations include: uncertainty about whether some clinical centers tested potentially eligible patients for an APA and excluded those with a positive result; that we did not measure the IgM subtype of anti-β2GP1 antibodies; that a substantial proportion of patients in the main study were not included in this substudy; that there were differences between those who were and were not included in the substudy, suggesting some selection bias; that evaluation of the association between an APA and recurrence was confined to patients who had stopped anticoagulant therapy in response to negative D-dimertesting; and that the study was unable to precisely identify recurrence risk in subgroups of patients, including those with different types of APAs. We also note that we tested for all 3 types of APAs on 2 occasions, rather than just repeating APA testing after a positive result; this will have increased the number of patients with a positive APA test on only 1 of the 2 occasions tested. Lastly, there is uncertainty about the APA level cutoff that should be used to define a positive test. If we had used a higher cutoff level of 40 units to define a positive ACA (IgG or IgM) or anti-β2GP1 test, fewer patients would have had a positive APA, and the association between an APA and recurrent VTE would have been weaker than we have reported (Figure 2; no patients with recurrent VTE had levels of these APAs >40 units).
What are the clinical implications of our findings? Current American College of Chest Physician guidelines suggest indefinite anticoagulation for patients with a first unprovoked proximal deep vein thrombosis or pulmonary embolism who are not at high risk for bleeding.17 If indefinite anticoagulation was planned, negative APA testing would not justify changing to a time-limited course of therapy. If a time-limited course of treatment is planned (eg, in a woman with a negative D-dimer after stopping therapy) or there is ambivalence about treatment duration, presence of an APA could justify choosing indefinite therapy.18 However, our findings suggest that a persistent APA or >1 type of APA is required for an APA to substantially increase the risk of recurrence.
In conclusion, in patients with a first episode of unprovoked VTE who had negative D-dimer results after stopping anticoagulant therapy and who were tested twice for an ACA, anti-β2GP1, and LA, having an APA on only 1 occasion was not associated with a substantially higher risk of recurrent VTE. However, having the same APA on 2 occasions or having >1 type of APA on the same or different occasions was associated with a higher the risk of recurrence and occurred in ∼10% of patients. The predictive value of an APA seemed to be independent of patient sex and posttreatment D-dimer levels. Therefore, if there is ambivalence about stopping or continuing anticoagulant therapy in patients with a first unprovoked VTE, APA testing may be helpful, because finding a persistent APA or >1 type of APA may tip the balance in favor of indefinite therapy.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Heart and Stroke Foundation of Ontario (GIA-50) and the Canadian Institutes of Health Research (MOP-84303). The DODS was funded by the Canadian Institutes of Health Research (GIA-50). The Clearview Simplify D-dimer assay was provided by Alere, Inc. (San Diego, CA), and the STA-Liatest D-dimer assay was provided by Diagnostica Stago (Asnières, France). C.K. is supported by an investigator award from the Heart and Stroke Foundation of Canada and the Jack Hirsh Professorship in Thromboembolism; S.R.L. is supported by grants from the National Institutes of Health and the American Society of Hematology; J.S.G. is supported by a career investigator award from the Heart and Stroke Foundation of Ontario and the David Braley and Nancy Gordon Chair for investigation of thromboembolic disease; and J.I.W. holds the Canada Research Chair in Thrombosis and the Heart and Stroke Foundation J. F. Mustard Chair in Cardiovascular Research.
The funding sources and assay providers had no role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Authorship
Contribution: C.K. was involved in study design, obtaining funding, data collection, data interpretation, writing, and critical review and final approval of the report; F.A.S., T.B., S.M.S., K.A.B., S.R.L., C.M.K., J.D.D., S.M., S.K., S.S., J.M.C., and J.S.G. were involved in study design, data collection, and critical review and final approval of the report; J.I.W., P.C.L., and J.A.J. were involved in study design, obtaining funding, data analysis, data interpretation, and critical review and final approval of the report; S.P. was involved in data analysis, data interpretation, and critical review and final approval of the report; L.S. was involved in study coordination, data analysis, and critical review and final approval of the report; and V.B. was involved in data interpretation and critical review and final approval of the report.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Clive Kearon, Thrombosis and Atherosclerosis Research Institute and Ontario Clinical Oncology Group, Juravinski Hospital, AE-73, 711 Concession St, Hamilton, ON L8V 1C3, Canada; e-mail: kearonc@mcmaster.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal