In this issue of Blood, Kearon et al show that the risk of recurrent venous thromboembolism (VTE) is significantly increased when several tests for antiphospholipid antibodies (APAs) of different types are positive.1
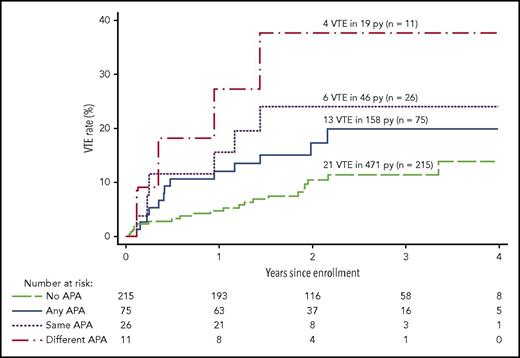
Recurrent VTE after stopping anticoagulants in patients with different APA profiles: patients with multiple tests positive and patients with persistent positivity are at increased risk for recurrent VTE. No APAs (all tests negative), any APAs (at least 1 test positive once), same APAs (positive test persistent for the same assay on 2 occasions), different APAs (multiple tests positive). See Figure 3 in the article by Kearon et al that begins on page 2151.
Recurrent VTE after stopping anticoagulants in patients with different APA profiles: patients with multiple tests positive and patients with persistent positivity are at increased risk for recurrent VTE. No APAs (all tests negative), any APAs (at least 1 test positive once), same APAs (positive test persistent for the same assay on 2 occasions), different APAs (multiple tests positive). See Figure 3 in the article by Kearon et al that begins on page 2151.
So-called APAs are a heterogeneous group of autoantibodies directed at plasma proteins such as prothrombin or β2 glycoprotein I.2 Routine tests to detect APAs include the lupus anticoagulant, anticardiolipin antibodies, and anti-β2 glycoprotein I antibodies. Although a significant association between APA and VTE had been previously demonstrated,3 the risk of recurrent VTE remained uncertain and debated.4
According to classification criteria, antiphospholipid syndrome (APS) is present when at least 1 of the defined clinical manifestations is documented (vascular thrombosis, pregnancy morbidity) and 1 routine test for APA is positive.5 Previous data indicate that all APS patients do not share the same risks; in particular, patients with triple positivity (all routine tests positive) seem to be at high risk for thrombotic events.6 Further risk stratification is obviously important to identify patients who are candidates for long-term anticoagulants.
Kearon and colleagues now report the characteristics of APA associated with an increased risk of recurrent thrombosis after a first unprovoked episode of VTE (see figure). The authors take advantage of the prospective D-dimer Optimal Duration Study,7 in which plasma samples were tested for APAs. Decisions about duration of anticoagulation were made without knowledge of the presence of APAs or not.
The results of the current study show the risk of recurrent thrombosis in 3 categories of patients. Although patients with any APA positivity at any time did not have a significant increase in the risk of recurrence, recurrence risk was significantly increased in 2 different laboratory profiles. Patients with the same type of APAs measured on 2 separate time points and thus consistent with Sapporo-Sydney laboratory classification criteria5 had almost a threefold higher risk for recurrence than patients without persistent APAs. In addition, patients with multiple APA positivity (Sapporo-Sydney laboratory category I) either at the same time or at different time points have a threefold higher risk than patients with only 1 test positive and a 4.5 higher risk than patients without APAs. These results may explain the reasons for previous controversies about the risk of recurrence because some earlier studies had performed only a single APA measurement, whereas others took into account persistent antibodies or multiple positivity.4,6
One other interesting result of this study is that patients with APAs did not have higher D-dimer levels than patients without APA. Furthermore, D-dimer levels were not significantly higher in patients with APAs or any specific type and recurrent VTE compared with patients with APA and without recurrence. These results confirm earlier results suggesting that D-dimers are not associated with an increased thrombotic risk in patients with APA.8
Some important issues are still to be considered. First, as indicated in this report, cutoff levels for APA positivity remain a matter of debate. In this study, cutoffs were indicated by manufacturers of commercially available tests, whereas Sydney-Sapporo suggest to use either the 99th percentile of a control population or the 40 immunoglobulin G (IgG) or IgM phospholipid units for the anticardiolipin assay to define cutoffs. The risk associated with these different cutoffs must be investigated in further studies, and sensitivity analyses should be performed to determine the risk of recurrence according to varying cutoffs. Second, it was not possible in this study to identify the risks associated with different types of APA: lupus anticoagulant compared with enzyme-linked immunosorbent assay (ELISA) tests or IgG and IgM isotypes of anticardiolipin and anti-β2 glycoprotein I antibodies. Finally, new ELISA tests and new techniques (chemiluminescence) are now available and have been evaluated in preliminary, mainly cross-sectional reports. Antiphosphatidylserine/prothrombin ELISA assays have been advocated because prothrombin is 1 of the main targets of APA.9 Tests for detection of antibodies against domain I of β2 glycoprotein I, in particular those recognizing the G40-R43 epitope of β2 glycoprotein I, are also being developed to identify pathogenic APA.9,10 These new tests should be included in future prospective studies and compared with classical APA tests.
The better characterization of APA and identification of profiles (persistent APA and multiple positivity) associated with a risk of recurrence in patients with a first unprovoked episode of VTE will help select candidates for indefinite prolongation of anticoagulant therapy. It is also important to consider these profiles for randomized controlled trials, in particular, when testing direct oral anticoagulants compared with traditional vitamin K antagonists in patients with APS.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal