TO THE EDITOR:
Intravascular large B-cell lymphoma (IVLBCL) is a rare variant of extranodal diffuse large B-cell lymphoma (DLBCL). It is characterized by proliferation of blastic, neoplastic B cells within the lumina of small- or intermediate-sized blood vessels and capillaries.1 IVLBCL may potentially involve any organ and is often widely disseminated. Two major patterns have been recognized. In Western patients, predominantly the skin and the central nervous system (CNS) are involved, whereas in Asian countries, the disease often involves multiple other organs and is accompanied by hemophagocytosis.2 Additionally, a cutaneous variant, with skin-limited disease at time of diagnosis, has been described in younger, Western women.3 Standard treatment of IVLBCL consists of rituximab-containing chemotherapeutic regimens, demonstrating an estimated 3-year overall survival (OS) of 60% to 81%.4,5
Previous patient reports and small case series have identified various translocations, sporadically involving an oncogene, such as BCL2, BCL6, or cyclin D1, but none have occurred in >1 patient.6-9 In 1 case, RNA expression profiling was similar to the non–germinal center B-cell (non-GCB) subtype of DLBCL.10 However, the mutational profile of IVLBCL is still unknown. Because of the phenotypic overlap with non-GCB DLBCL and frequent presentation in the skin and CNS, we hypothesized that IVLBCL may harbor NFκB-activating mutations.11-13 Therefore, we investigated the prevalence of a subset of DLBCL-associated genetic alterations in patients with IVLBCL and explored their association with survival.
For this retrospective study, we evaluated a unique and clinically well-annotated cohort of 25 patients who were diagnosed with IVLBCL according to the latest World Health Organization criteria in participating university and nonacademic regional hospitals in The Netherlands between 1985 and 2017.1 Central review was performed in the Leiden University Medical Center by A.M.R.S., P.M.J., and A.H.G.C. The study was performed in accordance with the Dutch Code Proper Secondary Use of Human Tissue and approved by the medical ethics committee of the Leiden University Medical Center (B16.048). Clinical data are summarized in Table 1. The group consisted of 16 women and 9 men, with a median age at diagnosis of 64 years (range, 49-85 years). The higher percentage of women in our cohort may be explained by the presence of 6 patients with skin-limited disease, which has a known female predominance.3 B symptoms were recorded in 18 of 22 (82%) patients. In total, 12 patients (48%) presented with skin lesions (Figure 1A-B), of whom 6 showed no signs of other affected organs on routine staging procedures. Other commonly involved organs were the bone marrow (8 of 24 [33%]), followed by the brain and lungs (7 of 24 [29%]). Nine patients had Ann Arbor stage IE disease (skin, brain, or kidney), and 15 patients had stage IV disease. Three cases were diagnosed at autopsy, and staging was not performed in 1 patient who declined additional diagnostic procedures.
Overview of clinical characteristics, treatment, follow-up, and results of 25 patients with IVLBCL
| Characteristic . | n/N (%) . |
|---|---|
| Sex | |
| Men | 9/25 (36) |
| Women | 16/25 (64) |
| Age at diagnosis, y | |
| Median | 64 |
| Range | 49-85 |
| Presence of B symptoms | 18/22 (82) |
| Disease localization | |
| Skin | 12/25 (48) |
| Bone marrow | 8/24 (33) |
| Brain | 7/24 (29) |
| Lung | 7/24 (29) |
| Spleen | 5/24 (21) |
| Liver | 4/24 (17) |
| Lymph nodes | 4/24 (17) |
| Kidney | 3/24 (13) |
| Other (thyroid, testis, [cardiac] muscle, soft tissue) | 5/24 (21) |
| Skin-limited disease | 6/24 (25) |
| Ann Arbor stage* | |
| IE (skin, brain, kidney) | 9/24 (38) |
| IV | 15/24 (63) |
| First-line therapy† | |
| Immunochemotherapy‡ | 6/22 (27) |
| Chemotherapy§ | 6/22 (27) |
| Immunotherapy (rituximab only) | 1/22 (5) |
| Radiotherapy | 3/22 (14) |
| Supportive care with/without steroids | 5/22 (23) |
| Surgery + watchful waiting (subcutaneous lipoma) | 1/22 (5) |
| Second-line therapy | |
| Immunochemotherapy‡ | 2/22 (9) |
| Chemotherapy§ | 1/22 (5) |
| Radiotherapy | 2/22 (9) |
| Survival status | |
| Died, disease related | 14/25 (56) |
| Died, disease unrelated | 5/25 (20) |
| Alive | 6/25 (24) |
| Median overall survival time, years‖ | 1.68 |
| Immunohistochemistry | |
| CD20 | 25/25 (100) |
| CD10 | 2/25 (8) |
| BCL6 | 14/24 (58) |
| MUM1 | 18/24 (75) |
| BCL2 | 23/24 (96) |
| IgM | 21/23 (91) |
| MYC | 15/22 (68) |
| CD5 | 13/25 (52) |
| Cyclin D1 | 0/25 (0) |
| CD30 | 0/24 (0) |
| EBER ISH | 0/24 (0) |
| MYC FISH | 0/15 (0) |
| Targeted next-generation sequencing | |
| MYD88 (exon 3/exon 5) | 11/25 (44) |
| CD79B (exon 5/exon 6) | 6/23 (26) |
| CD79A (exon 5) | 0/24 (0) |
| CARD11 (exon 6) | 0/24 (0) |
| EZH2 (exon 16) | 1/25 (4) |
| Characteristic . | n/N (%) . |
|---|---|
| Sex | |
| Men | 9/25 (36) |
| Women | 16/25 (64) |
| Age at diagnosis, y | |
| Median | 64 |
| Range | 49-85 |
| Presence of B symptoms | 18/22 (82) |
| Disease localization | |
| Skin | 12/25 (48) |
| Bone marrow | 8/24 (33) |
| Brain | 7/24 (29) |
| Lung | 7/24 (29) |
| Spleen | 5/24 (21) |
| Liver | 4/24 (17) |
| Lymph nodes | 4/24 (17) |
| Kidney | 3/24 (13) |
| Other (thyroid, testis, [cardiac] muscle, soft tissue) | 5/24 (21) |
| Skin-limited disease | 6/24 (25) |
| Ann Arbor stage* | |
| IE (skin, brain, kidney) | 9/24 (38) |
| IV | 15/24 (63) |
| First-line therapy† | |
| Immunochemotherapy‡ | 6/22 (27) |
| Chemotherapy§ | 6/22 (27) |
| Immunotherapy (rituximab only) | 1/22 (5) |
| Radiotherapy | 3/22 (14) |
| Supportive care with/without steroids | 5/22 (23) |
| Surgery + watchful waiting (subcutaneous lipoma) | 1/22 (5) |
| Second-line therapy | |
| Immunochemotherapy‡ | 2/22 (9) |
| Chemotherapy§ | 1/22 (5) |
| Radiotherapy | 2/22 (9) |
| Survival status | |
| Died, disease related | 14/25 (56) |
| Died, disease unrelated | 5/25 (20) |
| Alive | 6/25 (24) |
| Median overall survival time, years‖ | 1.68 |
| Immunohistochemistry | |
| CD20 | 25/25 (100) |
| CD10 | 2/25 (8) |
| BCL6 | 14/24 (58) |
| MUM1 | 18/24 (75) |
| BCL2 | 23/24 (96) |
| IgM | 21/23 (91) |
| MYC | 15/22 (68) |
| CD5 | 13/25 (52) |
| Cyclin D1 | 0/25 (0) |
| CD30 | 0/24 (0) |
| EBER ISH | 0/24 (0) |
| MYC FISH | 0/15 (0) |
| Targeted next-generation sequencing | |
| MYD88 (exon 3/exon 5) | 11/25 (44) |
| CD79B (exon 5/exon 6) | 6/23 (26) |
| CD79A (exon 5) | 0/24 (0) |
| CARD11 (exon 6) | 0/24 (0) |
| EZH2 (exon 16) | 1/25 (4) |
EBER-ISH, Epstein-Barr virus early RNA in situ hybridization; IgM, immunoglobulin M; MYC FISH, fluorescence in situ hybridization with breakapart probes for MYC.
Staging procedures were not performed in 1 patient with skin involvement.
Three patients were diagnosed at autopsy.
Immunochemotherapy consisted in first-line of R-CHOP (rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone; n = 4), R-CHOP with methotrexate (n = 1), and R-CVP (rituximab plus cyclophosphamide, vincristine, and prednisone; n = 1) and in second-line of R-CHOP (n = 1) and R-DHAP (rituximab plus dexamethasone, cytarabine, and cisplatin; n = 1).
Chemotherapy consisted in first-line of CHOP or CHOP-like regimens (n = 4), MBVP (methotrexate, carmustine, teniposide, and prednisone; n = 1), and vincristine with steroids (n = 1) and in second-line of IMVP (ifosfamide, methotrexate, etoposide, and prednisone) and DHAP followed by autologous stem-cell transplantation (n = 1).
Patients diagnosed at autopsy were excluded.
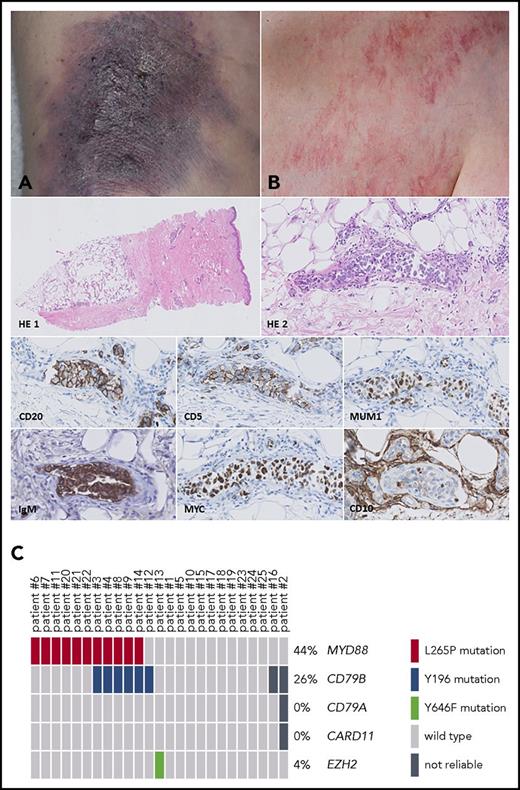
IVLBCL: skin lesions, histology, mutational status, and survival analysis. Skin lesions may present as bluish indurated plaques (A) or generalized telangiectasias (B). Histology of a representative skin biopsy shows a dermal blood vessel with intraluminal clustering of large, blastic cells (HE 1, HE 2), positive for CD20 (clone L26 from Dako, diluted 1:800), CD5 (clone 4C7 from Dako, diluted 1:400), MUM1 (clone MUM1p from Dako, diluted 1:100), IgM (polyclonal, from Dako, diluted 1:500), MYC (clone Y69 from ABCAM, diluted 1:100), and CD10 (clone 56C6 from Dako, diluted 1:20). Original magnification: ×50 for HE 1 and ×400 for HE 2, CD20, CD5, MUM1, IgM, MYC, and CD10. (C) OncoPrinter plot of the targeted next-generation sequencing data shows an MYD88 L265P mutation (NM_001172567) in 44%, a CD79B Y196 mutation (NM_001039933) in 26%, and an EZH2 Y646F mutation (NM_004456) in 4% of the patients. Patient numbers correspond to those in supplemental Table 1. In patients 16 and 2, the sequencing data of CD79B resp. CD79B/A and CARD11 were not reliable because of low read count (<100 reads).
IVLBCL: skin lesions, histology, mutational status, and survival analysis. Skin lesions may present as bluish indurated plaques (A) or generalized telangiectasias (B). Histology of a representative skin biopsy shows a dermal blood vessel with intraluminal clustering of large, blastic cells (HE 1, HE 2), positive for CD20 (clone L26 from Dako, diluted 1:800), CD5 (clone 4C7 from Dako, diluted 1:400), MUM1 (clone MUM1p from Dako, diluted 1:100), IgM (polyclonal, from Dako, diluted 1:500), MYC (clone Y69 from ABCAM, diluted 1:100), and CD10 (clone 56C6 from Dako, diluted 1:20). Original magnification: ×50 for HE 1 and ×400 for HE 2, CD20, CD5, MUM1, IgM, MYC, and CD10. (C) OncoPrinter plot of the targeted next-generation sequencing data shows an MYD88 L265P mutation (NM_001172567) in 44%, a CD79B Y196 mutation (NM_001039933) in 26%, and an EZH2 Y646F mutation (NM_004456) in 4% of the patients. Patient numbers correspond to those in supplemental Table 1. In patients 16 and 2, the sequencing data of CD79B resp. CD79B/A and CARD11 were not reliable because of low read count (<100 reads).
Histologic examination of the diagnostic pretreatment formalin-fixed, paraffin-embedded biopsies showed the presence of large blastic cells within the lumina of blood vessels. For 1 patient with skin-limited disease, only a skin biopsy of relapsed IVLBCL 6 years after diagnosis was available. Immunohistochemistry with antibodies against CD20, CD10, BCL6, MUM1, BCL2, IgM, MYC, CD5, cyclin D1, and CD30 was performed according to routine procedures (Table 1). In all cases, the tumor cells expressed CD20. Most cases were positive for BCL2 (23 of 24 [96%]), IgM (21 of 23 [91%]), and MUM1 (18 of 24 [75%]), whereas expression of CD5 (13 of 25 [52%]), MYC (15 of 22 [68%]), and BCL6 (14 of 24 [58%]) was variable. CD10 was weakly positive in 2 cases (8%), and all cases were negative for CD30 and cyclin D1. Expression of BCL2 and MUM1 in the vast majority of cases corresponded with that in previous studies14 and, together with expression of IgM, strongly suggested an activated B-cell phenotype. Also, aberrant coexpression of CD5 in a subset of patients with IVLBCL has been reported, without known clinical significance.14 Fluorescence in situ hybridization for MYC using Vysis Dual Color Break Apart Probes (Abbott) showed no rearrangement of the MYC gene in 15 evaluated patients, and in situ hybridization for Epstein-Barr virus early RNA was negative in 24 patients.
Additionally, we performed targeted next-generation sequencing covering hotspot regions of the genes MYD88 (exon 3/exon 5), CD79B (exon 5/exon 6), CD79A (exon 5), CARD11 (exon 6), and EZH2 (exon 16). As previously described, DNA of microdissected tumor cells was fully automatically isolated15 and sequenced16 with a single primer pool with Ampliseq amplicons covering hotspot regions of the selected genes. Pathogenic variants (class 5) and possibly pathogenic variants (class 4) with a variant allele frequency of ≥5% were reported. In 11 (44%) of 25 patients, MYD88 L265P mutations (NM_001172567) were detected. In 6 (26%) of 23 patients, mutations in CD79B Y196 (NM_001039933) were present, with cooccurrence of mutated MYD88 in 5 of these patients (Figure 1C). One patient had an EZH2 Y646F mutation (NM_004456), and no mutations were identified in the target regions of CD79A and CARD11. In 1 patient with a relapse after R-CHOP, the MYD88 L265P mutation was also identified in circulating free tumor DNA in the peripheral blood using digital droplet polymerase chain reaction (Bio-Rad). An overview of the results is presented in Table 1. Associated clinical and molecular features for each patient are listed in supplemental Table 1 (available on the Blood Web site).
To the best of our knowledge, this is the first study to report a mutational analysis of IVLBCL, revealing a high prevalence of MYD88 L265P mutations (44%) and CD79B Y196 mutations (26%). In DLBCL, activating mutations in MYD88 and/or CD79B have been identified as important molecular drivers of the Toll-like receptor and B-cell receptor pathways, respectively, resulting in constitutive activation of NFκB signaling.17,18 MYD88 mutations in particular are a hallmark of DLBCL presenting at immune-privileged sites, such as the testes and CNS, and primary cutaneous DLBCL leg type, with percentages up to 80%, in contrast to DLBCL in other sites (17%).12,13,19,20 Also, co-occurrence of MYD88 and CD79B mutations is a common phenomenon.11,13,17 Our results suggest that MYD88 and/or CD79B mutations are important molecular oncogenic drivers of IVLBCL.
As for the exploratory analysis of survival, Kaplan-Meier curves are shown in supplemental Figure 1. Because a significant number of patients received only supportive care or were treated in the prerituximab era, the 3-year OS in our cohort (43%) was lower than previously reported (60% to 81%).4,5 In line with literature, patients with skin-limited disease showed a trend in superior 5-year disease-specific survival compared with patients with systemic disease (log-rank P = .06).3 Mutational status of MYD88 and/or CD79B did not seem to influence disease-specific survival (log-rank P = .64), as was previously shown for other extranodal DLBCLs.12,21,22 Additionally, MYD88/CD79B mutations were detected in patients with skin-limited disease as well as those with systemic disease. Given our small patient number and heterogeneity of clinical characteristics and treatment regimens, our results indisputably need validation in larger, homogeneous (ie, R-CHOP–treated) IVLBCL cohorts, together with a broader evaluation of genetic aberrations, including other NFκB-activating mutations, such as TNFAIP3.
From a therapeutic perspective, our results suggest that a substantial number of patients with IVLBCL may be amenable to treatments targeting NFκB signaling. Recent studies have shown that patients with DLBCL with MYD88 and/or CD79B mutations are more sensitive to treatment with ibrutinib, a Bruton tyrosine kinase inhibitor that blocks the NFκB pathway.23-25 Additional studies are required to assess whether patients with IVLBCL may also benefit from these therapeutic approaches.
In conclusion, this is the first study to identify a high prevalence of MYD88 L265P and/or CD79B Y196 mutations as potential oncogenic drivers in patients with IVLBCL. This highlights the importance of investigating the mutational status of MYD88 and CD79B in larger, homogeneous cohorts and exploring the efficacy of molecularly targeted agents for these patients.
The online version of this article contains a data supplement.
Acknowledgments
This study was supported in part by research funding from Stichting Federatie Oncologie Holland (P.M.J., H.V., B.C.T., E.F.M.P., A.H.G.C., and J.S.P.V.).
Authorship
Contribution: A.M.R.S., P.M.J., R.W., M.H.V., H.V., A.H.G.C., and J.S.P.V. contributed to the design of the research; R.W., W.K., M.J.K., M.v.d.B., W.B.C.S., D.d.J., M.A.H., B.C.T., E.F.M.P., M.N., A.D., S.T.P., and J.S.P.V. contributed to the collection of patient material; A.M.R.S., P.M.J., A.-M.C.-J., S.F.S., R.v.E., W.K., and A.H.G.C. contributed to the analysis of the data; and A.M.R.S., P.M.J., R.W., A.H.G.C., and J.S.P.V. contributed to the writing of the manuscript.
Conflict-of-interest disclosure: M.H.V. is a member of an entity’s board of directors or advisory committee of the Innate Pharma safety board for IPH4102-101; M.J.K. and M.N. have received honoraria/research funding from Kite Pharma, Millennium/Takeda, Mundipharma, Gilead Sciences, Bristol-Myers Squibb, Roche, Celgene, Novartis Pharmaceuticals Corporation, Merck Sharp & Dohme, and Amgen. A.D. is a member of an advisory committee of Millennium/Takeda. The remaining authors declare no competing financial interests.
Correspondence: Joost S. P. Vermaat, Leiden University Medical Center, Department of Hematology, C2-R137, P.O. Box 9600, 2300 RC Leiden, The Netherlands; e-mail: j.s.p.vermaat@lumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal